Abstract
The present study aimed to evaluate the polysaccharides extracted from Toona sinensis seeds using ultrasound-assisted deep eutectic solvent (DES). The extracted polysaccharides (TSRP) were purified and studied for their hypoglycemic and antioxidant activities. The purified polysaccharides (TSRPs-1) were hydrolyzed and derivatized to study their monosaccharide composition. The optimum extraction conditions were DES (choline chloride to oxalic acid) at a molar ratio of 1.473:1, ultrasound time of 50.643 min, and a material-to-liquid ratio of 1:31.838 g/mL. The ultrasound-assisted DES extraction was more effective (56.1%) as compared to the water extraction (35.89%). The inhibition of α-glucosidase by TSRPs-1 was 42.77% at a concentration of 0.8 mg/mL. At 3.2 mg/mL, TSRPs-1 inhibited α-amylase by 34.38%. The clearance of DPPH by TSRPs-1 was 24.36% at 3.2 mg/mL. The IC50 for ABTS·+ clearance by TSRPs-1 was 0.233 mg/mL. It was shown that the monosaccharides of TSRPs-1 were composed of Man, Rha, GlcA, GalA, Glc, Gal, Xyl, and Ara in a molar ratio of 1:563.13:41.81:51.20:21.81:2244.58:3.73:1130.48. In this paper, the process of extracting TSRP with ultrasound-assisted DES was investigated and optimized. TSRPs-1 possesses certain hypoglycemic and antioxidant activities. Therefore, this paper can provide an experimental basis for the establishment of an industrialized production process of polysaccharides and the study of their biological activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed of Toona sinensis (A. Juss.) Roem. (TSR), known as the fruit of the Chinese Toona sinensis, is distributed widely in China [1]. It mainly contains flavones, polysaccharides, phenol, volatile oil, and terpenoids [2]. Due to the effect of anti-diarrhea, antibacterial activity, and relieving rheumatic pains, it has served as a medicinal material for almost a thousand years [3]. Toona sinensis (A. Juss.) Roem. polysaccharide (TSRP) is an important active substance in its active ingredient. Polysaccharides are glycoside-bonded sugar chains and are composed of at least 10 monosaccharides, widely used in medicine, food, and health care products due to their significant beneficial effect on the body [4]. How to improve the extraction rate of TSRP has a crucial influence in the research field of extraction of plant polysaccharide.
Deep eutectic solvent (DES) refers to a two-component or three-component low co-melting mixture composed of a hydrogen bond acceptor (such as quaternary ammonium salt) and a hydrogen bond donor (such as amide, carboxylic acid, and polyol), whose melt point is significantly lower than the melting point of the pure substance of each component [5]. It is a non-toxic, cheap, simple to prepare, and biodegradable green solvent that can be used to extract chemical components from different plants [6]. The viscosity and diffusion of DES systems affect the dissolution ability of polysaccharides, so water and the molar ratio of the component were adjusted to improve the solubility of polysaccharides [7]. At present, there are few studies on extraction using DES, but none of them focus on TSRP.
Polysaccharide extraction methods include enzyme extraction, solvent extraction, ultrasonic extraction, and microwave extraction. However, ultrasonic extraction combined with solvent extraction is common because ultrasonic machine produces powerful energy, effect, and cavitation to make the molecular thermal movement and penetration strong. This method has the advantages of short time-consuming, simple operation, and high extraction rate [8]. The response surface test is a method to optimize the extraction process.
In summary, the search for drugs and natural products with hypoglycemic and antioxidant activities are two hot research topics in the field of medicine. The polysaccharides of TSR have these two biological activities and are highly valuable for application and development. Therefore, the purpose of this study was to explore the optimal extraction conditions of TSRP with DESs through a single-factor experiment and response surface optimization experiment. Also, it aimed to study the antioxidant activity and hypoglycemic activity of TSRP in vitro and determine the monosaccharide composition of the polysaccharide, to provide a basis for the improvement and optimization of industrial extraction, healthcare, and food applications of TSRP.
Materials and methods
Materials and reagents
Toona sinensis was obtained from Guangdong Pharmaceutical University. n-butyl alcohol, glycol, malonic acid, glycerol, 1,4-butanediol, oxalic acid, acetyl propionic acid, urea, and citric acid were from Tianjin Zhiyuan Chemical Reagent Co., Ltd. (Tianjin China). Phenol reagents and concentrated sulfuric acid were obtained from Guangzhou Reagent Factory (Guangzhou, China). SephadexG-75 and DEAE-Sepharose were obtained from Lanxiao Technology and New Materials Co., Ltd. (Xian, China). Acarbose and p-nitrophenyl-α-d-glucopyranoside (PNPG) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Alpha-glucosidase, ABTS (2,2-diazo-di-3-ethylbenzothiazolin-6-sulfonic acid), d-( +)-anhydrous glucose, rhamnose, d-( +)-galacturonic acid, d-galactose, and d-glucuronic acid were obtained from Yuanye Biotechnology Co., Ltd. (Shanghai, China). α-amylase, DPPH (1,1-diphenyl-3-nitrophenylhydrazine), and PMP (1-phenyl-3-methyl-5-pyrazolone) were purchased from Maclin Biochemical Technology Co., Ltd. (Shanghai, China). DNS reagent was bought from Feijing Biotechnology Co., Ltd. (Fuzhou, China). l-Arabinose, d-mannose, d-( +)-xylose, and l-( −)-fucose were provided by Desi Biotechnology Co. Ltd. (Chengdu, China). All other chemicals and solvents were of analytical grade.
Determination of the polysaccharide extraction rate of TSRP
According to the previous method [9], the extraction process was carried out. 0.5 g of TSR powder was weighed in precision, and the extraction solvent was added with the corresponding material-to-liquid ratio. TSRP was extracted in an ultrasonic cleaner at the appropriate temperature and power conditions. After centrifugation, the filtrate was taken and concentrated. The TSRP precipitated by mixing with 95% ethanol till the alcohol meter was 80% and placed at room temperature for 12 h. The deposit was taken, dissolved in water, and mixed with Sevag regent (polysaccharide solution: 1,4-butanol:dichloromethane = 25:1:4). After centrifugation at 4000 r/min for 5 min, the precipitation was abandoned and the crude TSRP solution was acquired. Then, 100 µL of crude TSRP solution was transferred and 0.1 mL of 5% phenol solution and 0.5 mL of concentrated sulfuric acid solution, were added, shaking and waiting for 15 min. The absorbance was measured at 492 nm. According to the standard curve, the polysaccharide extraction rate was calculated according to the following formula:
where C is the mass concentration (mg/mL), N is the dilution multiple, V is the solvent volume (mL), and W is the mass of materials (g).
Screening test of DESs
The DES was prepared following the reported method [10]. Choline chloride (ChCl) was selected as the hydrogen bond receptor and mixed with 8 different hydrogen bond donors (ethylene glycol, malonic acid, glycerol, 1,4-butanediol, oxalic acid, urea, acetyl propionic acid, and citric acid) respectively at certain molar ratio (ChCl:ethylene glycol = 1:2, ChCl:malonic acid = 1:1, ChCl:glycerol = 1:2, ChCl:1,2-butanediol = 1:4, ChCl:oxalic acid = 2:1, ChCl:acetyl propionic acid = 1:2, ChCl:urea = 1:2 and ChCl:citric acid = 1:1). In 80 °C water bath, the material mixture were stirred to make a uniform transparent liquid mixture. After cooling to room temperature, the best DES with the highest extraction rate was chosen through the process of extracting the polysaccharides.
General water extraction method
0.5 g of TSR powder was accurately weighed, dissolved with 10 mL of distilled water, and ultrasonic for 40 min. The color was developed by the phenol–sulfuric acid method to determine the extraction rate. This experiment was performed in parallel three times.
Single-factor test
The material-to-liquid ratio, ultrasonic time, and molar ratio of DES were selected as the factors of extraction of TSRP. According to the experimental method of 2.2, the extraction process was carried out. The molar ratio included 1:1, 1.5:1, 2:1, 2.5:1, and 3:1. The ultrasonic time included 10, 30, 50, 70, 90 min. The material-to-liquid ratio included 10, 20, 30,40, and 50 mL/g. The best condition was fixed in subsequent experiments.
Effect of molar ratio of choline chloride:oxalic acid
Weighed 0.5 g of several portions of T. sinensis powder, added the material-to-liquid ratio of 1:20 g/mL according to the experimental method of 2.2, choline chloride:oxalic acid was designated as 1:1 to 3:1, and the adjusted ultrasonic time was set at 40 min. According to the experimental method of 2.2, the extraction process was carried out. The extract was transferred to a centrifuge tube and centrifuged to obtain the crude extract, which was then subjected to a color development reaction. The absorbance values at 490 nm were measured with a microplate reader, and TS's total polysaccharides extraction rate was calculated.
Effects of ultrasonic time
Weighed 0.5 g of several portions of T. sinensis powder, according to the experimental method of 2.2, added the material-to-liquid ratio 1:20 g/mL, the ultrasonication time was designated as 10 min to 90 min, and the molar ratio of choline chloride:oxalic acid was the optimal value selected from the above experiments. And pipetted the extract into a centrifuge tube for centrifugation to obtain the crude extract, then performed the color development reaction and measured the absorbance value at 490 nm with a microplate reader. The rate of extraction of all polysaccharides from TS was calculated.
Effects of material-to-liquid ratio
Precisely weigh 0.5 g of several portions of T. sinensis powder, according to the experimental method of 2.2, the material-to-liquid ratio of 1:10 g/mL to 1:50 g/mL was selected, and the ultrasonic time and the molar ratio of choline chloride:oxalic acid were the best values selected in the above experiments. The extracts were transferred to centrifuge tubes for centrifugation respectively to obtain crude extracts. Then the color development reaction was carried out, and the absorbance values were measured at 490 nm with a microplate reader to calculate the total polysaccharides extraction rate of TS.
Response surface experimental design
According to the results of 2.2.3, three influencing factors, the molar ratio of DES (A), ultrasonic time (B), and material-to-liquid ratio (C) were selected as the response variables, and the extraction rate of TSRP (Y) was taken as the response value. The level of the selected factors is shown in Table 1.
Determination and verification of the best process conditions
Under the optimal conditions obtained by the response surface optimization, the extraction was conducted to determine the best extraction yield. And the DES extraction was compared with water extraction.
Isolation and purification of crude polysaccharides
The purification was conducted, as described by the previous paper [11]. After concentrating the crude extract with a rotary meter, the concentrated solution was placed on an evaporation dish, and placed in a water bath for 60 °C heating and drying until constant weight. The sample solution of crude TSRP was prepared into 25 mg/mL and 10 mL of it was added into the DEAE-Sepharose Fast Flow chromatography column. With distilled water and gradient sodium chloride as elution agent, 8 mL of eluate per tube was collected at 1.8 mL/min, 50 tubes by water, and 80 tubes by salt washing. The elution curve was developed by the phenol–sulfuric acid method, and the eluate for the peak was collected and combined.
The eluate was concentrated, and put into a dialysis bag (retention amount is 3500 Da), with running water to dialysis for 24 h, and then concentrated and dried to acquire the material TSRPs. TSRPs were dissolved with distilled water to make a 10 mg / mL sample solution, and 5 mL of the solution was filtrated on the column in Sephadex G-75 column. Using distilled water as eluent, a flowing rate of 0.8 mL/min, 8 mL of eluate was collected in each tube, and collected 50 tubes. The elution curve was developed by the phenol–sulfuric acid method, and the eluate for the peak was collected and combined.
Purity calculation
The eluate was collected by Sephadex G-75, concentrated, and dried to make a material sample (named TPS-1), which was weighted and dissolved with distilled water to obtain test liquid for the determination of purity. The color was developed by the phenol–sulfuric acid method, and purity was calculated according to the following formula:
where c is polysaccharide concentration (mg/mL), V is the solution volume (mL) and m is the mass of the sample (mg).
Glucose-lowering activity test
α-Glucosidase inhibitory activity assay
To determine the inhibitory activity of α-glucosidase, the experimental protocol [12] was slightly changed. Briefly, 40 μL of sample solution (3200, 600, 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, and 1.5625 μg/mL) and 40 μL of α-glucosidase solution were mixed in 96-well plates and incubated at 37 °C for 10 min. After that, 20 μL of PNG solution was added and reacted at 37 °C for 30 min, and 100 μL of Na2CO3 solution was added to terminate the reaction. Acarbose as a positive control, the absorbance at 405 nm was measured three times in parallel, and α-glucosidase inhibition was calculated using the following equation:
where A0 is the absorbance of the blank, A1 is the absorbance of the sample and A2 is the background absorbance of the sample.
α-Amylase inhibition activity assay
The scheme was designed to refer to the reported method [13]. Briefly, 40 μL of sample solution (3200, 600, 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, and 1.5625 μg/mL) was mixed with 40 μL of α-amylase solution, and incubated at 37 °C for 10 min. Then, 20 μL of 1% starch solution was added and incubated at 37 °C for 10 min. DNS reagent was added and bathed in 100 °C to stop the reaction. Finally, the reaction liquid was diluted with 1 mL of distilled water and the absorbance was determined at 540 nm. Acarbose was a positive control. α-Amylase inhibition was calculated using the following equation:
where A0 is the absorbance of the blank, A1 is the absorbance of the sample and A2 is the background absorbance of the sample.
Antioxidant activity test
DPPH radical scavenging activity measurement
The method to determine the scavenging activity of TPS-1 was described by the reported method [14]. Briefly, 150 μg of sample solution (3200, 600, 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, and 1.5625 μg/mL) was mixed with 50 μg of DPPH-ethanol solution and reacted in the dark for 30 min, with Vc as positive control and distilled water as blank control. The absorbance was measured at 517 nm. The DPPH clearance rate is calculated as the formula described below:
where A0 is the absorbance of the blank, A1 is the absorbance of the sample and A2 is the background absorbance of the sample.
ABTS ·+ radical scavenging activity measurement
The method was adjusted by the reported method [15]. Forty microliters of sample solution (3200, 600, 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, and 1.5625 μg/mL) and 160 μL of ABTS·+ working solution were mixed and then reacted in the dark. VitC is a positive control, three times in parallel, the absorbance was measured at 734 nm, and ABTS·+ clearance was calculated by the following formula:
where A0 is the absorbance of the blank, A1 is the absorbance of the sample and A2 is the background absorbance of the sample.
The analysis of the monosaccharides component of TSRP-1
Derivatization of mixed monosaccharides
The method of derivatization was slightly modified from the previous method [16]. All the monosaccharides (glucose, mannose, arabinose, xylose, fucose, glucuronic acid, rhamnose, and galactose) were weighed precisely to make the monosaccharide control solution (2 mM). From every kind of control solution, 50 μL of it was taken, and mixed with 450 μL of 0.3 M NaOH solution and 450 μL of 0.5 M PMP-methanol solution, bathing in water at 70 °C. After cooling, 460 μL of HCl (0.3 M) to neutralize. 1 mL of chloroform was added and extracted three times to remove the extra PMP. The water layer was taken and filtered through a filter membrane (0.45 μm). And the mixed monosaccharides sample was obtained.
PMP derivatization of TSRP-1
5 mg of TSRP-1 was weighed precisely and combined with 2 mL of TFA (2 M) in a reacting tube of 10 mL, sealed, and bathed in the oil at 120 °C for 6 h to hydrolyze the polysaccharide into monosaccharides. The hydrolysis products were transferred into a 50 mL round-bottom flask, added 6 mL of methanol, and concentrated to dry at 65 °C with decompression. The above process was repeated three times to remove the TFA. Then 1 mL of deionized water was added to dissolve. 100 μL of the hydrolysis sample was taken into a 5 mL centrifuge tube for the derivatization, according to the method in 2.12.1.
In this experiment, HPLC was employed to analyze the component of polysaccharide, with the use of a C18 column (4.6 × 250 mm) and ultraviolet detector (250 nm). 10 μL of sample solution was injected at a flow rate of 1 mL/min. The mobile phase is 0.05 M phosphate buffer salt (KH2PO4-NaOH, pH 6.7). By applying HPLC, the monosaccharide composition of TSRP-1 was obtained.
Calculation method of the molar ratio of every single monosaccharide: calculate the relative correction factor f by using the glucose control in the mixed control as the internal standard, according to the following formula:
where ms is the quality of the glucose control, mi is the quality of the other monosaccharide fractions, As and \({A}_{i}{\prime}\) are the peak area of the glucose control article and the peak area of the other monosaccharides, respectively, A1–Ai is the peak area of each monosaccharide derivative of the hydrolyzed sample and M1–Mi is the molar mass of each monosaccharide derivative in the hydrolyzed sample.
Results and discussion
Screening result of DES
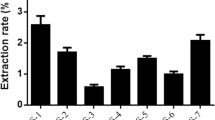
As shown in Fig. 1, the TSRP extracted with the DES composed of choline chloride and oxalic acid, reached the maximum value of extraction rate. So the choline chloride-oxalic acid with a molar ratio of 2:1 is the best one among the eight kinds of DES, and it achieves a higher yield than water extraction. Different compositions of DESs contributed to varied properties of DESs including the polarity, capability to dissolve natural compounds, viscosity, and mobility. Thus, the extraction rate was governed by these properties and varied when using different DESs (Panzella et al., 2020).
Results of single-factor test
The effect of the molar ratio of choline chloride and oxalate
Molar ratio is a crucial factor for a successful DES, because different proportions of its composition show different fluidity, viscosity, density, and different ability to dissolve polysaccharides, resulting in different extraction rates. As shown in Fig. 2A, at the ratio of 1.5:1, the polysaccharide extraction rate reached the maximum. When the molar ratio is 3:1, the extraction rate improved again, but the increasing molar ratio of DES tended to coagulate and crystallize, leading to a complicated process of the extraction of TSRP [17]. At this time, the viscosity and surface tension of DES reached the best value, so the extraction effect is better [18]. When the molar ratio was 1:1, the extraction rate was at a low level, which is probably because the components were more likely to crystalize at room temperature, thus hindering the dissolution of TSRP [8]. Thus, the best molar ratio of 1.5:1 was chosen for the subsequent test.
Effect of time on ultrasound
As shown in Fig. 2B, the extraction rate of TSRP reached the maximum value at 50 min, and the extraction rate gradually decreased with the ultrasound time. It is mainly because the ultrasonic wave has powerful energy, so destroyed the cell wall of TSR easily, promoted solvent penetration, and accelerated polysaccharides to dissolve. Part of the reason may be that proper time promoted the diffusion of dissolution of the polysaccharide with intensive interaction between the solvent and the RRT powder [19]. If ultrasonic time is too long, may destroy the polysaccharide structure of TSR, thus reducing the polysaccharide extraction rate [20]. In this experiment, 50 min of ultrasound time was used for the subsequent experiments.
The influence of material-to-liquid ratio
As shown in Fig. 2C, the extraction rate of 1:30 g/mL reached the maximum value, after which the extraction rate of TSRP showed a downward trend. This is due to the inability of a small amount of DES to completely wet the TSR powder, resulting in incomplete dissolution of the polysaccharide and a low extraction rate. Oppositely, a large solvent enabled the target compound to diffuse out of the cell thoroughly [5]. When the material-to-liquid is too large, too much low DES can dissolve other soluble components, which also affects the dissolution of TSRP to a certain extent, thus making the polysaccharides extraction effect decrease. In this experiment, the material-to-liquid ratio of 1:30 g/mL was determined for subsequent experiments.
Test results and analysis of the response surface
Based on the extraction results of single factor tests, the extraction rate of polysaccharide was taken as the response value, the molar ratio (A), ultrasonic time (B), and material-to-liquid ratio (C) as the response variables. The results of the response surface test designed according to the Box-Behnken principle are shown in Table 2.
Based on the results of the 17 test points of the BBD random test, the statistical analysis was performed using the Design Expert 11 software, as shown in Table 2. According to the experimental data obtained by the regression method, the polysaccharide extraction rate (Y), molar ratio (A), ultrasonic time (B), and material-to-liquid ratio (C) can be fitted to the following second-order polynomial equation: Y = 55.55 − 0.9288A + 0.8588B + 5.27C + 0.9773AB + 1.83AC − 1.09BC − 5.11A2 − 9.37B2 − 14C2, in which R2 is 0.9533, indicating a good fit.
The above models were significance-tested with ANOVA, and the results are shown in Table 3.
As shown in Table 3, the model of polysaccharide extraction rate was significant (P < 0.05), and the difference in P value of pure error was not significant (P > 0.05), indicating that the model had a good fitting effect on the independent variables. According to the P value of the interaction term, the interaction effect of AB and BC was not significant. From the F value, the order of effect is material-to-liquid ratio > molar ratio > ultrasound time; P values of A2, B2, and C2 are below 0.01, which shows that the three have a significant influence on the polysaccharide extraction rate.
BBD
Using Design-Expert 11 software, the response surface map and contour map corresponding to the interaction of each factor were obtained. As shown in Fig. 3A to C, to predict the interaction between variables, both types of plots can directly reflect the results of response values under the interaction of various factors. The steeper the response surface or the contour shape tends to be an ellipse, the more the interaction between the two factors is significant. The vertices of the response surface are the central points of the smallest ellipse in the contour line.
Within a certain range, with the increase of various factors, the extraction rate of TSRP gradually increases until the response surface has the maximum value, and then the various factors will gradually decrease. As can be seen from the figures, the steeper of AC is obvious, indicating that the interaction of AC is significant, while the interaction of AB and BC is not significant. This is consistent with the data from the ANOVA results. This was consistent with the single factors experiments. The contour plots were ellipse, which confirmed that these interacting variables affected the extraction rate.
Verification of the optimal conditions
After the analysis of the response surface, it was concluded that the optimal parameters for polysaccharide extraction of T. sinensis were a molar ratio of 1.473:1, time of 50.643 min, and material-to-liquid ratio of 31.838 mL/g, and the extraction rate of TSRP was 56.1%. For the convenience of operation, the molar ratio was adjusted to 1:47 g/mL, the time was 50 min, and the material-to-liquid ratio was 31 mL/g. After this test, the yield of TSRP was 55.9%, which was similar to the theoretical extraction rate, proving that the fitting degree of the model is acceptable. Compared with the water extraction rate of 35.89%, the optimization effect is good. This experiment not only indicated that the optimal results were reliable but also proved that the extraction method was feasible.
Therefore, according to this experiment and some other articles, DES is a practical and excellent solvent with no harm to the environment and high efficiency in extracting TSRP. In other words, this method had great potential to become a widely used way to extract TSRP, and to some extent, this paper provides an experimental foundation for the research of optimizing the extraction method of TSRP.
Isolation and purification results
Crude polysaccharides were separated by the DEAE column, and an elution peak appeared in the 0 ~ 0.8 M NaCl gradient. The elution map is shown in Fig. 4A. The peak components obtained from the DEAE column were separated and purified by the SephadexG-75 column, with a single elution peak (named TSRPs-1). The elution map is shown in Fig. 4B. The purified sample components in Fig. 4A and B were tested by the phenol-sulfate method and calculated with purity of 39.06% and 96.15%, respectively, which showed that TSRPs-1 was relatively pure.
Results and analysis of the hypoglycemic activity of TSRPs-1
As shown in Fig. 5A, when the concentration of polysaccharide and acarbose gradually increased, their activity against α-glucosidase was continuously increased. TSRPs-1 had an inhibiting effect on α-glucosidase in a concentration-dependent manner. At a concentration of 0.8 mg/mL, TSRPs-1 inhibited α-glucosidase by 42.77%. The IC50 of acarbose was 0.025 μg/mL. Therefore, TSRPs-1 inhibited α-glucosidase, but its effect was much weaker than that of acarbose.
As shown in Fig. 5B, when the concentration of TSRPs-1 and acarbose gradually increased, their inhibition effect on α-amylase was gradually enhanced. TSRPs-1 had an inhibiting effect on α-amylase in a concentration-dependent manner. At a concentration of 3.2 mg/mL, TSRPs-1 inhibited α-amylase by 34.38%. The IC50 of acarbose was 24.437 μg/mL. Therefore, TSRPs-1 inhibited α-amylase, but its effect was much weaker than that of acarbose. The α-amylase inhibitory activity of TSRPs-1 may be due to the presence of hydroxyl and carboxyl groups in the sugar chain of TSRPs-1, which bind to amino acid residues, thus binding to digestive enzymes and inhibit enzyme activity [21].
Therefore, T. sinensis polysaccharide has the potential to become an auxiliary hypoglycemic agent, or even replace traditional hypoglycemic drugs to make up for their defects, so it has a good development and application prospect. And further studies are in urgent need.
Results and analysis of the antioxidant activity of TSRPs-1
As shown in Fig. 6A, when the concentration of polysaccharide and Vit C gradually increased, the clearance effect of both on DPPH radicals gradually increased. TSRPs-1 had a scavenging effect on DPPH· in a concentration-dependent manner. At 3.2 mg/mL, TSRPs-1 inhibited DPPH· by 24.36%. The IC50 of Vit C was 4.541 μg/mL, so the TSRPs-1 had a DPPH radical removal effect, but its effect was weaker than that of Vit C.
As shown in Fig. 6B, when the concentration of polysaccharide and Vit C gradually increased, The removal effect of ABTS·+ radicals was constantly enhanced. TSRPs-1 had a scavenging effect on ABTS·+ in a concentration-dependent manner. At 3.2 mg/mL, TSRPs-1 inhibited DPPH· by 78.74%. The IC50 of TSRPs-1 was 0.233 mg/mL, significantly higher than VitC (1.015 μg/mL); therefore, TSRPs-1 pair cleared ABTS ·+ radicals have a scavenging effect that is much weaker than Vit C. By observing the activity of Brasenia schreberi polysaccharides against ABTS+ free radicals, Xiao et al. [22] showed that the IC50 value of BPL-1 was 5.46 mg/mL, which was higher than the IC50 of TSRPs-1. TSRPs-1 had a stronger scavenging effect on ABTS+ free radicals.
With the enhancement of health awareness, people tend to search for a relatively safe and convenient way to improve their physical conditions or prevent the occurrence of the disease, and TSRPs-1 has shown the possibility of helping people achieve this goal. Hence, attention should be paid to the development of the application of TSRPs-1, and further investigation should be conducted to clarify the relationship between the structure and the function of TSRPs-1.
Analysis of monosaccharide composition
Figure 7B shows the composition of TSRPs-1 measured by HPLC. Compared with mixed standard monosaccharides in Fig. 7A, the conclusion can be drawn that the TPSs-1 consisted of d-mannose (Man), l-rhamnose (Rha), d-glucuronic acid (GlcA), d-galacturonic acid (GalA), D ( +) anhydrous glucose (Glc), d-galactose (Gal), d-( +)-xylose (Xyl) and l-arabinose (Ara) composition, molar ratio was 1:563.13:41.81:51.20:21.81:2244.58:3.73:1130.48. The analysis of monosaccharide components is the first step in the analysis of polysaccharides. Since TSRPs-1 own some advantages and have great potential to be a supplemental agent, the analysis of their structure is needed, and the results of this experiment can offer some clues to further exploring their chemical structures and more important characteristics for the better application of TSRPs-1.
Ultrasound-assisted DES extraction is a non-toxic, inexpensive, simple to prepare, and biodegradable green solvent and can be used to extract chemical components from different food matrices. There are no studies on the use of response surface optimization DES for TSRP extraction. Ultrasonic-assisted DES extraction presented a higher yield of polysaccharides compared with water extraction. It was shown that the monosaccharide composition of TSRPs-1 was Man, Rha, GlcA, GalA, Glc, Gal, Xyl, and Ara, with a molar ratio of 1:563.13:41.81:51.20:21.81:2244.58:3.73:1130.48. TSRPs-1 is found for the first time. Moreover, TSRPs-1 showed certain anti-hyperglycemic and antioxidant activities.
Conclusions
In this paper, the ultrasound-assisted DES extraction conditions were optimized with a molar ratio of choline chloride to oxalic acid of 1.473:1, an ultrasound time of 50.643 min, and a material-to-liquid ratio of 1:31.838 g/mL, and the theoretical value of extraction rate was 56.1%. The ultrasound-assisted DES extraction was better compared with the water extraction of 35.89%. The inhibition of α-glucosidase by TSRPs-1 was 42.77% at a concentration of 0.8 mg/mL. At 3.2 mg/mL, TSRPs-1 inhibited α-amylase by 34.38%. The clearance of DPPH by TSRPs-1 was 24.36% at 3.2 mg/mL. The IC50 for ABTS·+ clearance by TSRPs-1 was 0.233 mg/mL. Therefore, the TSRPs-1 had certain hypoglycemic and antioxidant effects. It was shown that the monosaccharide composition of TSRPs-1 was Man, Rha, GlcA, GalA, Glc, Gal, Xyl, and Ara, with a molar ratio of 1:563.13:41.81:51.20:21.81:2244.58:3.73:1130.48. This offered a method for the development and utilization of T. sinensis polysaccharides. The structural analysis and the mechanism of hypoglycemic and antioxidant activities of TSRPs-1 need to be further investigated. It is hoped that more, in-depth and diverse reports on the preparation, structural analysis, and activity of Toona sinensis polysaccharides and their derivatives will be available in the future.
References
S. Ding, P. Su, D. Wang, X. Chen, C. Tang, J. Hou, L. Wu, LWT 173, 114400 (2023)
W. Peng, Y. Liu, M. Hu, M. Zhang, J. Yang, F. Liang, Q. Huang, C. Wu, Rev. Bras. Farmacogn. 29, 111–124 (2019)
Y. Yan, Y. Min, H. Min, C. Chao, Q. Ying, H. Zhi, J. Ethnopharmacol. 151, 176–182 (2014)
Q. Ren, J. Chen, Y. Ding, J. Cheng, S. Yang, Z. Ding, Q. Dai, Z. Ding, Int. J. Biol. Macromol. 124, 972–980 (2019)
Y. Zhang, L. He, Q. Li, J. Cheng, Y. Wang, J. Zhao, S. Yuan, Y. Chen, R. Shi, Sustain. Chem. Pharm. 30, 100855 (2022)
K.A. Avilés-Betanzos, J.V. Cauich-Rodríguez, M. González-Ávila, M. Scampicchio, K. Morozova, M.O. Ramírez-Sucre, I.M. Rodríguez-Buenfil, Processes 11, 1729 (2023)
L. Lomba, Á. Werner, B. Giner, C. Lafuente, Molecules 28, 6023 (2023)
N. Wang, Q. Li, Sustain. Chem. Pharm. 30, 100877 (2022)
J. Yao, J. Zeng, H. Tang, Y. Cheng, J. Tan, T. Li, X. Li, J. He, Y. Zhang, Sustain. Chem. Pharm. 34, 101166 (2023)
F. Chen, X. Su, J. Gao, Y. Liu, Q. Zhang, D. Luo, J. Chromatogr. A 1707, 464282 (2023)
Y. Fan, J. Ma, G. Wang, X. Li, Y. Liu, E. Xu, A. Luo, Arab. J. Chem. 16, 105033 (2023)
R. Xu, Y.-G. Bu, M.-L. Zhao, R. Tao, J. Luo, Y. Li, J. Funct. Foods 73, 104108 (2020)
F. Xia, S. Cao, M. Wang, Y. Sun, Food Biosci. 55, 103007 (2023)
X. Peng, J. Liu, N. Tang, J. Deng, C. Liu, H. Kan, P. Zhao, X. Zhang, Z. Shi, Y. Liu, Food Chem. X 17, 100621 (2023)
J.B. Baranyika, S. Bakire, P. Shoucheng, H. Hirwa, J. Uwagaba, S. Meihao, Results Chem. 5, 100989 (2023)
Q. Yuan, L. Zhao, Q. Cha, Y. Sun, H. Ye, X. Zeng, J. Agric. Food Chem. 63, 7986–7994 (2015)
M. Meenu, V. Bansal, S. Rana, N. Sharma, V. Kumar, V. Arora, M. Garg, Sustain. Chem. Pharm. 34, 101168 (2023)
S. Della Posta, V. Gallo, A. Gentili, C. Fanali, TrAC Trends Anal. Chem. 157, 116798 (2022)
Z. Tang, Y. Wang, G. Huang, H. Huang, Ultrason. Sonochem. 97, 106474 (2023)
B. Lin, S. Wang, A. Zhou, Q. Hu, G. Huang, Ultrason. Sonochem. 98, 106507 (2023)
C. Cao, Q. Huang, B. Zhang, C. Li, X. Fu, Int. J. Biol. Macromol. 109, 357–368 (2018)
H. Xiao, X. Cai, Y. Fan, A. Luo, Pharmacogn. Mag. 12, 193–197 (2016)
Funding
This research work was financially supported by the Science and Technology Program of Guangzhou, China (No. 202201010530) and the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110565).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, Y., Chen, G. & Zhang, X. Optimization of ultrasound-assisted deep eutectic solvent extraction, characterization, and biological studies of polysaccharide from seeds of Toona sinensis. Food Measure 18, 3255–3265 (2024). https://doi.org/10.1007/s11694-024-02400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02400-5