Abstract
Orlando tangelo fruits, originating from tropical and subtropical regions, have demonstrated a tendency to be susceptible to chilling injury when subjected to cold storage conditions. This study investigated the effects of methyl jasmonate (MeJA) (50 µM), γ-aminobutyric acid (GABA) (5 mM), and MeJA (50 µM) + GABA (5 mM) on chilling injury of Orlando tangelo fruits throughout cold storage (90 days at 3 ± 0.5 °C plus 5 days at 20 °C, shelf life). The findings demonstrated that all treatments considerably decreased fruit weight loss. Although all treatments significantly decreased chilling injury, MeJA treatment resulted in the least amount of chilling injury. Malonaldehyde was also significantly lower in the treated fruits in comparison to the control. Maximum phenol and flavonoid were observed in the MeJA treatment, which was 1.47 and 1.63 times higher than the control fruit, respectively. On average, the antioxidant activity of the treatments was 1.33 times higher than that of the control. The treatments increased the activity of catalase (CAT) and peroxidase (POD) enzymes in fruit juice and peel. Whereas, the highest activity of CAT and POD enzymes in the peel was observed in the treatments of MeJA and MeJA + GABA, respectively. Compared to other treatments, MeJA significantly increased the activity of the CAT enzyme in the fruit peel. Even though MeJA exhibited a more pronounced positive effect, the overall results indicate that both individual treatments and MeJA + GABA could be utilized to enhance the cold tolerance of Orlando tangelo fruit during extended periods of cold storage.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cold storage is utilized to increase the shelf life of different vegetables and fruits after harvest. On the other hand, a lot of tropical and subtropical fruits are extremely susceptible to chilling injury (CI) [1, 2]. Many citrus cultivars experience significant postharvest losses due to chilling damage (CI), which causes pitting, necrosis, and discoloration in the flavedo tissue (the colored outer layer of the peel) of the fruit [3]. The Orlando tangelo (C. paradisi Macfad. × C. reticulata Blanco.) is a cross between Duncan grapefruit and Dancy tangerine. One of the most serious postharvest issues affecting Orlando tangelo is their susceptibility to low temperatures, which causes chilling injury (CI). Citrus chilling damage is characterized by the most significant symptoms, which include peel browning, peel pitting, and superficial scald-like symptoms [1]. This physiological disorder reduces the fruit’s external quality and, as a result, its fresh market value. Therefore, adopting postharvest methods to maintain the quality and extend the shelf life of fruit in cold storage is crucial. Some elicitors have been more often used in recent years to help subtropical and tropical fruits develop tolerance to cold stress.
Jasmonic acid (JA) and MeJA, which are endogenous plant hormones, mainly affect plant defense responses such as antioxidant capacity against infections and abiotic stress [4, 5]. MeJA, a cyclopentanone-based molecule derived from a-linolenic acid, is regarded as a crucial plant hormone that can regulate inter- and cross-interactions because of its capacity to spread throughout biological system membranes and its volatile nature [4]. It has been claimed that the administration of MeJA slowed the onset of senescence in mandarins [6]. In previous research, it has been reported that MeJA had a beneficial impact on enhancing the antioxidant characteristics and postharvest quality of horticultural products [7].
γ-Aminobutyric acid (GABA), a naturally occurring signal molecule, is one of the effective and environmentally beneficial substances that prolong the shelf life of agricultural products. GABA is also regularly used to reduce fruit chilling injury, reduce oxidative damage, and increase disease resistance in horticultural crops [8]. It is a carbon-four non-protein amino acid that is synthesized in plants and accumulated to high levels under stress conditions such as heat, drought, UV radiation and chilling damage, salinity, and heavy metal toxicity [9, 10]. There is growing evidence that GABA protects many fruits from chilling injury in a crucial way. Because of GABA role in the TCA cycle, elimination of free radicals and reactive oxygen species, enhancement of antioxidant systems, and phenolic biosynthesis may contribute to the preservation of fresh fruits and vegetables for extended periods of storage [11].
Considering the role of MeJA and GABA as biological signaling molecules in plants, as well as the importance of safe and environmentally friendly compounds for human health and the environment, the use of these compounds is highly significant. On the other hand, due to the sensitivity of Orlando tangelo fruit to low temperatures and the high economic importance of preventing cold-induced damage in these citrus fruits during storage, the use of natural and non-chemical compounds can be employed as an effective and important solution. However, to the best of our knowledge, there is currently no research on the use of these treatments and their synergistic effect to preserve Orlando tangelo fruit quality in long-term cold storage. Subsequently, the purpose of this study was to determine the influence of these treatments on the quality and chilling injury of Orlando tangelo fruit during long-term cold storage.
Materials and methods
Fruit materials
Physiologically matured tangerine fruit (Orlando tangelo), were collected from a commercial orchard in Dasht Mosafar Abad, Ziyaratali village, Rodan city (57° 29′ E and 27° 59′ N), Hormozgan province, Iran. In terms of climate, this region has a temperature with an average temperature of 37 °C and a humidity of 64%. The fruits were brought to the lab in a plastic container within 2 h after being packed. Fruits with uniform size, color, and no mechanical damage or disease were selected for the following test.
Experimental strategy
The Orlando tangelo fruits were disinfected by sodium hypochlorite (0.05%) [12] and then immersed for 10 min in MeJA (50 µM), GABA (5 mM), and MeJA (50 µM) + GABA (5 mM). Control fruits were immersed in distilled water for 10 min. After drying at room temperature, the fruits were placed in plastic baskets and stored at 3 ± 0.5 °C and 80–90% RH for 90 days. For quality and biochemical analysis, sampling was done after 30, 60 and 90 days cold storage plus 5 days at ambient temperature. A portion of the samples were also stored at − 80 °C after frozen in liquid nitrogen.
Chilling index (CI)
Fruit chilling injury was evaluated by measuring the extent of browning and pitting area as described by [13], considering the following scales: 0 = lacking pitting spot, 1 = fewer than ¼ pitting spot, 2 = ¼ to ½ pitting, 3 = ½ to ¾ pitting, and 4 = more than ¾ pitting. Chilling injury was considered by the following formula (1):
where CIi is the number of fruits at the CI level and N is the total number of fruit in the treatment.
Malondialdehyde (MDA)
Orlando tangelo samples (0.25 g of peel and 500 µL of juice) were ground in 5 mL of trichloroacetic acid (1%), after centrifugation (10 min at 10,000 rpm), 500 μL of the extract was added to 2 trichloroacetic acid (20% containing 0.5% thiobarbituric acid) and heated at 95 °C for 30 min. The cooled mixture was centrifuged at 10,000 rpm for 10 min. The absorbance of the samples was read at a wavelength of 532 nm. Using the MDA extinction coefficient (0.155 mM−1 cm−1), the malondialdehyde (MDA) concentration per gram of fresh weight was computed and reported as μmol MDA/g fresh weight [14].
Determination of weight loss
Fruits were evaluated repeatedly on a computerized scale to measure the weight reduction of samples. Weight loss was determined as W0 − Wf/W0 × 100, where W0 is the initial weight, and Wf is the final weight [15].
where w0 represents the initial mass and w1 represents the final mass.
Total phenol content (TPC) and total flavonoid content (TFC)
The methanolic extract was prepared by mixing 500 µL of fruit juice with 1500 µL of methanol (85%) and after 24 h of rest, centrifugation (13,000 rpm) for 10 min. To measure the total phenol, total flavonoid and antioxidant activity of the extract, the method [16] was used with very minor changes. From each sample, 150 µL of methanolic extract was mixed with 750 µL of Folin–Ciocalteu reagent and allowed to rest for 5 min. Then 600 µL of 7% Na2CO3 was added. After putting the samples in the shaker for one and a half hours and in the dark, the absorbance at 750 nm was measured using a UV–visible spectrophotometer (CECIL-2501, England. The results were presented in milligrams of gallic acid equivalent (mg/g FW).
The flavonoid concentration was determined using the aluminum chloride colorimetric test [17]. Briefly, 200 μL of methanolic extract was mixed with 600 μL of methanol (85%), then 40 μL of 10% aluminum chloride, 40 μL of 1 M potassium acetate and 1120 μL of distilled water were added to the samples. After 30 min of placing the samples in the shaker and darkness, the UV–Vis spectrophotometer was used to test the absorption of the sample at 415 nm (CECIL-2501, England). The calibration curve was constructed utilizing quercetin amounts as a reference. The data were presented as (µg/mL).
Antioxidant capacity
We mostly adhered to the procedure created by [18] for the 2,2-Diphenyl-1-picrylidrazil (DPPH) radical scavenging test. As a result, 30 μL of methanolic extract was mixed with 1170 μL of DPPH and each sample was vortexed for 10 s. After being left in the dark for 30 min, the mixture’s absorbance at 517 nm was measured using a UV–VIS spectrophotometer. The DPPH radical scavenging activity was calculated as follows:
where AA is antioxidant activity (%), Ac is A control, and As is A sample.
Color measurement
Peel color based on the L*, a*, and b* color system was determined, where the L* value indicates darkness or lightness, the a* value is green (−) or red (+), and the b* value is yellow (+) or blue (−). These parameters were measured using a handheld colorimeter (CR-400) [19].
Catalase and polyphenol oxidase
For enzyme extraction, 0.5 g of fruit peel and 500 μL of fruit juice were mixed with 3 μL and 1500 μL of potassium phosphate buffer (pH 7.4, 1.5 mL 50 mM) containing 1 mM EDTA and 1% PVP (w/v), respectively, at 4 °C. The resulting mixture was homogenized and then centrifuged at 13,000×g at 4 °C for 20 min. The supernatant was used to estimate CAT and POD activity using a spectrophotometer (Spectrophotometer CECIL Model 3000, Cambridge, England).
POD activity (POD, EC: 1.11.1.7) was established using guaiacol as a substrate. For each sample, 100 μL of fruit juice extract and 20 μL of fruit peel extract were mixed with 50 μL of guaiacol and 150 μL of hydrogen peroxide (H2O2). The absorbance was measured at a wavelength of 460 nm for 1 min, and the change in OD 460 per gram of fresh weight per minute was used to identify POD activity. Each measurement was performed in triplicate [20].
Catalase (CAT, EC: 1.11.1.6) was evaluated using the method developed by [21]. For fruit juice, 200 μL of the extract was mixed with 1000 μL of 50 mM phosphate buffer (KH2PO4, pH 7.0) and 20 μL of hydrogen peroxide (H2O2) for each sample. For fruit peel, 10 μL of the extract was mixed with 1500 μL of 50 mM phosphate buffer (KH2PO4, pH 7.0) and 10 μL of H2O2 for each sample. The absorption of each sample was measured at a wavelength of 470 nm using a spectrophotometer (Cecil 2501, England). Each measurement was performed in triplicate.
Total soluble solid (TSS), pH and titratable acidity (TA)
The total soluble solids (TSS) of the fruit juice was determined using a handheld 173 refractometer (DBR95) manufactured in Thailand at 20 °C. The TSS values were expressed as a percentage (%), and three fruits were used for each replicate in the analysis. For titratable acidity (TA) quantitation, 5 mL of fruit juice was mixed with 20 mL of distilled water and titrated with 1% NaOH to pH 8.1. The result was expressed as a percentage of citric acid. The fruit juice pH was measured using a pH meter (HI-2211 Hanna, Romania).
Experimental design
The experiment was conducted as a factorial in the form of a completely randomized design with three replications. The data were analyzed using analysis of variance (ANOVA), and the mean values were compared with the LSD test at (P < 0.05) significance level using the SAS software (version 9.1). The principal component analysis (PCA) was performed using the XLSTAT program, version 2020 (www.xlstat.com, Addinsoft SARL). Information on hierarchical cluster analysis and Pearson correlation was carried out using (www.r-project.org).
Results
Weight loss in fruit
Over time, the weight loss of the fruit increased, but the treated fruit showed a significant reduction in weight loss. As shown in Fig. 1a, significant weight loss was observed in the control group compared to the treated fruits at 30 and 60 days after storage. At the end of the test, the MeJA treatment had the lowest weight loss (6.29%), while the control had the highest (8.97%). After 90 days of storage, the weight loss in the control fruit was 29.87% higher than in the MeJA-treated Orlando tangelo (Fig. 1a).
The effect of postharvest treatment of MeJA and GABA on weight loss (a) and chilling symptom (b) of Orlando tangelo fruits stored at 3 ± 0.5 °C and 80–90% RH for 90 days, followed by 5 days at 20 °C. The means with the same LSD test letters in each column are not statistically significant (P ≤ 0.05). Data are the average of three replications ± SD
Chilling injury (CI) index and MDA
The chilling injury index of Orlando tangelo fruit appeared to increase during storage. Compared to the control fruit, all treatments delayed and reduced chilling injury during cold storage, which manifested as external browning and pitting. The degree of chilling of the fruit after leaving the cold storage and the shelf life were measured (Fig. 1b). As observed in Fig. 1b, the first symptoms of chilling injury were observed in the fruits treated with MeJA, 60 days after storage, whereas the first symptoms of chilling injury in other treatments were observable at 30 days after storage. After 90 days, the least chilling injury (0.19) was observed in the MeJA treatments. Although, all treatments were effective in reducing chilling injury compared to the control (Fig. 1b).
The content of malondialdehyde (MDA) in the fruit peel and fruit juice of Orlando tangelo increased during storage. Significant reductions in MDA content were observed in the fruit peel of MeJA-treated samples compared to the control and other treatments at various stages of storage. Although the MeJA treatment also exhibited the minimum MDA content in the fruit peel after 90 days of storage, no significant differences were observed between the control and treatments on other days. The MDA levels in the control fruit were higher than those in the treated fruit during storage, the peel and juice of fruits treated with MeJA had 32.89% and 34.11% less MDA content, respectively, than the control fruit after 90 days of storage (Fig. 2a, b).
The effect of postharvest treatment of MeJA and GABA on (a) Malondialdehyde content of peel, and (b) Malondialdehyde content of fruit juice of Orlando tangelo fruits stored at 3 ± 0.5 °C and 80–90% RH for 90 days, followed by 5 days at 20 °C. The means with the same LSD test letters in each column are not statistically significant (P ≤ 0.05). Data are the average of three replications ± SD
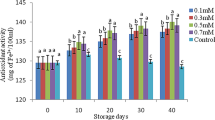
Determination of phenol, flavonoid and antioxidant
Total phenol showed a gradual decrease until 30 + 5 days, after which it remained relatively constant regardless of the treatments. After 90 days, the concentration of total phenolics in the GABA treatment was 47.74% higher than that of the control fruit of Orlando tangelo (Fig. 3a).
The effect of postharvest treatment of MeJA and GABA on a Phenol, b Flavonoid, and c Antioxidant activity of fruit juice of Orlando tangelo fruits stored at 3 ± 0.5 °C and 80–90% RH for 90 days, followed by 5 days at 20 °C. The means with the same LSD test letters in each column are not statistically significant (P ≤ 0.05). Data are the average of three replications ± SD
Flavonoid content decreased gradually until the 60 days of storage, then remained constant. After 90 days, GABA-treated fruit had the highest flavonoid concentration (1.80 mg/g), while the control fruit had the lowest (1.10 mg/g) (Fig. 3b).
The antioxidant capacity remained stable until 30 days of storage, then slightly decreased (Fig. 3c). After 90 days, treated Orlando tangelo samples showed higher antioxidant capacity than the control in the DPPH assay.
Fruit color
Color can be quantified by the lightness (L*) value, with higher values indicating lighter color. After 30 days, MeJA + GABA-treated fruit had higher L* values than the control (Fig. 4a). There was no significant difference in the a* values of treated and control fruit on the 60th and 90 days (data not shown). The b* value increased during storage, and MeJA treatment caused a significant increase in b* compared to the control (Fig. 4b).
The effect of postharvest treatment of MeJA and GABA on color parameters a L*, and b b* of Orlando tangelo fruits stored at 3 ± 0.5 °C and 80–90% RH for 90 days, followed by 5 days at 20 °C. The means with the same LSD test letters in each column are not statistically significant (P ≤ 0.05). Data are the average of three replications ± SD (Color figure online)
CAT and POD
The effect of treatment on the activity of antioxidant enzymes (CAT and POD in the fruit peel and juice) is shown in Fig. 5a–d. CAT and POD activities showed a comparable trend. CAT enzyme activity in the peel and juice increased until 30 days of storage, after which it remained relatively constant. The highest (130 U/mg FW) enzyme activity was observed in the MeJA treatment. In the fruit juice, the lowest enzyme activity was observed in the control (156 U/mg FW), while the treatments showed higher activity than the control. While the enzymatic activity in GABA + MeJA treatment showed a significant increase compared to the control and other treatments after 60 days of storage, it exhibited a significant decrease compared to the other two treatments, although higher than the control, after 90 days of storage.
The effect of postharvest treatment of MeJA and GABA on catalase activity of peel (a) and fruit juice (b) and peroxidase activity of peel (c) and fruit juice (d) of Orlando tangelo fruits stored at 3 ± 0.5 °C and 80–90% RH for 90 days, followed by 5 days at 20 °C. The means with the same LSD test letters in each column are not statistically significant (P ≤ 0.05). Data are the average of three replications ± SD
POD activity in the fruit peel showed little change during storage. The minimum enzyme activity was observed in the control during storage. After 60 days of storage, the highest enzyme activity in the peel was observed in the MeJA treatment, followed by the MeJA + GABA treatment, respectively. After 90 days of storage, the GABA and GABA + MeJA treatments showed a significant increase in POD activity compared to the control. However in fruit juice, enzyme activity gradually decreased. At the end of storage, minimal enzyme activity was observed in the control and other treatments showed a significant increase compared to the control. The highest enzyme activity was evidenced in MeJA, followed by MeJA + GABA (Fig. 5b, c).
Total soluble solid (TSS), pH and titratable acidity (TA)
As shown in Table 1, during cold storage, the TSS content exhibited an overall increase in all samples, although the rate of increase was comparatively lower in the treated fruits. Following 90 days of cold storage, the control group displayed the highest TSS content (9.63%), which significantly differed from the TSS content observed in the treated fruits. The pH of the fruit also slightly increased during cold storage. After 90 days of cold storage, significant increases in pH were observed in the treatments of GABA + MeJA and GABA compared to the control and MeJA. The TA decreased during cold storage. After 90 days of cold storage, the highest TA level (0.91%) was observed in the MeJA + GABA treatment, which showed a significant difference compared to the control and other treatments.
Correlation and principal component analysis
The heatmap obtained from Hierarchical Cluster Analysis (HCA) (Fig. 6a) showed that the measured parameters were divided into two groups. The clustering divided the parameters into two groups: (I) POD-Peel, TA, POD-Juice, L*, a*, Flavo, DPPH, and pH, and (II) WL, MDA-Juice, MDA-Peel, Chilling, TSS, CAT-Juice, b*, CAT-Peel, and Phenol (Fig. 6a). On the other hand, the cluster analysis grouped the treatments into two clusters: (IV): MeJA + GABAT4, GABAT4, MeJAT4, MeJA + GABA3, GABAT3, ControlT3, MeJAT3 and ControlT4. (V): MeJA + GABAT2, Control, MeJA + GABAT1, MeJAT1, ControlT1, GABAT1, GABAT2, and MeJAT2. A positive correlation was observed between CAT and MeJA + GABAT3, GABAT3, MeJAT4, GABAT4 and MeJA + GABAT4 treatment. The highest correlation was observed between phenol and the GABAT3 treatment (Fig. 6a). Additionally, a positive correlation was observed between CAT and the MeJA + GABAT3, GABAT3, MeJAT4, GABAT4, and MeJA + GABAT4 treatments. All 17 traits were loaded into PC1 and PC2, explaining 68.26% of the total variances (Fig. 6b). PC1 differentiated most traits and explained a higher proportion of variance (69%), while PC2 explained a lower proportion (12.71%) (Fig. 6b). MeJA + GABAT3, GABAT3, GABAT4, MeJAT4, MeJAT3, MeJA + GABAT2 treatments showed significant positive similarity with CAT-Juice, CAT-Peel, POD-Peel, pH, TA, TSS and b* traits (Fig. 6b). On the other hand, MDA, WL, and chilling showed the highest significant positive similarity with the control in 60 and 90 days (Fig. 6b).
a Hierarchical clustering analysis (HCA) of the edible coating treatments and variable trait relationships in Orlando tangelo fruit, including a heatmap of Pearson correlation coefficients (r values) for variable traits. The colored scale shows the r coefficient values (r = 0.5–1) indicating positive (red) and negative (blue) correlations, respectively. b The dendrogram clustering the lack of coating and treatment of edible coatings of MeJA, GABA, and MeJA + GABA in Orlando tangelo fruits. Principal component analysis (PCA) of treatments and variable trait relationships in Orlando tangelo fruit, including PCA loading plots of the examined variable traits (b). The circles indicate the variables with the highest correlations. PCA individual plots of edible coating treatments and untreated ones on Orlando tangelo fruit. The first number represents the time of storage (1, 2, and 3), and the second number represents the different treatments (0 = control, 1 = Methyl jasmonate, 2 = GABA, 3 = GABA + Methyl jasmonate). T1: Time 0 + 5, T2: 30 + 5, T3: 60 + 5, T4: 90 + 5 (Color figure online)
Discussion
According to a previous report, the delay in weight loss in treated fruit may be attributed to their effect on reducing respiration, reducing the severity of chilling damage, and preserving membrane integrity during cold storage [22]. All treatments reduced weight loss compared with the control fruit during storage. MeJA had a more positive impact on weight loss in Orlando tangelo compared to the control in our research. Similarly, to the previous study, the weight loss of ‘Kinnow’ mandarins treated with MeJA was less than the control throughout storage [6]. It has also been shown that GABA treatment had a positive effect on reducing weight loss in carambola fruit under both cold and non-chilling stresses [22].
In the present study, chilling injury (CI) symptoms, including browning and pitting, were observed on the peel of the control fruit. Other treatments displayed considerably less CI severity in Orlando tangelo fruit during 90 days of cold storage. MeJA has been shown to reduce chilling symptoms in fruits such as loquat [23] and ‘Kinnow’ mandarin [24]. Additionally, MeJA administration enhanced the intracellular concentrations of jasmonic acid (JA) and the genes involved in JA biosynthesis, suggesting that MeJA might effectively limit the rise in CI throughout the fruit’s cold storage [25]. It should be noted that no research has investigated the combined role of MeJA and GABA in fruits. In the results of this study, the combination of MeJA + GABA may improve the activity of the GABA shunt pathway. GABA treatment has been shown to significantly decrease the CI index, which represents the severity of chilling injury, in Aonla fruit by stimulating proline accumulation and maintaining membrane integrity [26]. Additionally, GABA may protect the pericarp of the fruit against cold temperatures by increasing the content of unsaturated fatty acids and decreasing saturated fatty acids [22].
Lipid peroxidation and other structural changes in membranes may also occur under cold stress. One of the low molecular weight byproducts of lipid peroxidation is malondialdehyde (MDA), and its concentration strongly correlates with the level of oxidative stress to membranes. Measurements of membrane degradation and oxidative stress induced in response to chilling and other environmental conditions in plants are often made using permeability and malondialdehyde (MDA) [27]. In this study, MDA levels generally increased with the development of chilling in Orlando tangelo fruit during cold storage. However, most treatments inhibited the increase of MDA, indicating that these treatments may reduce oxidative membrane damage. In a study, it was shown that MeJA decreased MDA levels in lemons (Citrus limon) [28]. In another study, the effect of GABA and storage time on the MDA content of pomegranate (Punica granatum L.) was significant (P = 0.01), and MDA levels increased with time [29]. In our study, GABA most likely preserved the superior membrane structure, inhibited the progression of MDA, and decreased oxidative damage, which ultimately led to increased chilling tolerance of Orlando tangelo fruit. The GABA treatment generally protects the membrane structure better, perhaps by preventing reactive oxygen species (ROS)-mediated changes in the fatty acids of the cellular membrane [10, 30, 31].
Flavonoids and other phenolic chemicals are part of the nonenzymatic antioxidant system. Phenolic compounds play a crucial role in preserving the nutritive qualities of fruits and vegetables, including their color, astringency, flavor, and bitterness. Phenolic compounds are crucial in preventing the negative consequences of free radicals and oxidative stress by mitigating their effects [32]. In the present study, GABA had an effective role in increasing phenolic content in fruits compared to the control. Additionally, the antioxidant content of fruits treated with GABA and MeJA showed an increase compared to the control.
The findings of other studies suggest that applying GABA to bananas [7] and mango [33] resulted in preserving the antioxidant capacity. GABA increases flavonoids by increasing the production of phenylalanine ammonia-lyase (PAL) enzyme, which is activated through the phenylpropanoid pathways [34]. Enzymes such as phenylalanine ammonia-lyase, which are important for polyphenol synthesis, were reported in earlier research to be increased by both pre and postharvest MeJA treatments [35,36,37,38,39]. MeJA has been shown to increase the activity of total antioxidants in Blood oranges and Chinese bayberries [40, 41]. GABA enhances antioxidant activity by promoting the biosynthesis of compounds like phenols and flavonoids and activating antioxidant enzymes [6]. In a study by Soleimani Aghdam et al. [42], the treatment of peaches with 4 and 6 mM GABA increased their DPPH scavenging capacity compared to the control.
CAT and POD are two enzymes that form the antioxidant system in plant cells. This system protects the cell membrane by removing excess reactive oxygen species (ROS) and reduces chilling injury [43]. Increased CAT activity contributes to the removal of superoxide anion (O2.−) and hydrogen peroxide (H2O2). The interaction between CAT and POD helps to eliminate excess hydrogen peroxide [44]. Pre-harvest MeJA treatments have been shown to enhance the functionality of antioxidant enzymes in plums [45] and pomegranates [45] during storage. Serna-Escolano et al. [46] reported that administering MeJA during the developmental stage of ‘Verna’ and ‘Fino’ lemons resulted in increased levels of phenolics and activity of CAT and POD enzymes in the juice and flavedo upon harvesting. Rabiei et al. [47] observed an increase in the functionality of ROS scavenging enzymes, including CAT, in cherry fruits treated with GABA. Also, the findings of the study reveal that the administration of exogenous GABA has a significant effect on the induction of POD activity in both Valencia and Newhall fruits [48].
The highest TSS was observed in control fruits. MeJA + GABA treatment had an efficient contribution in maintaining acidity, and the highest pH of the fruit was shown by GABA and MeJA + GABA treatments compared to the control. During the postharvest stage, MeJA is crucial for controlling the pH of the cell cytoplasm [38]. Since organic acid is used during metabolic processes, the pH of the juice typically increases with storage duration. Additionally, accelerated senescence, which refers to the natural deterioration of the fruit over time, can also contribute to a reduction in acidity [45].
Conclusion
This study investigated the effects of methyl jasmonate (MeJA), γ-aminobutyric acid (GABA), and their combination on chilling injury in Orlando tangelo fruits during cold storage. The results showed that all treatments reduced fruit weight loss and chilling injury, with the MeJA treatment being the most effective. The treated fruits had lower levels of malonaldehyde, indicating reduced oxidative stress. MeJA treatment also resulted in higher phenol and flavonoid content, as well as increased antioxidant activity compared to the control. The treatments enhanced the activity of catalase (CAT) and peroxidase (POD) enzymes in fruit juice and peel, with MeJA and MeJA + GABA treatments showing the highest activity in peel for CAT and POD enzymes, respectively. Overall, these findings suggest that individual treatments and the combination of MeJA + GABA can enhance the cold tolerance of Orlando tangelo fruits during prolonged cold storage. Also, further research is recommended to elucidate the molecular mechanisms underlying these treatments to enhance fruit quality during storage at low temperatures.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
L.S. Magwaza, U.L. Opara, P.J. Cronjé, S. Landahl, L.A. Terry, B.M. Nicola ï, Nonchilling physiological rind disorders in citrus fruit. Hortic. Rev. 41, 131–176 (2013)
A.A. Kader, E.M. Yahia, in Postharvest biology and technology of tropical and subtropical fruits (Woodhead Publishing, 2011), pp. 79–111
M.T. Lafuente, L. Zacarias, M.A. Martı́nez-Téllez, M.T. Sanchez-Ballesta, A. Granell, Phenylalanine ammonia-lyase and ethylene in relation to chilling injury as affected by fruit age in citrus. Postharvest Biol. Technol. 29(3), 309–318 (2003)
C. Wasternack, M. Strnad, Jasmonate signaling in plant stress responses and development–active and inactive compounds. New Biotechnol. 33(5), 604–613 (2016)
C. Wasternack, Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 100(4), 681–697 (2007)
A. Baswal, H. Dhaliwal, Z. Singh, B. Mahajan, K. Gill, Postharvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid extends the cold storage life and maintain the quality of ‘Kinnow’ mandarin (Citrus nobilis L. × C. deliciosa L.) fruit. Postharvest Biol. Technol. 161, 111064 (2020)
Y. Wang, Z. Luo, X. Huang, K. Yang, S. Gao, R. Du, Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 168, 132–137 (2014)
M.S. Aghdam, E.J. Flaherty, B.J. Shelp, γ-Aminobutyrate improves the postharvest marketability of horticultural commodities: advances and prospects. Front. Plant Sci. 13, 884572 (2022)
S.A. Ramesh, S.D. Tyerman, B. Xu, J. Bose, S. Kaur, V. Conn et al., GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 6(1), 1–10 (2015)
M.S. Aghdam, R. Naderi, M.A.A. Sarcheshmeh, M. Babalar, Amelioration of postharvest chilling injury in anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Postharvest Biol. Technol. 110, 70–76 (2015)
B.J. Shelp, M.S. Aghdam, E.J. Flaherty, γ-Aminobutyrate (GABA) regulated plant defense: mechanisms and opportunities. Plants 10(9), 1939 (2021)
V. Mishra, G.S. Abrol, N. Dubey, Sodium and calcium hypochlorite as postharvest disinfectants for fruits and vegetables, in Postharvest Disinfection of Fruits and Vegetables, vol. 1 (Academic Press, 2018), pp. 253–272
M. Bagheri, M. Esna-Ashari, A. Ershadi, Effect of postharvest calcium chloride treatment on the storage life and quality of persimmon fruits (Diospyros kaki Thunb.) cv. ‘Karaj.’ Int. J. Hortic. Sci. Technol. 2(1), 15–26 (2015)
J. Shi, J. Zuo, F. Zhou, L. Gao, Q. Wang, A. Jiang et al., Low-temperature conditioning enhances chilling tolerance and reduces damage in cold-stored eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 141, 33–38 (2018)
A. Lo’ay, M.A. Taher, Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati.’ Sci. Hortic. 235, 424–436 (2018)
V.L. Singleton, R. Orthofer, R.M. Lamuela-Raventós, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent, in Methods in Enzymology, vol. 299 (Academic press, 1999), pp. 152–178.
C.M. Mihai, L.A. Mărghitaş, O. Bobiş, D. Dezmirean, M. Tămaş, Estimation of flavonoid content in propolis by two different colorimetric methods. Sci. Pap.: Anim. Sci. Biotechnol. Timis. 43(1), 407–410 (2010)
W. Brand-Williams, M. Cuvelier, C. Berset, Antioxidative activity of phenolic composition of commercial extracts of sage and rosemary. LWT 28, 25–30 (1995)
R.G. McGuire, Reporting of objective color measurements. Sci. Hortic. 27(12), 1254–1255 (1992)
B. Chance, A. Maehly, Assay of catalases and peroxidases, in Methods of Biochemical Analysis, vol. 2 (1955), pp. 764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
H.E. Aebi, Catalase, in Methods of Enzymatic Analysis, ed. by H.U. Bergmeyer (Verlag Chemie, Weinhem, 1983), pp. 273–286.
F. Ngaffo Mekontso, W. Duan, E.H.M. Cisse, T. Chen, X. Xu, Alleviation of postharvest chilling injury of carambola fruit by γ-aminobutyric acid: physiological, biochemical, and structural characterization. Front. Nutr. 8, 752583 (2021)
S. Cao, Y. Zheng, K. Wang, P. Jin, H. Rui, Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 115(4), 1458–1463 (2009)
S. Ali, M.A. Anjum, S. Ejaz, S. Hussain, S. Ercisli, M.S. Saleem et al., Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’ mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 168, 77–85 (2021)
M.L. Zhao, J.N. Wang, W. Shan, J.G. Fan, J.F. Kuang, K.Q. Wu et al., Induction of jasmonate signalling regulators MaMYC2s and their physical interactions with MaICE1 in methyl jasmonate-induced chilling tolerance in banana fruit. Plant Cell Environ. 36(1), 30–51 (2013)
S. Ali, M.A. Anjum, A. Nawaz, S. Ejaz, R. Anwar, G. Khaliq et al., Postharvest γ-aminobutyric acid application mitigates chilling injury of aonla (Emblica officinalis Gaertn.) fruit during low temperature storage. Postharvest Biol. Technol. 185, 111803 (2022)
Z. Zhang, Q. Zhu, M. Hu, Z. Gao, F. An, M. Li et al., Low-temperature conditioning induces chilling tolerance in stored mango fruit. Food Chem. 219, 76–84 (2017)
X.I. Siboza, I. Bertling, A.O. Odindo, Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J. Plant Physiol. 171(18), 1722–1731 (2014)
F. Nazoori, E. ZamaniBahramabadi, S.H. Mirdehghan, A. Rafie, Extending the shelf life of pomegranate (Punica granatum L.) by GABA coating application. J. Food Meas. Charact. 14(5), 2760–2772 (2020)
F. Palma, F. Carvajal, R. Jiménez-Muñoz, A. Pulido, M. Jamilena, D. Garrido et al., Exogenous γ-aminobutyric acid treatment improves the cold tolerance of zucchini fruit during postharvest storage. Plant Physiol. Biochem. 136, 188–195 (2019)
Z. Niazi, F. Razavi, O. Khademi, M.S. Aghdam, Exogenous application of hydrogen sulfide and γ-aminobutyric acid alleviates chilling injury and preserves quality of persimmon fruit (Diospyros kaki, cv. Karaj) during cold storage. Sci. Hortic. 285, 110198 (2021)
R. Karimi, F. Mirzaei, M. Rasouli, Phenolic acids, flavonoids, antioxidant capacity and minerals content in fruit of five grapevine cultivars. Iran. J. Hortic. Sci. Technol. 18(1), 89–102 (2017)
S. Rastegar, H.H. Khankahdani, M. Rahimzadeh, Effect of γ-aminobutyric acid on the antioxidant system and biochemical changes of mango fruit during storage. J. Food Meas. Charact. 14(2), 778–789 (2020)
Y. Ma, P. Wang, M. Wang, M. Sun, Z. Gu, R. Yang, GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 270, 593–601 (2019)
D. Rudell, J. Mattheis, X. Fan, J.K. Fellman, Methyl jasmonate enhances anthocyanin accumulation and modifies production of phenolics and pigments in Fuji’ apples. J. Am. Soc. Hortic Sci. 127(3), 435–441 (2002)
P. Jin, Y. Zheng, S. Tang, H. Rui, C.Y. Wang, Enhancing disease resistance in peach fruit with methyl jasmonate. J. Sci. Food Agric. 89(5), 802–808 (2009)
M. Shafiq, Z. Singh, S.K. Ahmad, Pre-harvest spray application of methyl jasmonate improves red blush and flavonoid content in ‘cripps pink’ apple. J. Hortic. Sci. Biotechnol. 86(4), 422–430 (2011)
S. Yang, Y. Chen, L. Feng, E. Yang, X. Su, Y. Jiang et al., Effect of methyl jasmonate on pericarp browning of postharvest lychees. J. Food Process. Preserv. 35(4), 417–422 (2011)
Y. Ruiz-Garcia, I. Romero-Cascales, R. Gil-Munoz, J.I. Fernandez-Fernandez, J.M. Lopez-Roca, E. Gomez-Plaza et al., Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 60(5), 1283–1290 (2012)
F. Habibi, A. Ramezanian, F. Guillén, M. Serrano, D. Valero, Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 306, 125634 (2020)
K. Wang, P. Jin, S. Cao, H. Shang, Z. Yang, Y. Zheng, Methyl jasmonate reduces decay and enhances antioxidant capacity in Chinese bayberries. J. Agric. Food Chem. 57(3), 5809–5815 (2009)
M.S. Aghdam, F. Razavi, F. Karamneghad, Maintaining the postharvest nutritional quality of peach fruits by γ-Aminobutyric acid. Iran. J. Plant Physiol. 5(4), 1457–1463 (2016)
J. Sun, H. Lin, S. Zhang, Y. Lin, H. Wang, M. Lin et al., The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced pericarp browning and disease development of harvested longan fruit. Food Chem. 247, 16–22 (2018)
Y. Ge, B. Duan, C. Li, Q. Tang, X. Li, M. Wei et al., γ-Aminobutyric acid delays senescence of blueberry fruit by regulation of reactive oxygen species metabolism and phenylpropanoid pathway. Sci. Hortic. 240, 303–309 (2018)
M.S. Saleem, S. Ejaz, M.A. Anjum, A. Nawaz, S. Naz, S. Hussain et al., Postharvest application of gum arabic edible coating delays ripening and maintains quality of persimmon fruits during storage. J. Food Process. Preserv. 44(8), e14583 (2020)
V. Serna-Escolano, D. Martínez-Romero, M.J. Giménez, M. Serrano, S. García-Martínez, D. Valero, J.M. Valverde, P.J. Zapata, Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 338, 128044 (2021)
V. Rabiei, F. Kakavand, F. Zaare-Nahandi, F. Razavi, M.S. Aghdam, Nitric oxide and γ-aminobutyric acid treatments delay senescence of cornelian cherry fruits during postharvest cold storage by enhancing antioxidant system activity. Sci. Hortic. 243, 268–273 (2019)
L. Sheng, D. Shen, Y. Luo, X. Sun, J. Wang, T. Luo, Y. Zeng, J. Xu, X. Deng, Y. Cheng, Exogenous γ-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 216, 138–145 (2017)
Acknowledgements
This study as a research project (Code Number: 401/D/16877) was financially supported by the University of Hormozgan, Iran.
Author information
Authors and Affiliations
Contributions
SAD: formal analysis, investigation, writing—original draft. SR: funding acquisition, resources, project administration, review & editing. MM: resources, investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghaei Dargiri, S., Rastegar, S. & Mohammadi, M. Potential application of methyl jasmonate and γ-aminobutyric acid to preserve fruit quality and alleviate postharvest chilling in Orlando tangelo. Food Measure 18, 871–882 (2024). https://doi.org/10.1007/s11694-023-02201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02201-2