Abstract
Starches from two yam species, viz. Dioscorea alata and Dioscorea esculenta were subjected to heat moisture treatment (HMT) with 20 and 30% moisture at 110 °C for 3 h and annealing (ANN) at 1:2 and 1:4 starch to water ratio and 50 °C for 24 h, and their effect on functional, thermal, pasting, morphological, and rheological properties, and in vitro digestibility was investigated. HMT and ANN modifications decreased the swelling and solubility of the starches compared to the native starches. D. alata showed a higher amylose leaching than D. esculenta due to its higher amylose content. An increase in the transition enthalpy (∆H) after ANN and a decrease in ∆H after HMT were observed. ANN modification increased the setback viscosity, while HMT modification decreased the setback viscosity compared to the corresponding native starches of both the species. Both HMT and ANN decreased the peak viscosity, hot paste viscosity and breakdown viscosity of the starch granules. The steady shear and dynamic rheological properties of the starch paste at 25 °C were evaluated by the power-law model and indicated non-Newtonian flow properties with shear-thinning behaviour. ANN modified starch exhibited a greater impact than HMT on the rheological properties, indicating stronger molecular interactions in the pastes. An increase in slowly digestible and resistant starch with a decrease in rapidly digestible starch was noticed after HMT and ANN modifications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Dioscorea is an herbaceous, woody, and climber plant with underground tubers. Species of the genus Dioscorea are commonly referred to as ‘yams’ and only about a hundred species are edible either as such or after detoxification and long or quick cooking. Of the 50 species of Dioscorea found in India, about 19 are known to occur only in Assam [1]. Sharma and Hore [2] reported 28 species and 25 cultivars of the genus Dioscorea in northeastern India. In recent decades, this genus has emerged as one of the most important sources of diosgenin, a plant sapogenin used in the pharmaceutical industries for the synthesis of steroid drugs [3, 4]. However, starch, which is the most abundant carbohydrate in the rhizomes of different Dioscorea species, is always neglected and eliminated during the isolation of small bioactive molecules, resulting in a significant waste of starch resources. Although the starch content of Dioscorea species can be up to 80% on a dry weight basis [5], research on the food applications of these starches is limited.
Since some native starches do not have the requisite technological and functional properties desired by the target market, efforts are must to modify them as a value addition for local and industrial food applications [6]. Modified starches can provide desirable functional properties not found in native starches and can be used to improve viscosity, shelf life stability, processing parameters, particle integrity, texture, solubility, shape and emulsion, among other things. Physical modification of starch by moisture, heat, shear or radiation is increasingly applied as no chemical reagent by-products are found in modified starch. Heat-moisture treatment (HMT) and annealing (ANN) are physical modifications that alter the physicochemical properties of starch without destroying its granular morphology [7,8,9,10]. These two processes are related and both require control over the starch-to-moisture ratio, temperature, and heating time [11]. However, these treatments differ in the amount of moisture and the temperature being used for modification. HMT runs under limited moisture content (10–30%) and high temperatures (90–120 °C), whereas ANN works under large excess moisture (50–60%) and is accompanied by relatively low temperatures below the gelatinization point of starch [12]. Regardless of starch origin, HMT promotes an increase in the gelatinization transition temperature, widens the gelatinization temperature range, reduces granular swelling and amylose leaching, and increases the thermal stability of starch. However, depending on the botanical origin of the starch and the processing conditions, HMT can cause changes in X-ray patterns, formation of amylose–lipid complexes, disruption of crystalline structure, and increased or decreased enzyme sensitivity [13]. In addition, ANN modifies the physicochemical properties of starch, improves crystalline perfection and facilitates interactions between starch chains [14]. More specifically, ANN causes reorganization of starch molecules and the amylopectin duplex adopts a more organized configuration.
Starches of yams have been comparatively underutilized and underexploited in comparison to the starches from other tubers, roots, cereals, fruits and even legumes. Lack of adequate information on the structure, function and potential application of the starches is one of the limiting factors for industrial application of starches such as that from underutilized yams [15,16,17]. The physicochemical, functional, and pasting qualities of native and modified starches of cultivated Dioscorea species from northeastern India have not yet been thoroughly studied. Meanwhile, there is no report on the comparative study of HMT and ANN treatments on yam starch, and on the effect of moisture levels during these treatments on yam starch. Therefore, a study was designed to investigate and compare the effect of HMT and ANN (with different moisture levels) on the functional, thermal, pasting, morphological and rheological properties of underutilized yam starches.
Materials and methods
Materials
Two locally grown yams were collected from Kuthori Bagisha, Morigaon, Assam, India (26° 04′ 46.6″ N 92° 16′ 10.6″ E) and were identified to be species belonging to Dioscorea alata (purple yam) and Dioscorea esculenta (Lour.) Burkill. The harvesting period of the yams was in December before the Bihu festival of Assam. Amylose (potato), amylopectin (potato), α-amylase, and amyloglucosidase were purchased from Sigma-Aldrich Co. LLC., India. All other reagents used in the study were of analytical grade.
Starch isolation and modifications
Isolation of starches from the yams was carried out by the method described by Nindjin et al. [18] with modifications. The washed and cleaned tubers were cut into small pieces and immediately dipped in distilled water containing 0.2% (w/v) sodium metabisulphite. The pieces were crushed in a blender and suspended in excess distilled water containing 4% (w/v) NaCl to maintain a solid to liquid ratio of 1:5. The slurry was filtered through cheesecloth and the filtrate was centrifuged at 3000 rpm for 10 min using a laboratory centrifuge (Hermle Labortechnik, Germany). The obtained starch cake was re-suspended in distilled water and centrifuged. The process was repeated till no brownish layer on the surface was seen. The purified starch cake was dried in a tray drier overnight at 40 °C, followed by pulverization, sieving, and storage for further analysis. The native starches obtained from D. alata and D. esculenta were coded as 1YNS and 2YNS, respectively. The moisture content, protein content, lipid content, ash content and yield of 1YNS and 2YNS were 10.79 ± 0.13%, 1.53 ± 0.03%, 0.41 ± 0.05%, 0.15 ± 0.10% and 16.48 ± 0.86%, and 11.04 ± 0.11%, 0.85 ± 0.01%, 0.32 ± 0.04%, 0.16 ± 0.10% and 21.52 ± 1.14%, respectively.
For HMT treatment of the isolated starches, the method described by Piecyk et al. [19] was followed with slight modifications. Samples were adjusted to 20% and 30% (w/w) moisture levels and kept at 4 °C for 24 h in a sealed container. The sealed sample containers were then placed in a hot air oven at 110 °C for 3 h with occasional shaking of the containers. The treated samples were cooled down to ambient temperature and dried at 40 °C overnight. Accordingly, the treated starches were named 1HMT20, 1HMT30, 2HMT20, and 2HMT30, where the prefix ‘1’ and ‘2’ indicate the yam species D. alata and D. esculenta, respectively, and the suffix -20 and -30 indicate moisture levels.
Annealing of the starches was carried out using the method reported by Yeh and Lai [20] with modifications. Samples were suspended in distilled water (1:2 and 1: 4, w/v), mixed well in a sealed container for equilibration, and incubated at 50 °C in a water bath for 24 h. After incubation, the samples were cooled to ambient temperature and dried at 40 °C for 24 h. Accordingly, the treated starches were coded as 1ANN-12, 1ANN-14, 2ANN-12, and 2ANN-14, where the prefix ‘1’ and ‘2’ indicate the yam species D. alata and D. esculenta, respectively, and the suffix -12 and -14 indicate starch to water ratio of 1:2 and 1:4, respectively.
Total amylose content, amylose leaching, water and oil absorption capacity
Total amylose content (AM) was determined using a standard curve prepared by mixtures of amylose and amylopectin standards, according to the method described by Hoover and Ratnayake [21]. Amylose leaching (AML) of the native and modified starches was determined at 80 °C according to the method of Jayakody et al. [22] using the amylose content determination method of Hoover and Ratnayake [21]. Water absorption capacity (WAC) and oil absorption capacity (OAC) of the native and modified yam starches were determined following the method of Ashwar et al. [23].
Scanning electron microscopy
The native and modified starch powdered samples were dried initially at 40 ± 2 °C in a hot air oven for 4 h. The samples were first gently placed on a double-sided carbon tape attached to a metal stub. The stub along with the sample was later coated with a gold layer of 20 nm using a magnetron sputtering machine (Q150R ES, Quorum, England). The samples thus prepared, were morphologically examined under a scanning electron microscope (FESEM, Sigma 300, Zeiss, Germany) at 5 kV.
ATR-FTIR spectroscopy
The ATR-FTIR spectra of the samples were recorded using Spectrum-2 spectrometer (Perkin-Elmer, USA). The IR spectra were obtained with the help of Universal Attenuator Total Reflectance (UATR) and spectra in the range of 4000–400 cm−1 were recorded with a resolution of 4 cm−1 at ambient temperature.
Swelling power and solubility
Swelling power and solubility of the starches were measured by following the method reported by Bernardo et al. [24] with minor modifications. Briefly, 50 mg of starch was mixed with 10 ml of distilled water and heated at 55, 65, 75, 85 and 95 °C for 1 h. After cooling to room temperature, the mixture was centrifuged at 4000 g for 15 min. The supernatant was dried in a hot air oven at 105 °C for 4 h and the solubility was calculated as follows:
For determination of swelling power, the sediment was weighed and the swelling power was calculated as follows:
Thermal properties
Differential scanning calorimeter (Micro DSC III, Setaram, France) was used to analyze the starch gelatinization using the method reported by Liu et al. [25]. Starch samples (3 mg) in distilled water at a ratio of (1:3.5) were scanned from 30 to 150 °C at a heating rate of 10 °C/min in a sealed aluminum pan. An empty sealed DSC pan was used as reference. The onset temperature (To), peak temperature (Po), conclusion temperature (Tc), and endothermic enthalpy (ΔH) were determined.
Pasting properties
The pasting properties of the starch samples (12% w/v) were evaluated in a Rapid Visco Analyzer (StarcMaster2, Newport Scientific, Australia). The programmed cycle was set at 50 to 95 °C in 5 min (heating), 95 °C for 2 min (holding), 95 to 50 °C in 4 min (cooling), and 50 °C for 2 min (holding). Pasting temperature (PT), Peak viscosity (PV), Hot-paste viscosity (HPV), Final viscosity (FV), Breakdown (BD), and Setback (SB) were recorded.
Static and dynamic rheological parameters
The static and dynamic rheological properties of the flour or starch gelatinized paste (12% w/v) obtained from RVA were studied by following the procedures described by Qin et al. [26] using a rheometer (MCR 101, Anton Paar, Germany), which was equipped with parallel-plate geometry (50 mm diameter) with 0.5 mm gap at 30 °C. The steady shear (flow behavior) test was performed by varying the shear rate from 0 to 400 s−1 to measure the impact of shear rate on apparent viscosity and shear stress. The dynamic frequency sweep (frequency range of 0.1–100 rad/s) test was performed at a strain value of 1% (within the linear viscoelastic region) to record the loss modulus (G″), storage modulus (G′), and loss tangent (tan δ = G″/G′) as a function of frequency (ω). To further investigate the rheological behavior, Power-law as mentioned in Eqs. (1)–(3) were fitted to the experimental data of steady shear and oscillatory testing conducted on the starch pastes, respectively.
where σ = shear stress (Pa); γ = shear rate (1/s); k = consistency coefficient (Pa.sn); n = flow behaviour index (dimensionless).
where, k′, k″, n′ and n″ are the corresponding fitting parameters.
In vitro starch digestibility
The percentage of rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) of the native and modified yam starches were determined following the method of Huang et al. [27] with slight modification. Briefly, 200 mg starch was weighed in a centrifuge tube and 15 ml of phosphate buffer (pH 5.2 ± 0.2) was added to it. The mixture was allowed to boil in a water bath for 20 min and then equilibrated at 37 °C for 5 min. After equilibration, 5 ml of enzyme solution (1200 U/ml α-amylase and 15 U/ml amyloglucosidase) was added and incubated in a shaking water bath at 37 °C and 180 rpm. After 20 min and 120 min, 0.5 ml aliquots of hydrolyzed solution were mixed with 4 ml of ethanol (80%) in a centrifuge tube to stop the activity of the enzymes. Then, the tubes were centrifuged at 3000 g for 10 min and the supernatant was collected to measure the content of glucose using the Anthrone method. The percentage of hydrolyzed starch was determined by multiplying the glucose content with a factor of 0.9. The RDS (%), SDS (%), and RS (%) in each starch sample were determined using Eq. (4), (5) and (6), respectively.
where, G20 and G120 are the content of glucose determined after 20 min and 120 min of hydrolysis, respectively; FG is the free glucose content in starch; and TS is the total starch content.
Statistical analysis
All the tests of this study were conducted in triplicate. The results were expressed as mean ± standard deviation and evaluated for significance using one-way ANOVA and Turkey post hoc test at a significance level of p < 0.5 using IBM SPSS Statistic Version 20 software (IBM Corporation, Armonk, N.Y., USA). For the graphs and plots, OriginPro 8.5 software (OriginLab Corporation, Northampton, MA, USA) was used.
Results and discussion
Total AM content, AML, WAC and OAC
The data on amylose content, AML, WAC, and OAC of native and modified starches of yams are presented in Table 1. Amylose content of native and modified starches of D. alata (Y1) and D. esculenta (Y2) ranged from 26.58 to 33.93% and 21.34 to 29.53%, respectively. The above values of amylose content were within the range (9.9–47%) of amylose content reported for Dioscorea starches [28]. Amylose content of Y1 was found to be higher than Y2, and the species D. alata tend to have higher amounts of amylose compared to D. esculenta [29]. Various factors such as method of quantification, endogenous lipid content, environmental and agronomic practices may affect the content of amylose in yam starches [28]. A significant (p < 0.5) increase in amylose content was induced by HMT and ANN and with moisture levels of treatment. A similar increase in amylose content after HMT has been reported for buckwheat starches [30], and this increase in amylose content can be attributed to the interactions between amylose (AM) and amylopectin (AP) in the amorphous regions and degradation of AP during HMT or ANN [25, 31]. However, the amylose content was found to be higher after ANN compared to HMT. In hydrothermal treatments, heat and moisture disrupt the crystalline matrix of starch granules leading to AP degradation and causing an increase in the interactions within the amorphous matrix [32]. A significant (p < 0.5) reduction in the AML and OAC with an increase in the WAC was observed after HMT and ANN and with increase in moisture levels of treatment. Similar trends of WAC and OAC have been reported for HMT-modified buckwheat starch [33], amaranth starch [34], and sohphlang starch [35]; and ANN-modified maize starch [25] and white yam starch [36]. The decrease in AML after HMT treatment may be due to additional interactions between the amylose-amylopectin and the amylose-amylose chain during HMT [37]. The observed difference in AML of Y1 and Y2 was due to the total amylose content of the starches.
Scanning electron microscopy
Figure 1a illustrates the SEM images of the native, heat moisture treated, and annealed yam starches. D. esculenta (Y2) starches micrographs showed the presence of irregular polygonal or polyhedral shaped granules with smooth surfaces, and the length of the granules ranged in size from 1.6 to 6.2 µm. D. alata (Y1) starches micrographs showed the presence of oval, elongated or lenticular shaped starch granules with smooth surface, and the length of the granules ranged in size from 10.6 to 48.7 µm.
Scanning electron microscopy (SEM) of a native and modified starches of yam species 1 and 2 at ×500 and ×5000 magnification, and b 2HMT-20 and 2HMT-30 starch granules at ×15,000 magnification. YNS, HMT, and ANN indicate native, heat-moisture treated, and annealed yam starch, respectively. Prefix 1 and 2 indicate the two yam species. Suffix − 20, − 30, − 12 and − 14 indicate 20% moisture level, 30% moisture level, 1:2 starch to moisture ratio and 1:4 starch to moisture ratio, respectively. The fissures and cavities in (b) are encircled
2HMT-20 and 2HMT-30 starch granules showed the presence of cavities and fissures (Fig. 1b) on starch granules and granular aggregation, which may be linked to the partial gelatinization of starch induced by HMT. Similar studies have been reported for heat moisture-treated elephant foot yam starch [38] and pearl millet starch [39]. No cavities and fissures were found on the starch granules of 1HMT-20 and 1HMT-30 except for granular aggregation. A more aggregated structure of starch granules was observed for HMT granules compared to ANN granules. This changes in granular morphology might be due to the exposure of starch granules to high moisture content during hydrothermal treatment, which promotes the partial gelatinization and morphological changes of starch [40]. Similar observation on more aggregated HMT starch granules than ANN starch granules was reported on buckwheat starch by Liu et al. [31]. HMT and ANN starch granules had higher number of smaller granules in comparison to the native starches. These changes may be attributed to the compaction of granular matter induced by heating and pressure during HMT [31, 41]. Lower size of the ANN starch granules than native samples might be due to the strengthening of the granular structure and perfection of crystalline structure due to the thermal treatment [42]. Moreover, the morphology of starch depends on the treatment conditions of HMT and ANN, and starch source [25].
ATR-FTIR spectroscopy
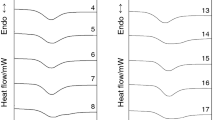
Figure 2 illustrates the ATR-FTIR spectra of the native, heat moisture treated, and annealed yam starches. HMT and annealing did not alter the ATR-FTIR spectra of the starch granules and exhibited similar vibration peaks at 2924 cm−1 (C–H stretching vibration), 1641 cm−1 (H–O–H bending vibration), 1150 cm−1 and 1077 cm−1 (C–O, C–C, and C–O–H stretching), 930 cm−1 (skeletal mode vibration of α-(1–4) glycosidic linkage), and 860 cm−1 (C–H and CH2 deformations) [38, 43, 44]. However, some variations in the peak intensities were observed in the ATR-FTIR spectra of the HMT and ANN starch granules. Higher intensities of the peak at 3293 cm−1 representing O–H stretching vibrations were observed in the annealed starches, and it indicates a stronger interaction of hydrogen bonds and more crystalline perfection [45]. HMT starches showed a decrease in the intensities of the peak at 3293 cm−1 indicating a weaker interaction of hydrogen bonds. HMT modification led to a decrease in the intensities of the peak at 1641 cm−1 which indicates lower moisture content in the amorphous region of HMT-modified starch granules, while no change in the intensities of the peak at 1641 cm−1 was observed for annealed starches [45]. A reduction in the intensities of the peak at 1000 cm−1 was observed in HMT and ANN starches indicating the breakdown of C-H bonding [46].
ATR-FTIR spectra of native and modified yam starches. YNS, HMT, and ANN indicate native, heat-moisture treated, and annealed yam starch, respectively. Prefix 1 and 2 indicate the two yam species. Suffix − 20, − 30, − 12 and − 14 indicate 20% moisture level, 30% moisture level, 1:2 starch to moisture ratio and 1:4 starch to moisture ratio, respectively
Swelling power and solubility
The effect of temperature on the native and modified starches of the yam species is presented in Fig. 3a–d. The swelling power and solubility of all the starches increased as the temperature increased from 55 to 95 °C, due to the gelatinization of starch. Native starches exhibited greater swelling power and solubility than the HMT and ANN-treated starches as the treatment temperature increased. The reduction in swelling power and solubility of HMT-treated starch in comparison to native counterparts might be due to the decrease in the amylose leaching as a result of increased interactions between amylose-amylose chains and amylose-amylopectin chains, and the formation of amylose–lipid complex or highly ordered amylopectin side-chain clusters leading to a stable and rigid granular structure of starch after HMT that prevents amylose leaching [30, 37, 38]. Furthermore, a decrease in the swelling power and solubility was noticed along with the increase in moisture levels of the HMT treatment of starch, which may be due to the greater mobility of the amylose chains resulting in enhanced amylose-amylose and amylose-amylopectin interactions [47, 48]. Similar observations on the decline of swelling power and solubility after HMT were noticed for mango kernel starches [49], maize starch [25], buckwheat starch [30], oat starch [50], and purple yam flour [47]. The decrease in swelling power and solubility of ANN-treated starch in comparison to their native counterparts might be due to the degree of crystalline perfections and interactions between amylose/amylose and/or amylose-amylopectin, which decreases the hydration of the amorphous regions of starch granules [51]. The strengthening of bonds between amylose/amylopectin or amylopectin/amylopectin molecules prevents amylose leaching, thereby reducing the solubility of the granules [52]. The increase in the moisture levels of ANN-treated starch decreased the swelling power and solubility and similar results on sohphlang starch [35] have been reported. The higher swelling power and solubility of ANN than HMT treated starch at higher temperatures might be due to the destruction of the starch’s internal structure at high temperatures and weaker interactions between the functional groups of starch [53]. The higher swelling power shown by Y2 starches than Y1 starches is probably influenced by crystallinity (D. esculenta > D. alata) and extent of interaction between AM-AP and AM-AM chains (D. esculenta > D. alata) [54].
Effect of temperature on the swelling power and solubility of native and modified yam starches. a and c are the swelling power vs. temperature curve of Y1 and Y2 yam starches, respectively. b and d are the solubility vs. temperature curve of Y1 and Y2 yam starches, respectively. YNS, HMT, and ANN indicate native, heat-moisture treated, and annealed yam starch, respectively. Prefix 1 and 2 indicate the two yam species. Suffix − 20, − 30, − 12 and − 14 indicate 20% moisture level, 30% moisture level, 1:2 starch to moisture ratio and 1:4 starch to moisture ratio, respectively
Thermal properties
Gelatinization, which is modulated by water and heat, can determine the starch quality. In the presence of an appropriate amount of water and high temperature, the semicrystalline regions in starch change to amorphous form; and induce changes in the functional properties such as viscosity and gel-forming ability of starch, which are desired for industrial food applications [55]. Table 2 presents the information on the gelatinization properties of the native starches, HMT, and ANN-treated starches. The gelatinization onset (To), peak (Tp) and conclusion (Tc) temperature for 1YNS were 71.16 ± 0.23 °C, 78.52 ± 0.18 °C, and 84.08 ± 0.19 °C, respectively, with a transition enthalpy (ΔH) of 17.58 ± 0.15 J/g. The gelatinization onset (To), peak (Tp) and conclusion (Tc) temperature for 2YNS were 72.34 ± 0.19 °C, 77.22 ± 0.18 °C, and 82.67 ± 0.11 °C, respectively, with a transition enthalpy (ΔH) of 17.87 ± 0.12 J/g. The gelatinization parameters of the native starches were in the range or close to that for Dioscorea esculenta (To, 72.30–72.55 °C; Tp, 75.00–75.73 °C; Tc, 81.65–85.40 °C; ΔH, 17.32–18.07 J/g) [54], Dioscorea alata (To, 68.80–75.80 °C; Tp, 73.50–82.40 °C; Tc, 79.90–88.40 °C; ΔH, 11.80–18.00 J/g) [56], and Dioscorea bulbifera (To, 71.50–75.26 °C; Tp, 75.69–78.10 °C; Tc, 82.50–93.05 °C) [57]. The gelatinization parameters in yam starches are found to be higher than in starches of potato, cassava, and sweet potato [28]. The long interblock chain length, external chain length, and a low number of building blocks per cluster of amylopectin in Dioscorea esculenta promote the parallel organization of adjacent double helices and lead to better packing of amylopectins in the starch granules with less branched backbone, thereby increasing ΔH and To [58]. HMT and ANN-treated starches had significantly higher To, Tp and Tc compared to their respective native starches. HMT treatments are reported to increase the gelatinization temperatures by a broadening of gelatinization range and decreasing the swelling power [59]. The reduced mobility of starch chains in the amorphous regions due to the interactions between amylose-amylose, amylose-amylopectin, and amylose-lipids could increase the gelatinization temperatures [13]. ANN reduces the swelling power and hydration capacity of the starch granules, leading to an increase in gelatinization temperature and narrowing of the gelatinization range, due to the higher alignment of amylopectin double helices and higher content of glassy amorphous regions in annealed starch [59].
There was a significant decline and increase in the ΔH of starch after HMT and ANN, respectively, compared to their respective native starches and with moisture levels. HMT treatments decrease the enthalpy of gelatinization, and reflect the unraveling of the double helix structure present in crystalline and non-crystalline regions of starch granules [33], whereas, the increase of ΔH in ANN indicates a more ordered arrangement of the double helices which forms a tighter starch network with higher crystallinity [55]. Meanwhile, partial gelatinization of less stable amylose and amylopectin molecules can also be linked with a reduced value of ΔH in HMT [25]. Similar results are reported after HMT for maize [25], elephant foot yam [38], and amaranth [34] starches; and after ANN for potato [60], wheat [52], and maize [25] starches. There was no gelatinization of native, HMT, and ANN starches below 70 °C; therefore, the starches can have potential applications in dough and bread making where an increase in dough volume and higher final bread volume is desired [61].
Pasting properties
The pasting parameters of the native and modified yam starches are summarized in Table 3. After HMT and with moisture levels, a significant (p < 0.5) increase in the pasting temperature and decrease in the PV, HPV, BD, SB, and FV values was observed (except SB for 1HMT-30). Similar trends were reported for HMT normal potato and pearl millet starches, which could be attributed to the decrease in swelling power and amylose leaching of starch granules, and stronger bonding between starch chains (AM-AM, AM-AMP, AMP-AMP) leading to increased granular rigidity [37, 62]. HMT promotes granular rigidity by strengthening granular bonding due to increased intermolecular association of starch chains, restricts swelling power and AML, and thus indicates a higher temperature requirement to disrupt the HMT starch granules to form paste [13, 37, 38]. The increase in moisture levels of HMT was found to increase the PT in elephant foot yam starch [38]. After ANN and with moisture levels, an increase in the PT, SB, and FV values with a decrease in the PV, HPV, and BD was observed. The increase in PT of ANN granules might be due to the complex changes in the crystalline regions and strengthening of intragranular binding forces in the granules, thereby rendering better thermal resistance [13, 60]. A remarkably higher decrease in the PV values was observed after HMT than ANN compared to native starch. This reduction in PV could be attributed to the reduction in the swelling power and AML of the starch granules induced by HMT and ANN. BD indicates the thermal stability and resistivity to shear or deformation of the starch granules during gelatinization at 95 °C under constant mixing and agitation. BD values were found to be significantly (p < 0.5) lower after HMT than ANN compared to the native starch, and with moisture levels. These results indicated the increased thermal stability of the HMT and ANN modified starches, and thus the extent of granular rupture was more pronounced in the native starches compared to the HMT and ANN modified starches. In comparison to the native starch, a decrease in moisture content and HPV of the starch granules after HMT and ANN was observed. Setback (retrogradation) depends on the extent of viscosity breakdown and size of the granules, and the presence of rigid and large granules embedded in leached amylose network can show a higher degree of SB [13, 54]. After ANN, the setback viscosity increased steadily, whereas a decrease in SB was observed after HMT, compared to the native starch. The final viscosity was found to be higher for ANN starches and lower for HMT starches as compared to the native starch granules. An increase in the FV after ANN could be attributed to insufficient gelatinization, decreased swelling power, and increased rigidity of the ANN granules that can cause the amount of leaching out starches to remain unchanged, leading to the formation of a continuous gel structure, and hence increased FV [14, 63]. These differences in the pasting and viscosity properties between the starches could be explained more clearly with molecular insights on the change of amylose/amylopectin ratio, chain length distribution, and internal helix arrangements during the modification processes, and hence needs further research.
Steady shear testing
The steady-state flow behaviors of the native and modified starch pastes at 25 °C are shown in Fig. 4a and b. The experimental data fitted with power-law models were used to explain the flow behavior of the sample pastes with high R2 values (0.9787–0.9980). Consistency coefficient (k), flow behavior index (n), and determination coefficient were the model parameters used to accurately analyze the flow curve of each starch paste (Table 4).
a and b are the flow curves of steady shear testing of Y1 and Y2 yam starches, respectively. YNS, HMT, and ANN indicate native, heat-moisture treated, and annealed yam starch, respectively. Prefix 1 and 2 indicate the two yam species. Suffix − 20, − 30, − 12 and − 14 indicate 20% moisture level, 30% moisture level, 1:2 starch to moisture ratio and 1:4 starch to moisture ratio, respectively
The flow curves of all the sample pastes were non-linear and behaved as non- Newtonian fluids as shown in Fig. 4. There was an increase in shear stress with an increase in the shear rate of all the sample pastes. However, after HMT treatment, there was a marked and significant decrease (p < 0.5) in the shear stress compared to the native sample paste. This reduction in shear stress might be due to the limited water absorption by starch granules during HMT treatment which leads to partial gelatinization of the starch granules, resulting in weaker molecular interactions in the starch pastes. Limited exudation of starch molecules, especially the amylose fractions, can also lower the shear stress required to achieve the same shear rate [64]. Compared to native starch, ANN-treated starch showed a significant (p < 0.5) higher value of shear stress, indicating stronger molecular interactions among starch granules in the pastes. All the sample pastes exhibited a pseudoplastic and shear-thinning fluid behavior (n < 1), and the observed values of flow behavior index (n), which indicates the shear-thinning behavior ranged between 0.48 and 0.63. There was no significant (p < 0.5) change in the n values of HMT treated starches compared to native starches; however the n values of ANN treated starches increased significantly (p < 0.5) compared to native starches, and also with moisture levels (except for 1ANN-12 and 1ANN-14), indicating lower shear thinning behavior than native and HMT treated starches. Similar results on increase of n values have been reported for annealed starches of potato, lentil, and wheat; and this might be attributed to the decrease in swelling power of the modified starches, indicating reduced susceptibility of the starch granules towards shear deformation and disintegration [65]. 1ANN-12 and 2ANN-14 had the highest n values of 0.58 ± 0.002 and 0.63 ± 0.003, respectively. 1YNS exhibited a higher shear-thinning tendency compared to 2YNS, and this behavior could be attributed to the higher amylose content of D. alata starches. Starch with higher amylose content contains more amylose pre-gel cluster, which contributes more micro-particles in the paste, and this eventually leads to higher severity of particle to shear thereby showing higher shear-thinning tendency [66]. The consistency coefficient (k) for native starch pastes was significantly reduced after the HMT treatment, indicating lower structural strength of the HMT-treated starch pastes. This reduction in the values of k may be due to the reduction in the swelling power of HMT-treated starch granules and a similar decrease was observed in HMT-treated potato, lentil, wheat, and pearl millet starches [62, 65]. Clearly, the results of this experiment showed a conspicuous decrease in swelling power for HMT-treated starches, and a reduction of k values can also be attributed to the decrease in AML from HMT-treated starches. Such flow properties contradicts earlier reports on sweet potato starch, where higher values of k and lower value of n has been reported [66]. This indicates that the type and source of starch can influence the flow properties, and efficient comparisons of consistency coefficient (k) between samples can only be done with a constant value of flow behavior index (n) in the samples. However, k values of all the ANN-modified starches (except for 1ANN-12) significantly (p < 0.5) increased compared to native starches and with moisture levels, indicating stronger structural strength due to enhanced starch structure interactions during ANN. Similar trends for k values were observed for ANN-modified starches of potato despite lower swelling power and AML [65]. After the starch granules reach the maximum viscosity, the extent of granular collapse and disintegration under shear might be less pronounced for annealed starches than native starches, thereby increasing the thermal stability of ANN-treated starches during the holding cycle (at 95 °C), which could lead to higher k values of ANN treated starches than their native counterparts. These results were consistent with the results of RVA shown in Table 3.
Oscillatory testing
Dynamic rheological properties such as storage modulus (G′), loss modulus (G″), and loss tangent (tan δ = G″/G′) as a function of frequency (ω) for the native and modified starch pastes at 25 °C are presented in Fig. 5a–f. Loss tangent (tan δ) > 1 indicates the predominance of viscous properties (behavior like liquid) whereas a tan δ < 1 indicates the predominance of elastic properties (behavior like solid) of a gel.
a, c and e are the storage modulus, loss modulus and tanδ vs. angular frequency curves, respectively, of oscillatory testing of Y1 yam starches. Likewise, b, d, and f of Y2 yam starches. YNS, HMT, and ANN indicate native, heat-moisture treated, and annealed yam starch, respectively. Prefix 1 and 2 indicate the two yam species. Suffix − 20, − 30, − 12 and − 14 indicate 20% moisture level, 30% moisture level, 1:2 starch to moisture ratio and 1:4 starch to moisture ratio, respectively
All the tested starch pastes showed a predominance of G′ over G″ and no crossover was observed at the frequency range of 0.1–100 rad/s, and both G′ and G″ increased with the increase in frequency, indicating weak gel characteristics of the starch pastes. Both the modulus (G′ and G″) for Y1 native and modified starches (except for 1HMT-20) were found to be higher than the moduli (G′ and G″) for Y2 native and modified starches, possibly due to its reduced swelling power and AML, and indicated better viscoelastic properties (Fig. 5a–d). Tan δ values (0.27–0.39) of the native and modified starches of Y1 were found to be lower than the tanδ values (0.30–0.53) of the native and modified starches of Y2, respectively (Fig. 5e, f). After HMT and with moisture levels, a conspicuous reduction in G′ (Fig. 5a, b) and G″ (Fig. 5c, d) was observed compared to their native counterparts. In addition, the HMT samples were highly viscous compared to native samples as indicated by the higher tanδ values (Fig. 5e, f) than native. A similar trend was reported for hydroxypropylated sweet potato starch where a reduction of G′ and G″ was observed with an increase in water uptake capacity of the starch granules, indicating weaker granular integrity [67]. Therefore, the results indicated that the decrease in moduli after HMT could be attributed to the decrease in granular integrity of the granules, and hence reduction of G′ and G″ with increased tan δ was noticed. This observation can also be correlated with the RVA results of lower setback and final viscosities of starch after HMT (Table 3), indicating lower retrogradation tendency (lower reassociation of amylose/amylopectin chains) leading to decreased gel strength. Moreover, granular rupture and increased water exudation from starch granules during gelatinization or shearing can reduce the frictional resistance to flow properties and make the adjacent starch granules easily slide each other, thereby can reduce G′ and G″. After ANN and with moisture levels, there was a remarkable increase in the moduli (G′ and G″) and decrease in tan δ, indicating stronger interactions between the starch chains in the starch pastes leading to increased gel strength. A higher value of G′ and G″ after ANN compared to their native counterparts may be due to their higher retrogradation as indicated by the higher setback and final viscosity during RVA analysis (Table 3). These findings are consistent with results reported for kithul starch after ANN, which could be associated with a higher rate of retrogradation due to stronger associations between amylose/amylopectin chains leached from starch granules leading to increased gel strength of starch pastes [45].
To further investigate, the frequency dependence of G′ and G″, the experimental data of G′ and G″ were fitted to power-law models (Eqs. 2 and 3) to obtain the model parameters k′, k″, n′ and n″, with high coefficients of determination (R2: 0.8184–0.9684 for G′ and R2: 0.9840–0.9989 for G″) as given in Table 4. A linear decrease in k′ (elastic) and k″ (viscous) was observed after HMT with increase in moisture levels compared to native starch whereas an opposite phenomenon was observed after ANN with increase in moisture levels (except for k″ of 2ANN-14). The n′ and n″ values of the starch pastes represent the increased or decreased susceptibility of G′ and G″, respectively, with changes in the angular frequency. An increase in the frequency dependence of G′ was noticed after HMT for Y1 starch compared to native starch, and either constant or decrease after HMT for Y2 starch. After ANN, a decrease in the frequency dependence of G′ was noticed compared to native starch for Y2 starches, and either constant or increase after HMT for Y2 starch. The frequency dependence of G″ was found to be decreasing after HMT and ANN for all the starches of Y1 (except constant for 1HMT30). The frequency dependence of G″ was found to be increasing at higher moisture levels after HMT and ANN for Y2 starches.
Therefore, based on the rheological results of the yam starches, HMT-modified yam starches can find suitable applications in food products such as bread making, ice-creams, puddings, salad dressing, etc., where a low viscosity is desired, whereas ANN-modified yam starches can be used in food products like jam, thickeners, etc. and more appropriately as an ingredient in making food products such as paneer to increase the viscosity as well as to improve the gel strength and consistency, which is the desired phenomenon to maintain the texture.
In vitro starch digestibility
The data on in vitro digestibility of native and modified yam starches are presented in Table 5. A significant (p < 0.5) increase in the SDS and RS and a decrease in the RDS content were found after HMT and ANN. ANN starches registered substantial increase in SDS and RS as compared to HMT starches. Among the ANN starches of Y1 and Y2, 1ANN-14 and 2ANN-14 had the highest SDS (21.26% and 24.18%, respectively) and RS (40.63% and 41.01%, respectively) content, while, in comparison, their native forms recorded SDS content of 15.68% and 13.35%, respectively and RS content of 22.32% and 15.51%, respectively. The increase in SDS and RS after HMT may be due to increased interactions between starch chains; perfection of existing starch crystallites; and reduced accessibility of enzymes for digestion [62, 68]. The decrease in RDS with an increase of SDS and RS after ANN could be attributed to a decrease in enzyme susceptibility; enhanced crystallites perfection; and increased amylose-amylose and/or amylose-amylopectin interactions [55]. An increase in starch granular porosity can decrease the content of RS during ANN, and no pores or fissures were seen on the ANN starch granules in this study. Many reports on the increase of SDS with a decrease of RS and decrease of SDS with an increase of RS after HMT and ANN have been published [11, 25, 31, 48, 69, 70]. Thus, the amount of SDS and RS produced after hydrothermal modifications appeared to be dependent on the moisture levels of starch during treatment, treatment temperature, treatment time, and source of starch. Moreover, the enzyme hydrolysis of starch depends on various factors like starch crystallinity, the morphology of starch granules, polymorphism, and amylose/amylopectin content [71]. Higher RS content starches with low digestibility are desired for the production of food products with the low glycemic index.
Conclusion
The effect of hydrothermal treatment on functional, thermal, pasting, morphological, and rheological properties of starches from two yam species was investigated in the present study. After HMT, a significant decrease in peak viscosity, hot paste viscosity, breakdown, setback, and final viscosity was observed, while after ANN, a significant decrease in peak viscosity, hot paste viscosity, and breakdown with a significant increase in the setback and final viscosity was observed. The consistency coefficient and flow behaviour index of the starch pastes increased after ANN, while a lower consistency coefficient of the starch pastes was noticed after HMT. Reduction in G′ and G″ was observed after HMT indicating a low retrogradation tendency of the starch granules, whereas an increase in G′ and G″ and decrease in tan δ was observed after ANN indicating a higher retrogradation tendency of the starch granules. ANN caused greater increase in slowly digestible and resistant starch with a decrease in rapidly digestible starch than HMT. Hence, in this study, ANN was found to be an effective modification method for the yam starches with increased gel strength. HMT modified yam starches can be used in products such as bread, ice-creams, puddings, salad dressing. ANN modified yam starches can be used in food products like jam, thickeners and as an ingredient in paneer. The suitability of HMT and ANN modified yam starches for the preparation of edible or biodegradable films can also be checked. Further investigations on in vitro digestibility of yam starches are required to study the influence of treatment conditions, and physicochemical properties, functional properties, size, shape, and other properties of starch granules on enzyme hydrolysis.
Data Availability
Data will be made available on request.
References
N. Goswami, S.K. Borthakur, D.K. Hore, Pleione 7, 73 (2013)
B.D. Sharma, D.K. Hore, India J. Hill Farm. 8, 145 (1995)
S. Kumar, G. Das, H.S. Shin, J.K. Patra, Front. Pharmacol. 8, 52 (2017)
R. Waris, S. Tripathi, A.C. Shukla, P. Agnihotri, Plant Sci. Today 8, 72 (2021)
J.E. Obidiegwu, J.B. Lyons, C.A. Chilaka, Foods 9, 1304 (2020)
D.K. Verma, P.P. Srivastav, Crit. Rev. Food Sci. Nutr. (2021). https://doi.org/10.1080/10408398.2021.1903383
V.M. Mathobo, H. Silungwe, S.E. Ramashia, T.A. Anyasi, J. Food Sci. Technol. 58, 412 (2021)
S. Park, Y.R. Kim, Food Sci. Biotechnol. 30, 1 (2021)
M. Schmiele, U. M. Sampaio, P. T. G. Gomes, M. T. Pedrosa Silva Clerici, in Starches for Food Application, ed. by M. Schmiele, M. T. Pedrosa Silva Clerici (Elsevier, US, 2019), pp. 223–269. https://doi.org/10.1016/B978-0-12-809440-2.00006-X
S. X. Xie, Q. Liu, and S. W. Cui, in Food Carbohydrates: Chemistry, Physical properties and Applications, ed. by S. W. Cui (CRC Press, Boca Raton, 2005), pp. 357–405. https://doi.org/10.1201/9780203485286
L.M. Fonseca, S.L.M. El Halal, A.R.G. Dias, E.R. Zavareze, Carbohydr. Polym. 274, 118665 (2021)
L. Harimu, M.J. Baari, L. Putri Ayuningtyas, A. Mukti Benita, D. Triastuti, IOP Conf. Ser. Earth Environ. Sci. 746, 012007 (2021)
A. Gunaratne, Phys Modif Starch (Springer, Singapore, 2018), pp.15–36
M. Iuga, S. Mironeasa, Crit. Rev. Food Sci. Nutr. 60, 3890 (2019). https://doi.org/10.1080/10408398.2019.1664978
B. Otegbayo, D. Oguniyan, O. Akinwumi, Starch Stärke 66, 235 (2014)
A.R. Tanimola, B.O. Otegbayo, R. Akinoso, Food Res. 6, 49 (2022)
J. Zou, Y. Li, X. Su, F. Wang, Q. Li, H. Xia, Molecules 2022(27), 2254 (2022)
C. Nindjin, G.N. Amani, M. Sindic, Carbohydr. Polym. 86, 1637 (2011)
M. Piecyk, B. Drużyńska, A. Ołtarzewska, R. Wołosiak, E. Worobiej, E. Ostrowska-Ligęza, Int. J. Biol. Macromol. 118, 2113 (2018)
Y. Yeh, L.S. Lai, Molecules 26, 4339 (2021)
R. Hoover, W.S. Ratnayake, Curr. Protoc. Food Anal. Chem. 00, E2.3.1 (2001)
L. Jayakody, R. Hoover, Q. Liu, E. Weber, Food Res. Int. 38, 615 (2005)
B.A. Ashwar, A. Gani, A. Shah, F.A. Masoodi, Int. J. Biol. Macromol. 105, 471 (2017)
C.O. Bernardo, J.L.R. Ascheri, D.W.H. Chávez, C.W.P. Carvalho, Starch Stärke 70, 1700185 (2018)
H. Liu, M. Lv, L. Wang, Y. Li, H. Fan, M. Wang, Starch Staerke 68, 1158 (2016)
Y. Qin, C. Liu, S. Jiang, J. Cao, L. Xiong, Q. Sun, PLoS ONE 11, e0160371 (2016)
T.T. Huang, D.N. Zhou, Z.Y. Jin, X.M. Xu, H.Q. Chen, Food Hydrocoll. 54, 202 (2016)
F. Zhu, Compr. Rev. Food Sci. Food Saf. 14, 357 (2015)
N.G. Amani, A. Buléon, A. Kamenan, P. Colonna, J. Sci. Food Agric. 84, 2085 (2004)
C. Goel, A.D. Semwal, A. Khan, S. Kumar, G.K. Sharma, J. Food Sci. Technol. 57, 2941 (2020)
H. Liu, X. Guo, W. Li, X. Wang, M. Lv, Q. Peng, M. Wang, Carbohydr. Polym. 132, 237 (2015)
I. Chakraborty, I. Govindaraju, S. Rongpipi, K.K. Mahato, N. Mazumder, Food Biophys. 16, 544 (2021)
R. Sindhu, A. Devi, B.S. Khatkar, J. Food Sci. Technol. 56, 2480 (2019)
R. Sindhu, A. Devi, B.S. Khatkar, Food Hydrocoll. 118, 106800 (2021)
V. Marboh, C.L. Mahanta, Int. J. Biol. Macromol. 168, 486 (2021)
K.O. Falade, O.E. Ayetigbo, Food Hydrocoll. 43, 529 (2015)
R. Colussi, D. Kringel, L. Kaur, E. da Rosa Zavareze, A.R.G. Dias, J. Singh, Food Chem. 318, 126475 (2020)
M. Suriya, C.K. Reddy, S. Haripriya, Int. J. Biol. Macromol. 137, 783 (2019)
S. Punia Bangar, M. Nehra, A.K. Siroha, M. Petrů, R.A. Ilyas, U. Devi, P. Devi, Foods 10, 1609 (2021)
E.D.R. Zavareze, A.R.G. Dias, Carbohydr. Polym. 83, 317 (2011)
Y. Watcharatewinkul, C. Puttanlek, V. Rungsardthong, D. Uttapap, Carbohydr. Polym. 75, 505 (2009)
H. Liu, L. Yu, G. Simon, K. Dean, L. Chen, Carbohydr. Polym. 77, 662 (2009)
F.J. Warren, M.J. Gidley, B.M. Flanagan, Carbohydr. Polym. 139, 35 (2016)
B.L. Karwasra, B.S. Gill, M. Kaur, Int. J. Food Prop. 20, S1093 (2017). https://doi.org/10.1080/10942912.2017.1328439
C. Sudheesh, K.V. Sunooj, M. Alom, S. Kumar, V.A. Sajeevkumar, J. George, J. Food Meas. Charact. 14, 1557 (2020)
S. Barua, K. Tudu, M. Rakshit, P.P. Srivastav, J. Food Process. Eng. 44, e13841 (2021)
A.N. Syarifin, A.S. Purnomo, A. Fudholi, Food Hydrocoll. 120, 106889 (2021)
H.J. Chung, Q. Liu, R. Hoover, Carbohydr. Polym. 75, 436 (2009)
I. Bharti, S. Singh, D.C. Saxena, LWT 110, 197 (2019)
M. Kaur, S. Singh, Int. J. Biol. Macromol. 122, 312 (2019)
J.S. Lee, J.H. Akanda, S.L. Fong, C.K. Siew, A.L. Ho, Molecules 27, 4838 (2022)
C. Su, A.S.M. Saleh, B. Zhang, K. Zhao, X. Ge, Q. Zhang, W. Li, Carbohydr. Polym. 247, 116675 (2020)
P.T.B. Trung, L.B.B. Ngoc, P.N. Hoa, N.N.T. Tien, P. Van Hung, Int. J. Biol. Macromol. 105, 1071 (2017)
L. Jayakody, R. Hoover, Q. Liu, E. Donner, Carbohydr. Polym. 69, 148 (2007)
T. Yao, Z. Sui, S. Janaswamy, Phys Modif Starch (Springer, Singapore, 2018), pp.37–49
C.C. Huang, M.C. Lin, C.C.R. Wang, Carbohydr. Polym. 64, 524 (2006)
Q. Jiang, W. Gao, X. Li, H. Wang, Y. Xia, P. Xiao, Starch Staerke 64, 531 (2012)
V. Vamadevan, E. Bertoft, K. Seetharaman, Carbohydr. Polym. 92, 1653 (2013)
S.X. Xie, Q. Liu, S.W. Cui, Starch Modification and Applications (CRC Press, Boca Raton, 2005)
M. Xu, A.S.M. Saleh, B. Gong, B. Li, L. Jing, M. Gou, H. Jiang, W. Li, Food Res. Int. 111, 324 (2018)
P. Megusar, D. Stopar, N.P. Ulrih, I. Dogsa, I. Prislan, Polymers 14, 3242 (2022)
K.S. Sandhu, A.K. Siroha, S. Punia, M. Nehra, Carbohydr. Polym. Technol. Appl. 1, 100002 (2020)
M.C.B.M. Almeida, S.S. Costa, M.T. Cavalcanti, E.L. Almeida, Starch Stärke 72, 1900137 (2020)
L. Chen, Y. Tian, Y. Bai, J. Wang, A. Jiao, Z. Jin, Food Hydrocoll. 77, 85 (2018)
R. Hoover, T. Vasanthan, J. Food Biochem. 18, 67 (1994)
L. Liao, H. Liu, Z. Gan, W. Wu, Int. J. Food Prop. 22, 1122 (2019). https://doi.org/10.1080/10942912.2019.1626418
H.L. Lee, B. Yoo, LWT Food Sci. Technol. 44, 765 (2011)
J. N. BeMiller, in Starch in food, ed. by M. Sjöö and L. Nilsson (Elsevier, Cambridge, 2018), pp. 223–253. https://doi.org/10.1016/B978-0-08-100868-3.00005-6
P. Van Hung, H.T. Chau, N.T.L. Phi, Food Chem. 191, 74 (2016)
F. Zeng, F. Ma, F. Kong, Q. Gao, S. Yu, Food Chem. 172, 92 (2015)
Q. Xie F. Zhu, in Physical modifications of starch, ed. By Z. Sui and X. Kong (Springer, Singapore, 2018), pp. 1–14. https://doi.org/10.1007/978-981-13-0725-6_1
Acknowledgements
The authors are thankful to the Department of Food Technology and Central Instrumental Facility, Jamia Hamdard for providing the instrumental and laboratory facilities.
Author information
Authors and Affiliations
Contributions
JB: conceptualization, methodology, formal analysis, investigation, visualization, validation, writing-original draft. NKM: investigation, validation. HAM: investigation, validation. CLM: conceptualization, resources, visualization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors of this present study declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bora, J., Mahnot, N.K., Makroo, H.A. et al. Impact of hydrothermal treatments on the functional, thermal, pasting, morphological and rheological properties of underutilized yam starches. Food Measure 17, 2285–2300 (2023). https://doi.org/10.1007/s11694-022-01789-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01789-1