Abstract
With hot water extraction and ethanol precipitation, three high molecular weight polysaccharides, LZLs (LZL-1, LZL-2 and LZL-3), were successfully purified from the edible bulbs of Lanzhou lily by DEAE-52 cellulose and sephadex G-200 column. The structure features of LZLs were characterized by gas chromatography, scanning electron microscope, high performance gel-permeation chromatography, IR and NMR, and the antioxidant activities of LZLs were assayed. The results showed that LZL-1, LZL-2 and LZL-3 were composed of glucose and mannose with the molar ratios of 2.4:2.5, 2.6:2.5 and 2.2:2.5, while their molecular weights were 2.33 × 106, 1.92 × 106 and 1.26 × 106, respectively. LZLs were mainly α-pyranose configurations with lamellar structure and triple helix conformation except LZL-3. LZLs had similar glycosidic linkage patterns with main substitution at O-6 of glucose or mannose but the different substitution positions at O-3 of them. Vitro bioassays showed that LZLs had effective scavenging abilities to free radicals, especially to DPPH and hydroxyl radicals. When the concentration reached 4 mg/mL, the scavenging rate of LZLs to DPPH and hydroxyl radicals were up to 60% and 70%, respectively. These findings provided more references for potential applications of Lanzhou lily polysaccharides as natural antioxidant agents in food and pharmaceuticals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lanzhou lily (Lilium davidii var. Unicolor cotton) is a variety of Lilium belonging to the genus Liliaceae. It has a large planting area, especially around Lanzhou city, Gansu province. Nowadays, the Lanzhou lily planting area has exceeded 7 million square meters, and the annual output has exceeded 20 thousand tons [1]. Lanzhou city has a semi-arid climate, high altitude, and large temperature difference between day and night, creating suitable conditions for the accumulation of sugar in plants. Therefore, the only sweet lily variety that can be used for both medicine and food has been cultivated and developed here [2]. The total sugar contents in Lanzhou lily are more than 13%, among which polysaccharides are beyond 15%. Lanzhou lily polysaccharides have obvious anti-fatigue, anti-oxidation, immune regulation, hypoglycemic, anti-cancer and other biological activities [3,4,5,6,7].
In recent years, most of the researches on Lanzhou lily polysaccharides have been focused on the small molecular weight but not that of high molecular weight. For example, Zhang and Gao found three Lanzhou lily polysaccharides, whose molecular weights were 5.17 × 104, 1.193 × 105 and 1.18 × 105 [8, 9]. Our team reported previously that the molecular weights of three Lanzhou lily polysaccharides were also within 1.93 × 105 [10, 11]. Therefore, no research have being concerned with high molecular weight polysaccharides (> 106) from Lanzhou lily until now.
Ethanol graded alcohol precipitation is the most convenient and effective method. Because of its simplicity, rapidity and easy concentration, it is often used to obtain polysaccharides of different sizes and molecular weights [12]. Generally, the molecular weight of polysaccharides will increase while the ethanol concentration decreases, and more importantly, the structural characteristics and biological activities of polysaccharides are associated with the concentration of ethanol precipitation [13,14,15]. The Lanzhou lily polysaccharides reported earlier were mainly precipitated by 80% and 70% ethanol, but there was no report of Lanzhou lily polysaccharide precipitated by the lower concentration of ethanol so far. Therefore, this study uses the hot water extraction and 50% ethanol precipitation to extract crude polysaccharides from the bulbs of Lanzhou lily, and further purifies them by gel permeation chromatography to obtain pure polysaccharides, namely three high molecular weight Lanzhou lily polysaccharides. Their preliminary structure and morphology characteristics have identified by gas chromatography (GC), high performance gel-permeation chromatography (HPGPC), IR, scanning electron microscope (SEM) and NMR. Meanwhile, their ability to scavenge free radicals has been evaluated by the vitro antioxidant assays. These findings will provide much more scientific references and feasible technical routes for better exploitation and utilization of the resources of Lanzhou lily.
Materials and methods
Material
Fresh bulbs of Lanzhou lily were harvested from the land of local farmers in the western orchard area of Lanzhou City, Gansu Province, China. The bulbs were ground into powder by a food grinder (FW100, Tianjin Taisite Instrument Co. Ltd., China) and stored in a vacuum dryer up until extraction.
Chemicals
D-glucose (Glc), D-glucuronic acid (GlcU), D-mannose (Man), D-xylose (Xyl), D-galactose (Gal), L-arabinose (Ara), L-rhamnose (Rha) and bovine serum albumin were bought from China Institute of Biological Products Verification. Sigma Aldrich (St. Louis, MO, USA) provided 2, 2-Azinobis-3-ethylbenzthiazoline-6-sulfonate acid (ABTS), Vitamin C and 1, 1-Diphenyl-2-picrylhydrazyl (DPPH). DEAE-52 cellulose was purchased from Pharmacia (Sweden), Sephadex G-200 were purchased from Yuanye Bio-Technology Co. (Shanghai, China). Dextran standards 1152, 11,600, 23,800, 48,600, 80,900, 148,000, 273,000, 409,800 were purchased from Sigma Aldrich (St. Louis, MO, USA). All other reagents used were of analytical grade.
Preparation of the LZL-1,LZL-2 and LZL-3
According to the method reported previously [10], dried powders of Lanzhou lily were refluxed with 95% ethanol for degreasing in a water bath. The dregs were decocted in distilled water at 90℃ with a liquid/solid ratio of 10:1 (mL/g) for three times (2 h/time), subsequently filtered by three layer gauze, the filtrate was merged and concentrated to 1/3 of the original volume under reduced pressure, then added the ethanol with a final concentration of 50% to precipitate the polysaccharides at 4℃. Followed by centrifuge, the sediments were collected and dried with a freeze drier (Labconco, FreeZone®, American) to obtain the crud polysaccharides (LZL). LZL was deproteinized by the Savage method [16], dialyzed against deionized water for 72 h. The dialysis liquid was collected and precipitated again by a final ethanol concentration of 50% at 4℃, then centrifuged, the precipitation was lyophilized and the crude polysaccharide was prepared. Subsequently, the crude polysaccharide was dissolved in distilled water and loaded on DEAE 52-cellulose column (4.5 cm × 60 cm) with gradient elution of 0–0.8 mol/L NaCl solution (1 mL/min) for separation, the elution fractions (6 mL/tube) were detected by phenol-sulfuric acid method [17], and the absorption curve was drew out with absorbance (Y)-tube numbers (X). The main peaks of the polysaccharide fraction were pooled, dialyzed and lyophilized, then continuously purified by Sephadex G-200 (4.2 cm × 57 cm) column eluted with distilled water at a flow rate of 0.5 mL/min, monitored as above method. Thereafter, collected and combied each peak, dialyzed and freeze-dried to obtain pure polysaccharides for the subsequent analysis.
Analysis of the physiochemical properties and purity of LZL-1, LZL-2 and LZL-3
The contents of total sugar, protein and uronic acid were measured by the phenol-sulfuric acid, Bradford’s method and carbazole-sulfuric acid method with D-glucose, bovine serum albumin and D-glucuronic acid as the standard, respectively [17,18,19]. Additionally, 1 mg/mL polysaccharide solution was prepared and scanned in the range of 190–900 nm with an ultraviolet spectrophotometer (UV-1700, Shimadzu Co. Japan) to detected whether the polysaccharides contained protein (280 nm) and nucleic acid (260 nm) conjugates.
The purity and homogeneity of LZL-1, LZL-2 and LZL-3 were detected by HPGPC adopted Waters HPLC module with Model 600 pump and RI detector (Model 2414). The column was a series combination of Shodex OH pak SB-804 and 803 (8.0 mm × 300 mm, Showa Denko k.k., Japan). The mobile phase was a mixed solution of 0.9% NaCl and 0.02% NaN3 with a flow rate of 1.0 mL/min, column temperature at 35 °C, and the injection volume was 20 µL.
The molecular weight determination of LZL-1, LZL-2 and LZL-3
HPGPC method was used to determine the molecular weight of LZL-1, LZL-2 and LZL-3. The measurement was conducted on the Shimadzu LC-10 A (Shimadzu Co. Japan) equipped with differential refractive index detector (DRI, Optilab Rex, Wyatt). The chromatographic column was combined with Shodex OH pak SB-804 and 803 (8.0 mm × 300 mm, Showa Denko k.k., Japan). The dextran standards were series of different relative molecular masses of dextran with 1152, 11,600, 23,800, 48,600, 80,900, 148,000, 273,000, 409,800, 535,600, 1,050,000, 2,655,000 (Sigma-Aldrich). The mobile phase was 0.05 mol/L NaCl solutions with a flow rate of 0.6 mL/min, and the temperature of the column oven was 40 °C.
The sample and standard was prepared to a 5 mg/mL solution, centrifuged at 12,000 rpm for 10 min, the supernatant was filtered with a 0.22 μm microporous membrane, and then transferred the samples to a 1.8 mL injection vial. The injection volume was 20 µL. The standard curve was plotted with different relative molecular masses of standard dextran, and the molecular weight of each polysaccharide was obtained according to the standard curve.
The monosaccharide composition determination of LZL-1, LZL-2 and LZL-3
Monosaccharide composition of LZL-1, LZL-2 and LZL-3 was analyzed by aldoononitrile acetate derivative method referred to our previous literature [10]. Briefly, took 5 mg of polysaccharide as samples, hydrolyzed and derivatized with nitrile acetate, then determined the monosaccharide composition by GC (Agilent 6890 N, santa Clara, CA, USA) fitted with a flame ionization detector (FID) and a quartz capillary column DB-1 (30 m×0.32 mm×0.3 μm). The injection port and detector temperature was 250℃ and 260℃, respectively. The column temperature programmed warming up from 100 ℃ (2 min) to 220 ℃ (5 min) at a rate of 3℃/min. The diffuse proportion was 50:1. The carrier gas was N2 and its flow rate was 2 mL/min. The internal standard was inositol.
FT-IR spectroscopic analysis of LZL-1, LZL-2 and LZL-3
2 mg of the dried samples were mixed with some dried potassium bromide powder to grind uniformly and press into tablet, then loaded to a Thermo Nicolet 5700 infrared spectrograph (Thermo-Electron, Madison, WI, US) for recording the FT-IR spectra from 4000 to 400 cm− 1.
Congo red test of LZL-1, LZL-2 and LZL-3
According to the reported method [20], the sample of polysaccharides (5.0 mg) was mixed with 2.0 mL distilled water and 2.0 mL Congo red solution (80 µmol/L), then gradually added the NaOH (1 mol/L) to the mixture, making the final concentration of NaOH change from 0 to 0.5 mol/L. The maximum absorption wavelength was scanned by a UV spectrophotometer (UV-1700, Shimadzu Co. Japan) in the range of 200–800 nm. The mixture without polysaccharide was used as a blank control.
SEM analysis of LZL-1, LZL-2 and LZL-3
Appropriate amount of thoroughly dried polysaccharide powders were stuck on the conductive glue of the experimental bench, blew off the floating samples with a suction ear ball, sprayed with a layer of gold in a vacuum sputter coater. Then the samples were observed on a field emission scanning electron microscope (JEOL, Ltd., Tokyo, Japan) under a high vacuum acceleration voltage of 5.0 kV form. The magnification of the image was set to 5000× and 10,000×, respectively.
NMR spectroscopy analysis of LZL-1, LZL-2 and LZL-3
The purified sample 40 mg was exchanged deuterium in D2O for three times, then lyophilized and re-dissolved in 0.5 mL D2O for NMR determination. The 1 H, 13 C NMR and DEPT135 spectra were recorded on a Bruker Advance III HD 600 MHz spectrometer (Rheinstetten, Germany) at 80℃.
Antioxidant activities of polysaccharides LZL-1, LZL-2 and LZL-3
DPPH free radical scavenging activity assay
The assay of DPPH radical scavenging activity was referred to the literature method and made slight changes [21]. DPPH solution (0.1 mmol/L) was prepared with 50% ethanol, 2 mL of freshly DPPH solution and 2 mL of Vc or polysaccharide solution with various concentrations (0.125–4.0 mg/mL) mixed to the test tube, shaken vigorously and let stand for 30 min at room temperature. The absorbance of the reaction solution was measured at 517 nm against a blank, and taken Vc as the positive control, the scavenging rate and IC50 were calculated. The DPPH radical scavenging rate was calculated as follows:
Scavenging rate=[1-(Ai−Aj)/ A0]×100%.
Aі represents the absorbance of the test sample mixed with the DPPH solution, A0 represents the absorbance of the DPPH solution containing no the sample, and Aj represents the absorbance of the sample not including DPPH solution.
Hydroxyl radical scavenging activity assay
The activity of hydroxyl radical scavenging was carried out according to the description of Fenton [22]. 2 mL of polysaccharide solution (0.125–4.0 mg/mL) was mixed with 2.0 mL of FeSO4 (6 mmol/L) and 2.0 mL of H2O2 (2.4mmol/L), shaken well and incubated at 37℃for 10 min. Then added to the 2.0 mL of salicylic acid (6 mmol/L), and shaken well again in 37℃ water bath for 30 min, centrifuged (3000 r/min, 10 min). Took the supernatant and measured the absorbance at 500 nm, and used Vc as reference antioxidant, the scavenging rate and IC50 were calculated. The hydroxyl radical scavenging rate was calculated as follows:
Scavenging rate =[1-(Ai−Aj)/ A0]×100%.
Aі represents absorbance of the test sample mingled with the reaction solution, Aj represents absorbance of salicylic acid solution replaced by water; A0 represents the absorbance of the blank solution reaction.
ABTS free radical scavenging activity assay
The activity of ABTS free radical scavenging was conducted by modified TEAC method [23]. 7 mmol/L ABTS solution mixed with 140 mmol/L sodium persulfate to react and generate ABTS radical cation in dark at room temperature for 16 h. Before use, the ABTS solution was diluted with PBS (pH 7.0) to an absorbance of 0.70 ± 0.05 at 734 nm. Put 0.1 mL of samples solution with various concentrations (0.125–4.0 mg/mL) into 5 mL disposable centrifuge tube, then added 3.9 mL of the ABTS solution and mixed evenly with strong shaking. After the reaction, the mixture stood at room temperature for 6 min, and immediately measured the absorbance at 734 nm, and taken Vc as a control, the scavenging rate and IC50 were calculated. The ABTS radical scavenging rate was calculated as follows:
Scavenging rate=[1-(Ai−Aj)/A0]×100%.
Where Aі expresses the absorbance of the test sample blended with the ABTS solution; Aj expresses the absorbance of the sample not including ABTS solution and A0 is the absorbance of the control reaction.
Determination of total reducing power
The total reducing power was assessed according to the method reported in the literature with slightly modification [24]. 2 mL of polysaccharide solution with various concentrations (0.125–4.0 mg/mL) was mixed with 3.0 mL of phosphate buffer solution (0.2 mol/L, pH 6.8) and 2.5 mL of 1% K3Fe(CN)6 solution, blended thoroughly. The reaction mixture was incubated in a 45℃ water bath for 30 min. After rapid cooling, 3.0 mL of 10% trichloroacetic acid was added to the reaction solution, mixed well, and centrifuged at 6000 r/min for 10 min. Then took 3.0 mL of the supernatant, added 2.0 mL of distilled water and 1.0 mL of 0.1% FeCl3, and let the reaction mixture stood at room temperature for 10 min, measured the absorbance at 700 nm. Taken Vc as a control.
Statistical analysis
The experiments were repeated in triplicate and all values were expressed as mean ± SD. SPSS 18.0 software package was carried out for statistical analysis by using one-way ANOVA and Duncan’s new multiple range tests. P < 0.05 represented the difference was statistically significant.
Results and discussion
Purification and physiochemical properties of polysaccharides
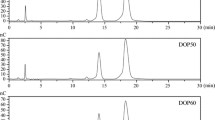
The polysaccharide LZL was separated by DEAE-52 ion-exchange column chromatography to obtain three main fractions, as shown in Fig. 1a, named as LZL-1, LZL-2 and LZL-3 (LZLs), collected and freeze-dried, respectively. Subsequently, LZLs were further purified by Sephadex G-200 gel column chromatography, and each fraction appeared as a single peak (Fig. 1b, c and d), respectively. HPGPC diagrams of LZLs also showed a single symmetrical peak (Fig. 1e and f g). Those results indicated that the LZL-1, LZL-2 and LZL-3 were homogeneous polysaccharides.
The physicochemical characteristics of three purified polysaccharides LZLs were shown in Table 1. The total carbohydrate contents of three polysaccharides were more than 95%, and contained a trace of protein. Additionally, a small amount of uronic acid was found in polysaccharide LZL-1. Three purified polysaccharides LZLs have no obvious absorption peaks on the UV scanning spectrum at 260 and 280 nm (Fig. S1), suggesting that the three purified polysaccharides were free of nucleic acids.
Molecular weight of polysaccharides
After HPGPC determination, the standard curve was obtained by plotting the logarithm of the retention time and molecular weight of the series of dextran standards measured on the gel column (logMW=−0.1973RT + 12.456, R2 = 0.9957, where MW is the average molecular weight, RT is retention time). Bring the RT value of the retention time of three purified polysaccharides into the standard curve, the relative molecular weights of LZL-1, LZL-2 and LZL-3 were calculated to be as 2.33 × 106, 1.92 × 106 and 1.26 × 106, respectively. The molecular weights of Lanzhou lily polysaccharides reported earlier were all less than 1.93 × 105 [8,9,10,11]. Obviously, LZLs were high molecular weight polysaccharides, which were correlated with the ethanol concentration of precipitated polysaccharides.
Monosaccharide composition analysis
LZLs were hydrolyzed and derivatized with nitrile acetate and then measured by GC, whose results were shown in Fig. 2b, c and d; Table 1. Three high molecular weights of polysaccharides LZL-1, LZL-2 and LZL-3 were mainly composed of glucose and mannose with the molar ratios of 2.4:2.5, 2.6:2.5 and 2.2:2.5, respectively. Compared to previous reports [10, 11], the composition of polysaccharides LZLs were analogous to the previous lily polysaccharides, but the monosaccharide ratio was completely different, the contents of glucose in LZLs were greatly reduced and gradually approached to that of mannose. This indicated that the concentration of ethanol precipitation affected the chemical composition of polysaccharides, also suggested the diversity of polysaccharides in Lanzhou lily.
FT-IR spectroscopy analysis
The FT-IR spectra of LZLs (Fig. 3a, b and c) were similar, and showed the characteristic absorption of typical polysaccharides. The broad peak at 3397‒3426 cm− 1 was –OH stretching vibration, while the absorption peak at 2919‒2931 cm− 1 was the C–H stretching vibration, and the peak at 1729‒1737 cm− 1 belonged to the C = O stretching vibration on the carboxyl group [25, 26]. LZL-1 had a weak absorption peak at 1737 cm− 1, which indicated that LZL-1 was a weak acidic polysaccharide. These findings were consistent with the results of uronic acid determination. 1617‒1654 cm− 1 was the absorption peak of the amorphous region of bound water, while 1441‒1460 cm− 1 was the C–H curve-vibration absorption peak [27, 28]. Three absorption peaks appearing at 1250‒900 cm− 1 were the C–O–C or C–O stretching vibration of pyranose ring, and the absorption at 860‒871 and 806‒813 cm− 1 were the characteristic absorption peak of glucomannan [26,27,28], and the peaks at 759‒772 cm− 1 indicated the presence of α-pyranose [26,27,28,29,30]. Combined with the physiochemical and composition analysis, the polysaccharides LZLs mainly existed as α-pyran glucomannan.
Congo red test analysis
As shown in Fig. S2, the curves of the three polysaccharides were different from those of Congo red itself. With the increase of sodium hydroxide concentration, the maximum absorption wavelength of Congo red solution gradually decreased, while the maximum wavelength of the three polysaccharides-Congo red solution increased first and then decreased, which was similar to the change curve of Lanzhou lily polysaccharide previously reported by our team [10]. Compared to the Congo red solution, the maximum absorption wavelength of LZLs were all increased, and the order of increase was LZL-1 > LZL-2 > LZL-3. Congo red assays suggested that the three polysaccharides LZLs might have a triple helical structure, as LZLs formed a complex with Congo red to produce a metastable zone, respectively, which caused a certain red shift in ultraviolet absorption. When the concentration of sodium hydroxide continued to increase, the maximum absorption wavelength decreased sharply, indicating that the three-strand helix structure of the polysaccharide begun to crack and become into a random coil. When the sodium hydroxide concentration reached 0.45 mol/L, the three-strand helix structure of the polysaccharide was completely broken into random curls, and kept in a state of maximum disorder, with the maximum absorption wavelength tended to be stable at finally [31]. Studies have shown that polysaccharides with a triple helix structure generally had strong biological activity [32,33,34]. Therefore, the biological activities of LZLs needed to be researched.
Scanning Electron microscope analysis
Scanning electron microscopy can be used to qualitatively analyze the surface morphology changes of polysaccharides, and obtain morphological information of the surface microstructure of polysaccharides [35,36,37]. The scanning electron microscopy images of the three polysaccharides LZLs were shown in Fig. 4, and LZLs had different morphological characteristics. Under 5000×, the surface of LZL-1 was curled and interwoven into a porous network structure (Fig. 4a), while the surface of LZL-2 formed wrinkles with a large number of particles gathering on the surface (Fig. 4c), and LZL-3 possessed a disorderly accumulation of lamellar structures with wrinkles or depressions on the surface (Fig. 4e). Under 10,000×, it can be clearly observed that the porous network structure of LZL-1 was interwoven with dendritic structures, and a large number of lamellae fragments were attached to the dendrites (Fig. 4b). While the surface of LZL-2 showed a compact accumulation of spherical particles, and some particles were stacked and connected to form a tree root (Fig. 4d), indicating that the cross-links between the molecules were relatively close and the mutual force were relatively strong. The lamellar structures of LZL-3 were pie-shaped with a large number of ravines on the surface (Fig. 4f). In short, the three polysaccharides LZLs were mainly manifested as lamellar structures randomly stacked into honeycomb, spherical, strip and rod-like shapes.
NMR analysis
NMR is a common analytical method for identification the structure of polysaccharide. Generally, the anomeric 1 H chemical shift of α-pyranoside is greater than 4.9 ppm but that of β-pyranoside less than 4.9 ppm in the 1 H NMR, and the anomeric 13 C chemical shift of α-pyranoside is less than 100 ppm while that of β-pyranoside greater than 100 ppm in the 13 C NMR [27,28,29]. All three polysaccharides LZLs had the similar strong signal peak at 4.9 ppm and weak signal peaks at 5.2, 5.3 ppm in the 1 H NMR spectra (Fig. 5a and d g), indicating that LZLs were mainly contained α-pyranose. This was consistent with the result of IR and confirmed by the strong carbon signal at 97.7 ppm in the 13 C NMR spectra (Fig. 5b and e h), which was attributed to the α-1,6-D-Glcp or α-1,6-D-Manp according to the reported literature [29, 30]. Moreover, 13 C NMR spectrum showed no carbon signal in the range of 82‒85 ppm, suggesting that LZLs had no furanose, which further verified the presence of pyranose. There were no anomeric carbon difference among the LZLs in the 13 C NMR spectra, but the significant difference between them was observed in the DEPT 135 spectra (Fig. 5c, f and i). Compared with LZL-2, LZL-1 increased signal at 99.6 ppm, while LZL-3 increased signals at 99.6 and 95.6 ppm in the DEPT 135 spectra (Fig. 5c, f and i), which corresponded to α-1,3,6-D-Glcp and α-1,3,6-D-Manp, respectively, as reference for the previous reports [27, 29, 30, 35]. Obviously, LZL-1 contained the residue α-1,3,6-D-Glcp, and LZL-3 contained both the residues α-1,3,6-D-Glcp and α-1,3,6-D-Manp except for the same sugar residue. Additionally, a weak signal at 20.1 ppm in 13 C NMR spectrum (Fig. 5b) and 1.2 ppm in 1 H NMR spectrum (Fig. 5a) showed that O-acetyl group existed in the LZL-1, which further proved the results of uronic acid determination. Unfortunately, the 2D NMR of three polysaccharides LZLs have been obtained difficultly due to their poor solubility, which could be used for good interpretation of the shifts that should otherwise have been present. All the main chemical shift assignments of 1 H and 13 C NMR for LZLs were summarized in Table 2.
Based on the above analysis, LZLs were α-pyran glucomannans with the main substitution at O-6 of the mannose and glucose. Meanwhile, LZL-1 substituted at O-3 of the glucose, and LZL-3 substituted at O-3 of the glucose and mannose, the terminal residue was α-D-Glcp or α-D-Manp. As for the fine chemical structure of LZLs, further study should be carried out in the coming day.
Antioxidant activities analysis
DPPH radical scavenging activity
DPPH was a kind of nitrogen radicals, which existed stably and easily underwent electron transfer reaction to compounds with hydrogen bond donors. Once the reaction happened, the absorbance of the solution would change. It was one of the commonly used methods for evaluating the antioxidant activity of natural products in laboratories [38]. The results of LZLs on DPPH free radicals scavenging were shown in Fig. 6a. The three kinds of polysaccharides had a certain scavenging effect on DPPH free radicals, and the elimination ability on DPPH free radicals gradually enhanced with the continuous increase of the concentration of polysaccharides, showing certain dose-effect dependence. When the concentration of polysaccharides reached to 1 mg/mL, the scavenging rates of LZL-1, LZL-2 and LZL-3 on DPPH free radicals were 37.18%, 32.5% and 38.9%, respectively. The IC50 of LZL-1, LZL-2 and LZL-3 were 2.09 mg/mL, 2.55 mg/mL and 2.13 mg/mL, respectively, which were greater than the IC50 value of Vc (0.02 mg/mL). The results showed that the three polysaccharides scavenging DPPH free radicals were weaker than that of Vc but gradually approach to Vc.
Hydroxyl radical scavenging activity
Hydroxyl radicals (·OH), as the most active free radicals, which reacted with biomolecules in living cells, such as lipids, peptides, proteins, nucleic acids, etc. The ·OH would produce the strongest oxidative damage effect on biological macromolecules, and cause oxidative damage to cells and tissues, leading to organ disease and body aging [39,40,41,42]. As shown in Fig. 6b, the ability to remove ·OH of the three polysaccharides was gradually trended upward when the concentration of polysaccharides increased. As the concentration reached 1.0 mg/mL, the clearance rates of ·OH by LZL-1, LZL-2 and LZL-3 were 75.91%, 61.99% and 65.40%, respectively. The IC50 of LZL-1, LZL-2 and LZL-3 were 0.04 mg/mL, 0.19 mg/mL, 0.57 mg/mL, respectively, and the IC50 of Vc was 0.143 mg/mL. It can be seen that the IC50 of LZL-1 was significantly smaller than Vc, and the IC50 of LZL-2 and LZL-3 was close to Vc. When the concentration was less than 0.25 mg/mL, the order of the scavenging ability of LZLs on ·OH was LZL-1 > LZL-2 > Vc > LZL-3, while the order of ·OH scavenging ability was Vc > LZL-1 > LZL-3 > LZL-2 since the concentration exceeded 0.5 mg/mL. In short, the three polysaccharides had a stronger scavenging ability on ·OH than that of Vc at lower concentrations, which was similar to the scavenging effect on ·OH of the polysaccharide LPR in Lanzhou lily root [11].
ABTS radical scavenging activity
ABTS+ was widely used to test the antioxidant activity of natural products, which formed a metastable free radical cation under the peroxidation of the oxidant. ABTS+ free radicals were partially eliminated when antioxidant substances were present, and the absorbance value of the solution decreased accordingly [43]. The three polysaccharides LZLs had certain scavenging ability on ABTS+ free radicals, which were weaker than Vc as shown in Fig. 6c. In the experimental concentration range, the ability of LZLs to scavenge ABTS+ slowly increased with the increase of polysaccharide concentration. The IC50 of LZL-1, LZL-2 and LZL-3 were 265.6 mg/mL, 165.1 mg/mL and 37.3 mg/mL, respectively, which were far greater than the IC50 value of Vc (0.07 mg/mL). The order of the three polysaccharides to remove ABTS+ free radicals was LZL-3 > LZL-2 > LZL-1, showing that their ability to remove ABTS+ free radicals was inversely proportional to the molecular weight of the three polysaccharides.
Total reducing power
The reducing power was an important potential activity index. The higher the absorbance value was, the stronger the reduction ability was. As shown in Fig. 6d, the reducing power of LZL-3 increased with increasing concentration, while the reducing power of LZL-1 and LZL-2 first increased and then decreased, subsequently started to increase again when the concentration was beyond 2 mg/mL. At low concentrations (< 1 mg/mL), the order of reducing power was LZL-2 > LZL-1 > LZL-3. When the concentration reached 4.0 mg/mL, the order of reducing power was LZL-1 > LZL-3 > LZL-2, and the absorbance value of LZL-1 was 0.51, which was gradually close to Vc (0.59). The results indicated that LZLs had strong reducing power, but were still weaker than Vc.
Conclusion
Lanzhou lily, as a medicinal and edible resource, its bulbs were rich in sugar. In this paper, we extracted polysaccharides from Lanzhou lily bulbs with hot water and 50% ethanol precipitation, then successively purified by DEAE-cellulose anion exchange and G-200 gel chromatography, three kinds of high molecular weight polysaccharides LZLs were obtained from Lanzhou lily bulbs for the first time. The results of physicochemical, composition and IR analysis showed that LZLs were α-pyran glucomannans, which were similar to the Lanzhou lily polysaccharides previously reported by our team, but different in the molecular weights and glycosidic linkage. The molecular weights of LZLs all reached more than one million, which were higher than that of lily polysaccharides previous reports. Meanwhile, the composition ratio of monosaccharides was also different. Compared with previous reports, the molar ratio of mannose to glucose gradually approached in the LZLs, and the content of mannose had increased in the three high molecular weight polysaccharides. The main linkage in LZLs was 1,6-linked-α-D-Glcp and 1,6-linked-α-D-Manp, which was different from the 1,4-linked-α-Glcp and 1,4-linked-α-Manp in the previous reports [10, 11]. SEM analysis showed that the three high molecular weight polysaccharides LZLs had different morphological characteristics, mainly forming a lamellar structure, which was similar to the Lanzhou lily polysaccharides in our previous reports. The Congo red test showed that LZLs might have a triple helix structure. Vitro antioxidant assays indicated that the three high molecular weight polysaccharides had strong free radical scavenging ability, especially to the hydroxyl free radicals at low concentrations (< 0.25 mg/mL), and the antioxidant activity order of three high molecular weight polysaccharides were LZL-1 > LZL-3 > LZL-2. Overall, the antioxidant activities of LZLs at the same concentration were close to the lily polysaccharides reported previously, but the reducing power of LZLs was stronger than that of previous reports, which might be related to the higher molecular weight and different linkage type in the structure of LZLs from the previous reports [7, 8, 10, 11]. The polysaccharides LZLs had greater potential antioxidant activity with broad application prospects as antioxidants in food, medicine, cosmetics and other industries.
References
D.D. Gao, W.Q. An, H. Chen, In vitro study of the growth, Response surface optimization of extraction of polysaccharides from Lanzhou Lily, Science and Technology of Food Industry. 5, 226–229 (2013)
Q.Y. Gao, B.T. Zhao, Q.Y. Shang et al., Study on the polysaccharide from Lilium davidii var.unicolor Salisb with ultrasonic- assisted compound enzyme hydrolysis method. Food and Fermentation Industries. 40, 263–267 (2014)
X.J. You, C.Y. Xie, K.L. Liu et al., Isolation of non-starch polysaccharides from bulb of tiger lily (Lilium lancifolium Thunb.) with fermentation of Saccharomyces cerevisiae. Carbohydr. Polym. 81, 35–40 (2010)
Y.F. Li, J. Ming, L.G. Wang et al., Research Progress on Basic Nutritional and Bioactive Substances of Lily. CHINA VEGETABLES 24, 7–13 (2012)
L. Jin, Y.L. Zhang, L.M. Yan et al., Phenolic Compounds and Antioxidant Activity of Bulb Extracts of Six Lilium Species. Molecules. 17, 9361–9378 (2012)
X. Li, F. Zhang, Y.C. Li et al., Comparative study on the polysaccharide contents and antioxidant activities of different parts of Lanzhou Lily, Science and Technology of Food Industry. 24, 88–91 (2012)
B.T. Zhao, J. Zhang, X. Guo et al., Microwave-assisted extraction, chemical characterization of polysaccharides from Lilium davidii var. unicolor Salisb and its antioxidant activities evaluation. Food Hydrocoll. 31, 346–356 (2013)
J. Zhang, Y.X. Gao, X.J. Zhou et al., Chemical characterization of polysaccharides from Lilium davidii. Nat. Prod. Res. 24, 357–369 (2010)
T.Y. Gao, B.T. Zhao, Y.Q. Shang et al., Study on the polysaccharide from Lilium davidii var.unicolor Salisb with ultrasonic-assisted compound enzyme hydrolysis method. Food and Fermentation Idustries. 40, 263–267 (2014)
H.P. Hui, X.Z. Li, H. Jin et al., Structural characterization, antioxidant and antibacterial activities of two heteropolysaccharides purified from the bulbs of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 133, 306–315 (2019)
H.P. Hui, H. Jin, X.Z. Li et al., Purification, characterization and antioxidant activities of a polysaccharide from the roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 135, 1208–1216 (2019)
M. Jin, Q. Huang, K. Zhao et al., Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 54, 16–23 (2013)
S.P. Xing, X.F. Zhang, H.N. Ke et al., Physicochemical properties of polysaccharides from Dendrobium ofcinale by fractional precipitation and their preliminary antioxidant and anti-HepG2 cells activities in vitro. Chem. Cent. J. 12, 1–10 (2018)
J. Xu, R.Q. Yue, J. Liu et al., Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int. J. Biol. Macromol. 67, 205–209 (2014)
H.F. Li, S.B. Guo, S.L. Man et al., Graded ethanol precipitation method on physicochemical properties and antioxidant activities of polysaccharides extracted from Astragalus Radix. China J. Chin. Materia Med. 40, 2112–2116 (2015)
A. Staub, Removal of protein-Sevag method. Method Carbohydr. Chem. 5, 5–6 (1965)
M. Dubois, K.A. Gilles, J.K. Hamilton et al., Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
M. Bradford, A rapid and sensitive method for the quantiWcation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254 (1976)
N. Blumenkrantz, G. Asboe-Hansen, New method for quantitative determination of uronic acids. Anal. Biochem. 54, 484–489 (1973)
C.Z.P. Nie, P.L. Zhu, M.C. Wang et al., Optimization of water-soluble polysaccharides from stem lettuce by response surface methodology and study on its characterization and bioactivities. Int. J. Biol. Macromol. 105, 912–923 (2017)
J.F. Yuan, Z.Q. Zhang, Z.C. Fan et al., Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohydr. Polym. 74, 822–827 (2008)
A. Luo, X. He, S. Zhou et al., In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 45, 359–363 (2009)
J. Wang, Y. Wang, X. Liu et al., Free radical scavenging and immunomodulatory activities of Ganoderma lucidum polysaccharides derivatives. Carbohydr. Polym. 91, 33–38 (2013)
W.C. Zeng, B. Shi, Common methods of antioxidant activity evaluation for natural products: A review. Chem. Ind. Eng. Progress 32, 1205–1247 (2013)
F. Krichen, W. Karoud, A. Sila et al., Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins. Int. J. Biol. Macromol. 75, 283–289 (2015)
J. Gao, T. Zhang, Z.Y. Jin et al., Structural characterization, physicochemical properties and antioxidant activity of polysaccharide from Lilium lancifolium Thunb. Food Chem. 169, 430–438 (2015)
R.K. Suryawanshi, N. Kango, Production of mannooligosaccharides from various mannans and evaluation of their prebiotic potential. Food Chem. 334, 127–428 (2021)
C. Sabater, A. Blanco-Doval, A. Montilla et al. Optimisation of an enzymatic method to obtain modified artichoke pectin and pectic oligosaccharides using artificial neural network tools. In silico and in vitro assessment of the antioxidant activity, Food Hydrocolloids. 110, 106–161 (2021)
A.V. Samrot, J. Lavanya Agnes Angalene, S.M. Roshini et al., Purification, characterization and utilization of polysaccharide of Araucaria heterophylla gum for the synthesis of curcumin loaded nanocarrier. Int. J. Biol. Macromol. 140, 393–400 (2019)
J.J. Wu, Q.L. Ming, X.Zhai, et al. Structure of a polysaccharide from Trichoderma atroviride and its promotion on tanshinones production in Salvia miltiorrhiza hairy roots. Carbohydr. Polym. 223, 115–125 (2019)
M. Wang, P. Zhu, S. Zhao et al., Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 95, 809–817 (2017)
Y. Zhang, S. Li, X. Wang et al., Advances in lentinan: isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 25, 196–206 (2011)
R. Huang, J.H. Xie, Y. Yu et al., Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 155, 1262–1269 (2020)
R. Zhang, X.X. Zhang, Y.X. Tang et al., Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 228, 115–381 (2020)
Z.F. Zhang, G.Y. Lv, J.W. Cheng et al., Characterization and biological activities of polysaccharides from artificially cultivated Phellinus baumii. Int. J. Biol. Macromol. 129, 861–868 (2019)
Y.D. Sun, Z.H. Wang, Q.S. Ye, Composition analysis and anti-proliferation activity of polysaccharides from Dendrobium chrysotoxum. Int. J. Biol. Macromol. 62, 291–295 (2013)
F.Y. Ma, D.K. Wang, Y. Zhang et al. Carina Tikkanen-Kaukanenc, X.H. Liu, A.E. Belld, Characterisation of the mucilage polysaccharides from Dioscorea opposite Thunb. With enzymatic hydrolysis, Food Chemistry. 245, 13–21 (2018)
Q.Y. Wu, H.S. Qu, J.Q. Jia et al., Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr. Polym. 132, 31–40 (2015)
S. Zhang, B. He, J. Ge et al., Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int. J. Biol. Macromol. 47, 546–550 (2010)
I. Khemakhem, O. Abdelhedi, I. Trigui et al., Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 106, 425–432 (2018)
H.H. Yang, Y.J. Wu, C.J. Gan et al., Characterization and antioxidant activity of a novel polysaccharide from Pholidota chinensis Lindl. Carbohydr. Polym. 138, 327–334 (2016)
Y.Q. Xu, X.J. Niu, N.Y. Liu et al., Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 243, 26–35 (2018)
J. Jiang, F. Kong, N. Li et al., Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 147, 365–371 (2016)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21775154), and the Gansu Province Higher Education Innovation Fund of China (2021 A-260).
Author information
Authors and Affiliations
Contributions
Heping Hui and Bo Qin designed the research. Bo Qin supervised the research process and provided financial support for the conduct of the research. Yanxiao Yang and Hui Jin conducted the data analysis and provided a number of experimental instruments. Heping Hui performed most of the experiments and wrote the manuscript. Weijun Gao revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hui, H., Jin, H., Yang, X. et al. The structure and antioxidant activities of three high molecular weight polysaccharides purified from the bulbs of Lanzhou lily. Food Measure 17, 800–812 (2023). https://doi.org/10.1007/s11694-022-01654-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01654-1