Abstract
The effects of methyl jasmonate (MeJA) treatment on the mold decay and disease resistance of Ginkgo biloba seeds in response to pathogen attack were assessed. G. biloba seeds were exposed to different concentrations of MeJA vapor for 12 h before being inoculated with Penicillium oxalicum. Results showed that MeJA at different concentrations could markedly decrease the disease incidence and inhibit membrane damage of G. biloba seeds compared with the control, especially at the concentrations of 100 and 150 μmol L−1. The H2O2 content and activities of H2O2-metabolising related enzymes were induced to a higher level with MeJA. Defense related enzyme activities were also significantly enhanced in G. biloba seeds treated with 100 and 150 μmol L−1 of MeJA. These results together indicated that MeJA stimulated the disease resistance of G. biloba seeds by regulating the activation of H2O2 accumulation and inducing activities of defense-related enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginkgo biloba seed, as a type of nut, is increasingly becoming popular food mainly due to its nutritional contents, such as carbohydrates, protein, amino acids, vitamin C, flavonoids, and lactones [1]. G. biloba seed is also a traditional Chinese medicine and used as a raw material for directly cooking or making processed food that benefits human health. The active ingredients of ginkgo seeds such as flavanol glycosides, terpene trilactones, ginkgolic acids and peptides were reported to treat fever, coughs, sputum and skin disease effectively [2]. Modern medical research has proven that ginkgo seeds exhibit good effects on cardiovascular and cerebrovascular system diseases; they could discourage clot formation, improve blood circulation, as well as protect nerve cells [3,4,5]. Nowadays, consumption of G. biloba seed is increasing with the consumer’s demands mainly due to its nutritional contents and functional components. However, G. biloba seeds are susceptible to mold infection by Penicillium during long-term storage.
Blue mold decay, which is caused by pathogens belonging to Penicillium genus, is a common and major postharvest disease that limits the storage of many horticulture crops [6,7,8,9]. G. biloba seeds are prone to the mold decay caused by Penicillium oxalicum, which is one of the pathogens isolated from decayed seeds. Various methods have been used for preserving G. biloba seeds, such as cold storage, SO2 fumigation, chemical fungicide, chitosan and nanomaterial coatings [10,11,12]. However, these preservation treatments still have limitations, including limited antifungal effect, residence of chemical fungicides, and complicated operation, which may result in threat to human health or increased cost.
One of the possible options to reduce disease development is inducing the defense mechanisms in plants [13]. Methyl jasmonate (MeJA) is a fragrant volatile compound that is distributed ubiquitously in fruit and plants. MeJA and its free-acid jasmonic belong to jasmonates, which are synthesized in plants via the octadecanoid pathway from α-linolenic acid. As a natural plant signaling compound, MeJA is closely related to developmental processes and the defense responses against biotic and abiotic stresses in fruit and plants [7, 14]. Moreover, the defense-related genes in fruit, which are involved in jasmonate biosynthesis, cell wall formation, secondary metabolism, and those encoding stress protective and defense proteins, can be upregulated after MeJA treatment. Therefore, MeJA treatment has been widely used for controlling postharvest diseases, maintaining quality, and prolonging the shelf life of fruit and vegetables [15]. The treatment of MeJA vapors has been found to significantly reduce the incidence of anthracnose and gray mold diseases on avocados and table grapes [8, 16]. Moreover, it has been applied on postharvest apple, pear, and peach fruit to control blue mold decay [17,18,19]. The mechanism involved in the disease-resistant process may be not only related to the transient direct inhibitory effect against the pathogens, but also related to the ability to induce resistance in fruit by MeJA. The results showed that MeJA at different concentrations of 100, 150, and 200 μmol L−1 could inhibit the mycelial growth of P. expansum and are efficient in controlling blue mold decay [17,18,19]. The concentrations of MeJA most commonly used in previous studies were 10, 50, and 100 μmol L−1 [8]. Besides, most studies of fruit treated with MeJA focus on the defense mechanism represented by defense-related enzymes, including phenylalanine ammonia-lyase (PAL), chitinase (CHI), and β-1,3-glucanase (GLU) [17,18,19]. PAL is a crucial enzyme involved in the phenylpropanoid pathway. PAL could induce the production of secondary metabolites in plants to build barriers and limit the infection process. CHI and GLU, which consist of chitin and β-1,3-glucan, respectively, could hydrolyze the polymers of fungal cell walls [8].
However, to the best of our knowledge, the literature on the effect of MeJA on the blue mold disease susceptibility of G. biloba seeds is limited. Therefore, this research aimed to investigate the effects of MeJA vapors at different concentrations (0, 50, 100, and 150 μmol L−1) on the following: (i) disease incidence, (ii) activities of antioxidant enzymes, and (iii) defense-related enzyme activities in postharvest G. biloba seeds kept at 25 °C, with a relative humidity (RH) 90%, for 24 d.
Materials and methods
Materials
G. biloba seeds at commercial maturity were collected from trees cultured in Nanjing Forestry University, China. The seeds without episperm were washed with tap water and air-dried until their water content was below 70%, following the process of removing the mesosperm. Afterwards, seeds were selected for absence of blemishes or disease prior to MeJA treatment and pathogen inoculation.
Pathogen
P. oxalicum (Gene bank accession number: MK419111) was isolated from the infected G. biloba seeds and incubated on a potato dextrose agar medium (PDA: HB0233-12, Qingdao Hope Bio-Technology Co., Ltd) for 7 d. Afterwards, the spores were flushed with sterile water, and the suspensions were prepared by filtering with four layers of lens wiping paper. The desired concentration of spores (1 × 105 conidia mL−1) was obtained by using a hemocytometer.
Measurement of MeJA on spore germination of P. oxalicum in vitro
The effects of MeJA on the spore germination of P. oxalicum were measured in the potato dextrose broth (PDB) according to a previous report [18]. A final concentration of 106 spores mL−1 was obtained with PDB, which contained various concentrations of MeJA (0, 50, 100, and 150 µmol L−1). The PDB culture mediums were incubated at table concentrator with a speed of 100 g at 25 °C for 24 h. The germination rate was determined by observing approximately 100 spores of pathogen for each treatment, with three replications.
MeJA treatment and pathogen inoculation
A total of 500 seeds for one treatment with three replicates were placed in 40 air-tight containers. MeJAs (Sigma–Aldrich, USA) at three concentrations of 50, 100, and 150 µmol L−1 were deposited on Petri dishes at the bottom of the container [20]. All treatments, including the MeJA at the concentration of 0 (control), were immediately sealed in containers and exposed to MeJA vapor for 12 h at 10.0 ± 0.5 °C. After MeJA fumigation, the containers were opened and ventilated at 25 °C for 4 h. Before pathogen inoculation, all seeds in the MeJA treatments and the control were surface disinfected with 75% ethanol for 30 s. Afterwards, they were surface wounded (1 mm diameter × 1 mm deep) and inoculated with 10 µL of spore suspension, with the same amount of sterile water as the control. Afterward, all seeds were maintained at 25 °C with 90% RH for 24 d. Samples were collected and frozen by liquid nitrogen immediately, and then stored at − 80 °C for further analysis. The quality parameters and physico-chemical characteristics were determined every 6 d.
Disease incidence determination
The disease incidence was determined by calculating the amount of diseased seeds exhibiting visible fungal infection, and the results were expressed as the percentage of infected seeds compared with total number of seeds in each treatment [20].
Measurement of relative conductivity and malondialdehyde (MDA) content
The relative conductivity was determined according to the previous method [21]. The initial conductivity (L0) of the tissues after water bath shaking at 25 °C for 30 min and another reading (L1) of the tissue conductivity after boiling for 20 min and then cooling down to 25 °C were measured using a conductivity meter (DDS-307, Shanghai INESA Scientific Instrument Co., Ltd., Shanghai, China). The relative conductivity was calculated using the following equation:
Relative conductivity (%) = L0/Lt × 100. (E1).
The MDA content was measured as described in a previous report [22] and the results were calculated using the following formula based on fresh weight basis: C (mmol kg−1) = 6.45 × (OD532 – OD600) – 0.56 × OD450. (E2).
Determination of H2O2 content and antioxidant enzymes activities
For H2O2 determination, 2 g of frozen tissues were homogenized with 1 mL of 0.1% cold trichloroacetic acid solution, and then maintained in an ice bath for 10 min. The supernatant was obtained after centrifugation at 12,000 g for 15 min at 4 °C. The reaction mixture consisted 0.5 mL of supernatant, 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0), and 1 mL of 1 mol L−1 potassium iodide. The absorbance was measured at 390 nm, and the H2O2 content was extrapolated from a standard curve expressed as μmol g−1 [23]. The superoxide dismutase (SOD), the peroxidase (POD), and the catalase (CAT) were measured according to previous reports [24,25,26].
Determination of defense related enzymes activities
The PAL activity was estimated according to the reported method [27]. The reaction system included 75 µL of crude enzyme extract and 150 µL of borate buffer (50 mM, pH 8.8) containing l-phenylalanine (20 mM). After incubated at 37 °C for 60 min, the reaction was ended with the addition of 75 µL of HCl (1 M). The absorption was measured at 290 nm, and the enzyme activity was recorded as a nmol cinnamic acid h−1 mg of protein−1.
The chitinase activity was determined according to Abeles et al. [28]. The enzyme extract (600 µL) mixed with 2% (w/v) dye-labelled chitin azure (125 µL) and sodium acetate buffer (pH 5.0, 50 mM) was incubated at 40 °C for 2 h, and then the reaction system was stopped by adding 25 µL of HCl (1 mol L−1). The supernatant was measured at 550 nm, and the unit of enzyme activity was defined as a nM product h−1 mg of protein−1.
The β-1,3-glucanase activity was assayed according to Sellamuthu et al. [29]. The reaction system was as follows: 100 µL of enzyme extract, 100 µL of 2% (w/v) laminarin, and 25 µL of 3,5-dinitrosalicyclic. The reaction was ceased after the 5-min boiling water bath, and one enzyme unit was defined as the 1 mol glucose equiv. h−1 mg of protein−1.
Statistical analysis
All data presented as mean (n = 3) ± standard deviation (SD) were evaluated by one-way ANOVA, and the differences (p < 0.05) were assessed using Duncan’s multiple range test using the IBM SPSS 19.0 Statistics analytical software program.
Results and discussion
Effect of MeJA at different concentrations on inhibiting blue mold decay and spore germination
Concerned to the effects of MeJA fumigation on the growth of P. oxalicum in vitro, we designed the in vitro test to find the changes of spore germination in response to MeJA treatment. As shown in Fig. 1. the spore germination of P. oxalicum of all the treatments were similar, indicating that MeJA of different concentrations had little effect on the spore germination of P. oxalicum (p > 0.05). This finding is similar to the study of Zhang et al. [18], who observed that MeJA at all tested concentrations could not suppress the spore germination of P. expansum, which indicated that MeJA could not directly influence the growth of some fungal pathogens.
Spore germination of at 25 °C for 24 h. Seeds were organised into the following treatments: (i) control (MeJA 0); (ii) treated with methyl jasmonate (MeJA) with concentrations of 50, 100 and 150 µmol L−1 (MeJA 50, MeJA 100 and MeJA 150). Different letters indicate significant differences (p < 0.05) due to different treatments at the same storage period. Each value represented the mean of three replicates, data were presented as mean ± standard deviation (SD)
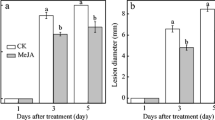
Figure 2 and Fig. 3 showed that blue mold disease occurred in control and all treated G. biloba seeds during postharvest storage. The disease incidence increased rapidly in the control samples, whereas significantly (p < 0.05) reduced in seeds fumigated with different concentrations of MeJA vapor. G. biloba seeds treated with 100 and 150 µmol L−1 MeJA vapors exhibited significant lower disease incidences with the values of 32.99 and 33.96% compared with control (63.27%) at the end of the storage. However, no statistically significant difference was observed between the groups of MeJA 100 µmol L−1 and MeJA 150 µmol L−1 at the end of storage. Our result was consistent with the observation of Yao and Tian [19], who found that MeJA could control postharvest blue mold decay, but the inhibitory ability is limited. In other studies, MeJA treatment at 100 or 200 µmol L−1 could significantly reduce blue mold rot lesion diameter of pear fruits, the combination of MeJA at 100 µmol L−1 and antagonistic yeast was more effective to reduce the lesion diameter of blue mold decay of apples [17].
Disease incidence of infected G. biloba seeds stored at 25 °C with RH 90% for 24 d. Seeds were organised into the following treatments: (i) control (MeJA 0); (ii) treated with methyl jasmonate (MeJA) with concentrations of 50, 100 and 150 µmol L−1 (MeJA 50, MeJA 100 and MeJA 150). Different letters indicate significant differences (p < 0.05) due to different treatments at the same storage period. Each value represented the mean of three replicates, data were presented as mean ± standard deviation (SD)
Effect of MeJA at different concentrations on the relative conductivity and malondialdehyde (MDA) content
All MeJA vapor treatments could suppress the increasement of relative conductivity compared to the control during most of the storage time (Fig. 4A). The relative conductivity calculated with E1 in all treated G. biloba seeds showed no statistically significant differences (p > 0.05). However, at the end of storage, G. biloba seeds treated with MeJA at concentrations of 100 and 150 µmol L−1 exhibited the lowest values of relative conductivities without significant differences. The values of MDA content were calculated according to E2. In Fig. 4B, the MDA content of the G. biloba seeds without MeJA increased approximately 1.46-, 1.99-, and 2.18-fold in 24 d compared with those with MeJA at 50, 100, and 150 µmol L−1, respectively (p < 0.05). Changes of the relative conductivity and MDA content in G. biloba seeds fumigated with MeJA together indicated that MeJA could maintain the stability of cell membrane to avoid the infection of P. oxalicum, especially.
Relative conductivity (A) and MDA content (B) of infected G. biloba seeds stored at 25 °C with RH 90% for 24 d. Seeds were organised into the following treatments: (i) control; (ii) treated with methyl jasmonate (MeJA) with concentrations of 50, 100 and 150 µmol L−1. Different letters indicate significant differences (p < 0.05) due to different treatments at the same storage period. Each value represented the mean of three replicates, data were presented as mean ± standard deviation (SD) of triplicate experiments
Effect of MeJA at different concentrations on H2O2 content and antioxidant enzymes (SOD, POD and CAT) activities
In Fig. 5A, the H2O2 content increased in the control and treated seeds with the extension of storage period. After 18 d of storage, G. biloba seeds treated with MeJA at the concentrations of 100 and 150 µmol L−1 showed remarkably higher H2O2 contents than the control (p < 0.05). The difference between the 50 µmol L−1 MeJA treated seeds and the control was not significant (p > 0.05) after 18 d of storage. Moreover, the H2O2 content of MeJA at 50 µmol L−1 remained at a relatively lower level than those of MeJA at 100 µmol L−1 and 150 µmol L−1 after 18 d of storage.
H2O2 content (A), SOD (B), POD (C) and CAT activities (D) of infected G. biloba seeds stored at 25 °C with RH 90% for 24 d. Seeds were organised into the following treatments: (i) control; (ii) treated with methyl jasmonate (MeJA) with concentrations of 50, 100 and 150 µmol L−1. Different letters indicate significant differences (p < 0.05) due to different treatments at the same storage period. Each value represented the mean of three replicates, data were presented as mean ± standard deviation (SD) of triplicate experiments
The antioxidant enzymes activities varied with a similar trend of first increasing and then decreasing (Fig. 5B–D). The group of MeJA 150 showed a more rapid ascent of SOD activity during the 12 d after inoculation than all the other groups (p < 0.05). The highest SOD activity was noted in the seeds of MeJA 100 at day 18 (p < 0.05). At the end of the storage, the exposure of the seeds to MeJA at 100 µmol L−1 resulted in higher SOD activity, exhibiting a significant difference from the other treatments of MeJA (50 µmol L−1, 150 µmol L−1) and control (p < 0.05). The activity of POD was markedly increased in seeds treated with MeJA at 150 µmol L−1 from day 6 and maintained a high level in all treated seeds for the following storage period (p < 0.05). The CAT activity also showed a similar tendency as POD, showing an increase in activity from day 6 and declining from day 18 in all groups. A higher CAT activity was observed in seeds exposed to MeJA at different concentrations than those untreated from inoculation for 12 d (p < 0.05). The G. biloba seeds exposed to MeJA at 100 and 150 µmol L−1 showed significantly (p < 0.05) higher CAT activities than those of the untreated (control) and the other MeJA treatment during the whole storage.
Effect of MeJA at different concentrations on defense-related enzymes (PAL, Chitinase and β-1,3-Glucanase) activities
The PAL enzyme activity in all G. biloba seeds reached the top values over 12 d of storage, and then declined during the rest of the period (Fig. 6A). All the MeJA treated seeds exhibited significantly higher enzyme activities than those seeds without any treatment after 12 d of storage (p < 0.05). However, during the latter part of the storage period, MeJA at 150 µmol L−1maintained higher PAL enzyme activities than MeJA at 50 µmol L−1. Moreover, at the end of storage, the PAL enzyme activity was maintained at a markedly higher level in MeJA 100-treated seeds. During most of the storage time, the CHI activity was found to be significantly increased in MeJA exposed seeds, especially at 100 and 150 µmol L−1 (p < 0.05). Compared to the control samples, all MeJA treated seeds could maintain the CHI activity at significant high values after storage (Fig. 6B). The β-1,3-glucanase activity was higher in MeJA-treated G. biloba seeds than in the untreated control fruit (p < 0.05) from day 12 after inoculation (Fig. 6C). At the end of storage, the β-1,3-glucanase activity of MeJA at 50 µmol L−1 and MeJA at 100 µmol L−1 was significantly higher than that of MeJA at 150 µmol L−1and control (p < 0.05).
PAL (A), CHI (B) and β-1,3-glucanase (C) activities of infected G. biloba seeds stored at 25 °C with RH 90% for 24 d. Seeds were organised into the following treatments: (i) control; (ii) treated with methyl jasmonate (MeJA) with concentrations of 50, 100 and 150 µmol L−1. Different letters indicate significant differences (p < 0.05) due to different treatments at the same storage period. Each value represented the mean of three replicates, data were presented as mean ± standard deviation (SD) of triplicate experiments
Evidently, MeJA effectively reduced the disease incidence of blue mold on postharvest G. biloba seeds after a long storage time, especially for MeJA at 100 and 150 µmol L−1. A high level of MeJA concentration could accelerate the ripening and senescence of G. biloba seeds (data not shown). Relative research showed that the most effective concentrations focused on the range of 1–1000 µmol L−1, which was dependent on many factors, including the varieties of fruit and treatment methods of MeJA [30]. Our result is consistent with those previous studies. For instance, the disease incidence could be effectively reduced by MeJA treatment in sweet cherry fruit inoculated with P. expansum during 5 d of storage [9] and in avocado fruit at 100 µmol L−1 prior to being stored for 14 d followed by 6–7 d shelf-life, confirming the effect of MeJA treatment on enhancing the disease resistance of G. biloba seeds [8]. However, MeJA reportedly does not have a direct effect on delaying the spore germination of P. expansum and P. digitatum and the growth of C. acutatum [6, 9, 31]. Consistent with these reports, our study also demonstrated that MeJA showed no direct inhibition of the spore germination of P. oxalicum (Fig. 1). Therefore, the defense effect of G. biloba seeds was not directly achieved by inhibiting the growth of pathogens by MeJA treatment but mainly due to the induced activation of a highly coordinated biochemical and structural defense system that could inhibit the spread of pathogens [32]. Besides, our study also showed the same phenomenon as previously reported, MeJA could alleviate the membrane lipid peroxidation and electrolyte leakage [33].
The induction of the disease resistance in postharvest horticultural crops is an important strategy to avoid infection of pathogen. They can rely on their own defense mechanisms and thus reduce the incidence of diseases. The possible induction of the disease resistance of postharvest plants involves complex reaction systems as shown in Fig. 7. It involves the activation of a series of biochemical and structural defense systems against the invasion of fungal pathogens, such as reactive oxygen species (ROS), and the phenylpropanoid pathway [34, 35]. ROS plays a role on regulating plant resistance responses to corresponding pathogen infections. It accumulates with a so-called oxidative burst as one of the earliest cellular responses following a successful pathogen recognition. ROS reportedly strengthened the host cell walls by cross-linking glycoproteins [36]. In the present study, the H2O2 content was activated to a higher level after the MeJA treatment and the inoculation by the pathogen, inducing defense responses probably by priming the tissue to promote ROS accumulation. A previous study indicated that the accumulation of ROS was closely associated with decreased fruit susceptibility to decay during postharvest storage [30]. This result was consistent with the study of the MeJA induced responses of sweet cherry against P. expansum [9]. Therefore, ROS might act as a central part in the MeJA primed responses against pathogen infection.
However, a substantial production of ROS burst can break the structure of host cells, resulting in cell death. Therefore, host tissues need a ROS scavenging system to protect themselves from injuries. High activities of antioxidant enzymes, including CAT, SOD, and POD, were reportedly involved in enhancing fruit disease resistance [30, 37]. Our results are consistent with those of these studies, MeJA (100 and 150 µmol L−1) significantly increased the SOD, CAT, and POD activities. The CAT and POD activities were induced to high level first and then quickly declined at the later period of storage. The probable reason may be that CAT and POD were involved in the plant-derived ROS scavenging due to the accumulation of H2O2. SOD can convert superoxide radicals (O2•−) into H2O2, and excessive H2O2 will be scavenged by intracellular CAT and POD, suggesting that the high levels of antioxidant enzymes induced by MeJA might play important roles in reducing oxidative stress and restraining the infection by the fungal pathogen. Moreover, the high activities of antioxidant enzymes induced by MeJA contribute to maintaining the balance of the redox state in the cells. Otherwise, hypersensitivity (HR) may occur due to the redox state alteration and lead to cell death. Therefore, one of the mechanisms of MeJA in inducing disease resistance in G. biloba seeds may be the increase of ROS and the activation of defenses following infection (Fig. 7).
In our research, the activities of defense-related enzymes, including PAL, CHI, and GLU, were all enhanced by MeJA treatment, especially at 100 and 150 µmol L−1. Similar results were also obtained in peach and sweet cherry fruit [30, 32]. CHI and GLU can hydrolyze polymers in the cell wall of the pathogenic fungi to prevent fungal damage, and some metabolites can induce the accumulation of enzymes related to the disease resistance reaction. PAL is a key enzyme in the phenylpropanoid pathway associated with the biosynthesis of secondary metabolites, including phytoalexins, phenolic compounds, and lignin, which are involved in enhancing disease resistance processes [31]. These enzymes could directly or indirectly break or inhibit pathogens, contributing to the reduction of disease incidence. These defense-related enzymes exhibited various functions in the defense system, which may be collectively and coordinately induced by MeJA in G. biloba seeds. Hence, the activation of defense-related enzymes activities to higher levels may be another part of the mechanisms of MeJA in inducing disease resistance in G. biloba seeds (Fig. 7).
Conclusion
In summary, MeJA treatment, especially at concentrations of 100 and 150 µmol L−1, could effectively reduce the disease incidence in G. biloba seeds. Exposure to these MeJA treatments promotes the accumulation of ROS as important signals for mediating the defense system activation and enhances the activities of antioxidant and defense-related enzymes. Two probable action mechanisms of MeJA involve increased ROS and defense enzymes activities. Both results suggest that MeJA induces high disease resistances in G. biloba seeds, resulting in less disease infection. These results provide a reference for further application on the commercial storage of G. biloba seeds.
References
W. Huang, Q. Deng, B. Xie et al., Purification and characterization of an antioxidant protein from Ginkgo biloba seeds. Food Res. Int. 43, 86–94 (2010)
P.D. Tredici, Ginkgos and people - a thousand years of interaction. Arnoldia. 51, 2–15 (1991)
D.O. Kennedy, A.B. Scholey, K.A. Wesnes, The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacology. 151, 416–423 (2000)
K. Nakanishi, Terpene trilactones from Gingko biloba: from ancient times to the 21st century. Bioorgan. Med. Chem. 13, 4987–5000 (2005)
J. Polich, R. Gloria, Cognitive effects of a Ginkgo biloba/vinpocetine compound in normal adults: systematic assessment of perception, attention and memory. Hum. Psychopharm Clin. 16, 409–416 (2001)
S. Cao, Y. Zheng, Z. Yang et al., Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol. Tec. 49, 301–307 (2008)
M. Glowacz, D. Rees, Using jasmonates and salicylates to reduce losses within the fruit supply chain. Eur. Food Res. Technol. 242, 143–156 (2016)
M. Glowacz, N. Roets, D. Sivakumar, Control of anthracnose disease via increased activity of defence related enzymes in ‘Hass’ avocado fruit treated with methyl jasmonate and methyl salicylate. Food Chem. 234, 163–167 (2017)
L. Wang, P. Jin, J. Wang, Methyl jasmonate primed defense responses against Penicillium expansum in sweet cherry fruit. Plant Mol. Biol. Rep. 33, 1464–1471 (2015)
F. Tian, W.L. Chen, G.J. Fan et al., Effect of Ginkgo biloba seed exopleura extract and chitosan coating on the postharvest quality of ginkgo seed. J. Sci. Food Agr. 99, 3124–3133 (2019)
F. Tian, W.L. Chen, C.E. Wu et al., Preservation of Ginkgo biloba seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films. Int. J. Biol. Macromol. 126, 917–925 (2019)
Y. Li, C.E. Wu, G.J. Fan et al., Antimicrobial and preservative effects of natamycin on ginkgo fruits. Food. Sci. 35, 220–225 (2014)
G. Romanazzi, S.M. Sanzani, Y. Bi et al., Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Tec. 122, 82–94 (2016)
R.A. Creelman, J.E. Mullet, Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol Biol Rep. 48, 355–381 (1997)
C. Ding, C. Wang, K.C. Gross et al., Reduction of chilling injury and transcript accumulation of heat shock proteins in tomato fruit by methyl jasmonate and methyl salicylate. Plant Sci. 161, 1153–1159 (2001)
L. Jiang, P. Jin, L. Wang et al., Methyl jasmonate primes defense responses against Botrytis cinerea and reduces disease development in harvested table grapes. Sci. Hortic. 192, 218–223 (2015)
L. bvEbrahimi, H.R. Etebarian, H. Aminian et al., Effect of Metschnikowia pulcherrima and methyl jasmonate on apple blue; mold disease and the possible mechanisms involved. Phytoparasitica. 41, 515–519 (2013)
H. Zhang, L. Ma, M. Turner et al., Methyl jasmonate enhances biocontrol efficacy of Rhodotorula glutinis to postharvest blue mold decay of pears. Food Chem. 117, 621–626 (2009)
H.J. Yao, S.P. Tian, Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J. Appl. Microbiol. 98, 941–950 (2005)
M.J. Giménez, J.M. Valverde, D. Valero et al., Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Tec. 117, 102–109 (2016)
J.Y. Chen, L.H. He, Y.M. Jiang et al., Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol. Plant. 132, 318–328 (2008)
G. Yuan, B. Sun, J. Yuan et al., Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 118, 774–781 (2010)
B. Patterson, E. Mackae, I. Ferguson, Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal. Biochem. 139, 487–492 (1984)
S. Lurie, E. Fallik, A. Handros et al., The possible involvement of peroxidase in resistance to Botrytis cinerea in heat treated tomato fruit. Physiol. Mol. Plant. 50, 141–149 (1997)
M.V. Rao, G. Paliyath, D.P. Ormrod, Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. J. Plant Physiol. 110, 125–136 (1996)
B. Chance, A.C. Maehly, Assay of catalases and peroxidases. Method Enzymol. 2, 764–775 (1955)
J.S. Assis, R. Maldonado, T. Muñoz et al., Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Tec. 23, 33–39 (2001)
F.B. Abeles, R.P. Bosshart, L.E. Forrence et al., Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 47, 129–134 (1971)
P.S. Sellamuthu, D. Sivakumar, P. Soundy et al., Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Tec. 81, 66–72 (2013)
P. Jin, Y. Zheng, S. Tang et al., Enhancing disease resistance in peach fruit with methyl jasmonate. J. Sci. Food Agr. 89, 802–808 (2009)
J. Guo, W. Fang, H. Lu et al., Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol. Tec. 88, 72–78 (2014)
H. Yao, S. Tian, Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Tec. 35, 253–262 (2005)
M. Rehman, Z. Singh, T. Khurshid, Methyl jasmonate alleviates chilling injury and regulates fruit quality in ‘Midknight’ Valencia orange. Postharvest Biol. Tec. 141, 58–62 (2018)
C.L. Wilson, A.E. Ghaouth, E. Chalutz et al., Potential of induced resistance to control postharvest diseases of fruits and vegetables. Plant Dis. 78, 837–844 (1994)
J. Kuć, Concepts and direction of induced systemic resistance in plants and its application. Eur. J. Plant Pathol. 107, 7–12 (2001)
M.A. Torres, J.D.G. Jones, J.L. Dangl, Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378 (2006)
M.J. Tareen, N.A. Abbasi, I.A. Hafiz, Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci. Hortic. 142, 221–228 (2012)
Acknowledgements
This research was financially supported by National Key R&D Program of China [2019YFD1002300], North Jiangsu Science and Technology Project [XZ-SZ201929], Research Project of Jiangxi Forestry Bureau [No.202012], Postgraduate Research & Practice Innovation Program of Jiangsu Province [No. KYCX17_0836]. The authors also acknowledged the support of Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All author delares that there is no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, F., Wu, C., Kou, X. et al. Postharvest methyl jasmonate treatment inhibits blue mold decay in Ginkgo biloba seeds by inducing antioxidant and defense systems. Food Measure 17, 1199–1207 (2023). https://doi.org/10.1007/s11694-022-01662-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01662-1