Abstract
Globally, vinegar is a widely used acidic condiment with unique flavors and rich nutritional value. Aroma can be used as an important factor to measure the quality of vinegar and affect consumers’ choices and preferences. Volatile components provide different aroma characteristics of vinegar and have an important impact on its sensory quality. In this study, the volatile components in solid-state fermented vinegar and liquid-state fermented vinegar were investigated by headspace solid-phase microextraction followed by gas chromatography—mass spectrometry. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) for evaluating and distinguishing between solid-state fermented vinegar and liquid-state fermented vinegar based on their composition of volatile components were carried out. The results showed that a total of 38 different volatile compounds were identified, including 5 alcohols, 17 ethers, 3 acids, 4 heterocyclic compounds, 4 aldehydes, and 5 other volatiles. PCA and HCA have proven to be effective methods for identifying volatile compounds as well as evaluating and comparing of solid-state fermented vinegars and liquid-state fermented vinegars. Multivariate statistical analysis revealed substantial variation between solid-state and liquid-state fermented vinegars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vinegar has become an indispensable condiment on the table due to its unique flavors and rich nutritional value [1]. Recent studies have shown that high-quality vinegars possess antibacterial [2], anti-infective [3], antioxidant [4] and anticancer properties [5]. Due to different raw materials and processes of vinegar production, there are a wide range of commercially available vinegar products. Generally, western countries brew vinegar with fruit and wine, such as Italian balsamic vinegar and Spanish Sydney vinegar [6]. Eastern countries brew vinegar from grain, such as grain vinegar in China and Japan [7]. Presently, commercially available vinegars can be classified into solid-state fermentation (SSF) vinegars and liquid-state fermentation (LSF) vinegars in terms of production technology. SSF is the fermentation of microorganisms on a solid substrate with little or no free water, while LSF refers to the fermentation method in which the state of the material appears liquid in the acetic acid fermentation stage [8]. These processes lead to different kinds of vinegar with their own unique flavor characteristics.
Aroma has long been considered a vital feature of consumers’ perception of vinegar quality, which directly affects consumers choices and preferences [9]. Volatile compounds provide different aroma characteristics of vinegar and have an important impact on the sensory quality of vinegar. The volatile compounds in vinegar mainly include alcohols, esters, aldehydes, acids, lactones, phenols and so on [10]. These compounds usually have little content in vinegar, but they can give vinegar a special aroma in an appropriate proportion. The flavor of vinegar is an important factor impacting its quality. The amount of vinegar flavor is of great significance to the nutritional value and hygienic indices of vinegar. Hence, the determination of aroma components of vinegar is conducive to the identification of the quality of vinegar. The types of aroma components in vinegar are complex and easy to change. Different raw materials, origins, climates and brewing technologies make the flavor and taste of vinegar very different [11]. Therefore, research on volatile flavor substances in vinegar is essential for quality control.
Given the important role of volatile flavor substances in vinegar analysis, numerous reports have been conducted. Previous studies have mainly targeted the effects of different brewing processes on the volatile flavor substances of vinegar [12], the changes in volatile flavor substances in the process of vinegar production [13], the characteristics of volatile flavor substances of vinegar with different acidities [14], the changes in volatile flavor substances of vinegar during the aging process [15], and the characteristics of volatile flavor substances of certain types of vinegar [16,17,18]. However, to the best of our knowledge, research evaluating the volatile flavor substances of SSF vinegar and LSF vinegar has been inadequately investigated. Hence, there is a large demand for fast and reliable methods to evaluate and compare the volatile organic compounds (VOCs) between SSF and LSF vinegars.
Headspace solid-phase microextraction (HS-SPME) is a solvent-free sample preparation technique that integrates preconcentration, extraction, and sample introduction into one step [19]. Combined with gas chromatography-mass spectrometry (GC–MS), it can objectively, accurately and quickly evaluate VOCs in samples. With its good reproducibility and stability, this technique has been extensively used in the separation and analysis of volatile and semivolatile samples, and this method is widely utilized in the study of vinegar volatile aroma [20]. Nevertheless, the use of HS–SPME–GC–MS coupled with multivariate statistical analysis for characterizing and distinguishing Chinese-style SSF vinegar and LSF vinegar has rarely been studied.
In this work, HS-SPME followed by GC–MS was used to analyze and identify the volatile compounds of four SSF vinegars and four LSF vinegars. The types and contents of the volatile compounds of vinegar samples were comprehensively compared via multivariate statistical analysis. Hierarchical cluster analysis and principal component analysis were carried out based on the composition of volatile components. These results were expected to make a significant contribution to elucidating the differentiation of the composition and content of volatiles in SSF vinegars and LSF vinegars. By extension, this study also laid the foundation for future research on the mechanism of VOCs generation, thereby promoting the genetic improvement of vinegar flavor.
Materials and methods
Materials
Four kinds of SSF vinegar samples were numbered SF1–SF4, and four kinds of LSF vinegar samples were numbered LF1–LF4. These samples were all commercially widely consumed brands in China. Information about the vinegar samples is presented in Table 1.
HS-SPME extraction
According to the method reported in the literature, HS-SPME extraction was carried out with a few modifications [21, 22]. In this study, to strengthen the signal peak of the total ion chromatograms, the sample volume was increased to 8 mL and then pipetted accurately by a pipette (BRAND Shanghai Trading Co., Ltd., China) into a 15 mL headspace vial sealed with sealing film (Nanjing Dahu Scientific Instrument Co., Ltd., China). Subsequently, the fiber (50/30 μm polydimethylsiloxane/carboxen/divinylbenzene (PDMS/CAR/DVB)) of the manual SPME device (Supelco, USA) was exposed in the upper space of the sealed vial. New fiber needs to be aged at the GC inlet according to the instructions before use. Moreover, the sealed vial was heated in an HH.S21-6 electrothermal constant temperature water bath pot (Shanghai Boxun Industrial Co., Ltd. Medical Equipment Factory China) at a increased temperature of 50 °C for 30 min. Thereafter, the fiber was withdrawn and immediately inserted into the GC inlet. Finally, desorption was accomplished at 250 °C for 3 min. GC/MS analysis was performed. The sample was analyzed in triplicate to verify the signal stability and obtain a sufficient amount of data. An illustration of the extraction process for HS-SPME is presented in Fig. 1.
Analysis of volatile compounds by GC–MS
The analysis of volatile compounds was implemented using a Trace ISQ 1300 GC–MS with a standard mass spectrometry library (NIST 2014) workstation (Thermo Fisher Scientific, USA). The GC–MS conditions were employed after optimization. The DB-5MS (30 m × 0.25 mm i.d. × 0.25 μm) capillary column was employed. The operating system was Xcalibur software (v. 2.2, Thermo Fisher Scientific, USA). High-purity helium (purity > 99.999%) was employed as the carrier gas at a constant flow rate of 1.0 mL/min. The inlet temperature was set and maintained at 250 °C. The injection mode was nondiversion injection. Originally, the oven temperature was set at 40 °C for 1 min, then programmed at 5 °C/min to 130 °C, and finally raised to 220 °C at a rate of 10 °C/min and finally maintained for 2 min.
The mass spectrometer was operated in electron ionization (EI) mode with the electron energy set at 70 eV. The transmission line temperature and ion source temperature were both set and maintained at 250 °C. The mass spectra was acquired using the full-scan monitoring mode. The spectra were collected in a mass range of m/z 33–450. The solvent delay time was 1 min.
Identification of volatile compounds
The total ion chromatogram obtained by GC–MS analysis was searched by computer and matched with the NIST 14 standard mass spectral databases. The main volatile components in vinegar samples were further determined by the retention index (RI) of compounds, which were calculated under the same chromatographic conditions after the injection of a C8–C40 n-alkane series (Shanghai Jieli Biotechnology Co., Ltd., China) [23].
The relative content of volatile flavor compounds in vinegars was quantified by the area normalization method (component peak area accounting for the total peak area). The test results were expressed as percentages (%).
Data processing
The mean and standard deviation were calculated using Excel software (Office 2019, Microsoft Corporation, USA). Values are expressed as the mean ± standard deviation (SD) resulting from the analysis of three parallel samples. The percentage histogram was obtained by Origin 2021 software. Principal component analysis and cluster analysis were performed using IBM SPSS statistics 21 software.
Results and discussion
Analysis of volatile compounds in different fermented vinegar samples
The GC–MS instrument (Thermo Fisher Scientific, Trace ISQ 1300) used in this study can perform complete scans in an accurate and rapid manner, providing reliable and substantive molecular weight and structural details. The volatile compounds of 4 kinds of solid fermented vinegar and 4 kinds of liquid fermented vinegar produced in China with high sales volume were extracted by HS-SPME and analyzed by GC–MS. A total of 38 different volatile compounds were identified by NIST library combined with RI, including 5 alcohols, 17 ethers, 3 acids, 4 heterocyclic compounds, 4 aldehydes, and 5 other volatiles. The detailed information of the identified compounds is denoted in Table 2, given emphasis on the metadata which include retention times, compound name, CAS number, relative contents expressed as the mean ± SD, retention index, and literature retention index.
The volatile components in vinegar are affected by raw materials, starters, and processing technologies, which constitute their different characteristics [24]. As shown in Figs. 2 and 3, the numbers and content of volatiles in the SSF vinegar and LSF vinegar samples exhibited great diversity. In terms of the types of compounds (Table 2, Fig. 2), SSF vinegar contained a wider variety of compounds than LSF vinegar. All vinegar samples contained the largest number of ester compounds. The results were in accordance with the data reported in previous studies [25]. Further comparison for each category of volatiles in terms of volatile compound contents (Fig. 2) showed that SSF vinegar contained a higher relative content of heterocyclic compounds than LSF vinegar, which accounted for 34–52% of the total volatiles. In addition, there are higher content of acids (ranging from 16 to 32%) and esters (ranging from 10 to 32%) in SSF vinegar, followed by alcohols (ranging from 5 to 12%). In comparison to that in SSF vinegar, the content of acids had the largest proportion (ranging from 42 to 98%), followed by esters (ranging from 2 to 52%) in LSF vinegar. Yu et al. reported similar results [26].
Some characteristic and bioactive volatile components of vinegars reported in the literature could also be authenticated in this study [27]. For instance, phenylethyl alcohol, detected in both SSF and LSF vinegars, has a soft, pleasant and long-lasting rose aroma and good antibacterial activity [28]. Compounds such as benzaldehyde, and benzeneacetaldehyde detected in the samples were aromatic aldehydes with antioxidant and anti-inflammatory activities [29]. Furthermore, esters, which usually have typical fruit flavor characteristics, are the main substances that constitute the flavor of vinegar. The esters in vinegar are an important indicator for judging the quality of vinegar [30]. For example, 2-phenylethyl acetate, as a highly valued natural volatile ester with a rose-like odor that is widely used to add scent or flavor to cosmetics, soaps, foods and drinks, which was detected in both SSF and LSF vinegars [31]. Heterocyclic compounds in vinegar are mainly produced by microbial fermentation, and generally have important bioactive activities and the flavors of nuts, coke and baking [32]. Compounds such as trimethyl-pyrazine, and tetramethyl-pyrazine detected in sectional vinegar samples were reported as bioactive alkaloids. These compounds have been proved to have pharmacological effects in clinical application for more than 30 years and having played an important role in anti-cardiovascular disease [33]. There are also other VOCs in vinegar, that are usually less abundant but have an important effect on the quality and flavor of vinegar. For example, phenolic compounds can play a fragrant and aromatic role, which are especially important to the quality of vinegar [34]. The structures of the major volatile compounds identified in the vinegar are presented in Fig. 4.
Dendrogram classification of hierarchical cluster analysis (HCA)
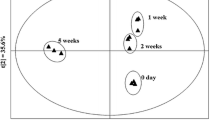
When many indicators are used to distinguish different samples, unsupervised data processing as a visualization protocol is often used to evaluate the clustering trend [35]. HCA is a numerical data integration method that can accurately describe the differences between different vinegar varieties. It is conducive to locating the homogeneity among vinegar varieties to a certain extent according to the volatiles with similar characteristics. Euclidean distance was used as the measurement standard, and the intergroup connection method was used as the characteristic of the systematic clustering model [36]. The clustering data were processed by hierarchical tree. The horizontal axis represents the Euclidean distance between populations, and the vertical axis represents the degree of flavor similarity and diversity among populations. Taking 38 volatile compounds and their contents as the elements of the new data matrix, a matrix containing 8 samples and 38 variables (volatile compounds) was constructed for HCA.
The similarity of samples could be represented by the size of the Euclidean distance. The smaller the Euclidean distance is, the greater the similarity. Figure 5 that the eight vinegar varieties can be obviously divided into two groups when the Euclidean distance is 6. In the cluster diagram of volatile compounds, four kinds of SSF vinegar belonged to one group at the minimum distance, indicating that they had the greatest similarity. Four kinds of LSF vinegars belonged to another group. However, the Euclidean distance between the sample LF2 and the other three LSF vinegars was larger, indicating that the volatile components in LF2 were different from those in the other three LSF vinegars. This result was consistent with the result that the amount and content of volatile components in LF2 were lower. The HCA results showed that it could discriminate different kinds of vinegars by responding specifically to VOCs from samples.
Principal component analysis (PCA)
The VOCs of vinegar are rich in variety and quantity. Usually, subtle changes in these compounds will affect the flavor of vinegar. Therefore, it is of great importance to study the relationship between the differences in VOCs in vinegar and the variety and quality of vinegar. PCA is also an unsupervised clustering method, that does not need a priori dataset knowledge [37]. It is the most widely used data dimensionality reduction algorithm, which can sequentially find a group of mutually orthogonal coordinate axes from the original space. The selection of new coordinate axes is closely related to the data itself. PCA has been widely used in food flavor analysis to show the relationship between samples [38]. For example, Zhu et al. quantitatively analyzed the VOCs in Shanxi aged vinegar by SPME–GC–MS, and verified its linearity, repeatability, reproducibility and accuracy. The difference and similarity between Shanxi aged vinegar samples was studied in combination with PCA [39].
The Kaiser–Meyer–Olkin (KMO) and Bartlett sphericity tests were first performed on the original data in Table 2. The closer the KMO value is to 1, the stronger the correlation between variables. In this study, the original data show that KMO = 0.68 > 0.5, and the significant value of Bartlett's sphericity test was 0. The results indicated that the data of this study were applicable to PCA [40]. The two-dimensional data matrix was then imported into SPSS 21 software for PCA. Figure 6 shows that when the extraction score was 3, the cumulative eigenvalue reached 97.546%. The results can reflect the vast majority of odor information of all samples [40]. Hence, three principal components were extracted. As shown in Fig. 5, the spatial distribution of different points shows that the eight samples were obviously divided into two parts: one part was concentrated in the upper part of LF1, LF2, LF3 and LF4, and some samples overlapped, indicating that their volatile composition was very similar. The other part was gathered in the lower area. The results also indicated that VOCs in SSF vinegars and LSF vinegars were significantly different. The main difference was that SSF vinegars contained a wider variety of VOCs than LSF vinegars and SSF vinegars contained a higher relative content of heterocyclic compounds than LSF vinegars (Table 2, Fig. 2). The PCA results and HCA results confirmed each other, which can better distinguish SSF vinegar and LSF vinegar.
Conclusion
The volatile components of four kinds of SSF vinegars and four kinds of LSF vinegars were studied by HS–SPME–GC–MS coupled with multivariate statistical analysis. The analysis results indicated that there were obvious differences in the types and contents of volatile components in the two different ways of fermenting vinegars. The volatile components mainly included acids, esters, heterocyclic compounds, alcohols, aldehydes and other compounds, which likely resulted in different aroma characteristics and qualities of vinegar. PCA and HCA were successfully applied to evaluate and distinguish between SSF vinegars and LSF vinegars based on a database of volatile components. This study not only provides a feasible method for distinguishing different vinegar varieties, but also lays a theoretical foundation for the improvement of vinegar flavor.
References
N.H. Budak, E. Aykin, A.C. Seydim, A.K. Greene, Z.B. Guzel-Seydim, J. Food Sci. 79(5), R757–R764 (2014)
S.I. Makino, H.I. Cheun, H. Tabuchi, T. Shirahata, J. Vet. Med. Sci. 62(8), 893–895 (2000)
A. Heshmati, F. Mehri, A. Nili-Ahmadabadi, A. Mousavi Khaneghah, Int. J. Environ. An. Ch. (2021). https://doi.org/10.1080/03067319.2021.1946526
M. Koyama, Y. Ogasawara, K. Endou, H. Akano, T. Nakajima, T. Aoyama, K. Nakamura, Int. J. Food Prop. 20(4), 888–898 (2017)
Z. Ali, J. Li, Y. Zhang, N. Naeem, S. Younas, F. Javeed, Food Rev. Int. 1–28 (2020)
D. Gajewska, P.K. Kęszycka, M. Sandzewicz, P. Kozłowski, J. Myszkowska-Ryciak, Nutrients 12(9), 2727 (2020)
C.Y. Wang, J. Zhang, Z.Z. Gui, Genet. Mole. Res. 14(2), 5054–5064 (2015)
Y. Jiang, X. Lv, C. Zhang, Y. Zheng, B. Zheng, X. Duan, Y. Tian, Food Res. Int. 125, 108531 (2019)
J. Li, Y. Fu, X. Bao, H. Li, J. Zuo, M. Zhang, J. Wang, J. Food Meas. Charact. 14(1), 465–475 (2020)
X.L. Zhang, Y. Zheng, M.L. Xia, Y.N. Wu, X.J. Liu, S.K. Xie, Y.F. Wu, M. Wang, Foods 9(2), 166 (2020)
G.Y. Fang, L.J. Chai, X.Z. Zhong, Y.J. Jiang, Int. J. Food Microbiol. 341, 109070 (2021)
S. Al-Dalali, F.P. Zheng, B.G. Sun, C.X. Zhou, M. Li, F. Chen, LWT-Food. Sci. Technol. 133, 109969 (2020)
S. Al-Dalali, F.P. Zheng, B.G. Sun, F. Chen, P. Wang, W.P. Wang, J. Food Meas. Charact. 14(1), 535–547 (2020)
D. Jo, G.R. Kim, S.H. Yeo, Y.J. Jeong, B.S. Noh, J.H. Kwon, Food Sci. Biotechnol. 22(6), 1559–1565 (2013)
S. Al-Dalali, F.P. Zheng, B.G. Sun, F. Chen, Food Anal. Method. 12(2), 544–557 (2018)
X. Zhang, P. Wang, D.D. Xu, W.P. Wang, Y. Zhao, Food Res. Int. 119, 398–410 (2019)
G.Z. Zhao, G.L. Kuang, J.J. Li, H. Hadiatullah, Z.J. Chen, X.W. Wang, Y.P. Yao, Z.H. Pan, Y.R. Wang, Food Res. Int. 129, 108879 (2020)
T.G. Nam, J.Y. Lee, B.K. Kim, N.E. Song, H.W. Jang, Int. J. Food Prop. 22(1), 1195–1204 (2019)
A.N. Yu, B.G. Sun, D.T. Tian, W.Y. Qu, Food Chem. 110, 233–238 (2008)
C. Dadalı, Y. Elmacı, J. Food Meas. Charact. 16(1), 687–699 (2022)
L.C. Liu, H.Y. Hu, Y.P. Yu, J.H. Zhao, L.L. Yuan, S.S. Liu, S.S. Zhao, R. Huang, J.H. Xie, M.Y. Shen, J. Food Biochem. 45, 20 (2021). https://doi.org/10.1111/jfbc.13670
S. Al-Dalali, F.P. Zheng, H.H. Li, M.Q. Huang, F. Chen, LWT-Food Sci. Technol. 112, 108264 (2019)
X. Ji, J. Essent. Oil Bear. Pl. 23(5), 1105–1117 (2020)
Y. Wu, L. Wang, Y. Bian, Z. Zhou, Y. Wang, L. Cao, S. Gu, J. Food Meas. Charact. 14(4), 2262–2270 (2020)
H. Gao, Y. Zhao, W. Wang, D. Xu, Y. Sun, J. Li, X. Zhang, Food Anal. Method. 1–14 (2022)
Y.J. Yu, Z.M. Lu, N.H. Yu, W. Xu, G.Q. Li, J.S. Shi, Z.H. Xu, J. I. Brewing 118(1), 133–141 (2012)
X. Liang, J.H. Wu, Q.Y. Zhao, X.P. Dong, L. Dong, L. Qin, X.B. Xu, M. Du, Anal. Method. 11, 2443–2449 (2019)
T. Oshima, M. Ito, J. Nat. Med. 75, 319–325 (2021)
G.L. Petretto, C.I.G. Tuberoso, M.A. Fenu, J.P. Rourke, O. Belhaj, G. Pintore, Int. J. Food Prop. 20, 2016–2027 (2017)
S. Malherbe, V. Watts, H.H. Nieuwoudt, F.F. Bauer, M. Du Toit, J. Agr. Food Chem. 57, 5161–5166 (2009)
C.H. Kuo, S.H. Chiang, H.Y. Ju, Y.M. Chen, M.Y. Liao, Y.C. Liu, C.J. Shieh, J. Sci. Food Agr. 92, 2141–2147 (2012)
R.N. Marín, R.C. Mejías, M.V.G.H. Moreno, J. Chromatogr. A. 967, 261–267 (2002)
J.C. Chen, Q.H. Chen, Q. Guo, S. Ruan, H. Ruan, G.Q. He, Q. Gu, Food Chem. 122, 1247–1252 (2010)
B. Zhang, T.W. Xia, H. Duan, Z.J. Zhang, Y. Li, B. Fang, M.L. Xia, M. Wang, Molecules 24, 3799 (2019)
C. Xiong, Y.J. Zheng, Y.N. Xing, S.J. Chen, Y.T. Zeng, G.H. Ruan, Food Anal. Method. 9, 768–776 (2016)
D. Jo, G.R. Kim, S.H. Yeo, Y.J. Jeong, B.S. Noh, J.H. Kwon, Food Sci. Biotechnol. 22, 1559–1565 (2013)
Z.B. Xiao, S.P. Dai, Y.W. Niu, H.Y. Yu, J.C. Zhu, H.X. Tian, Y.B. Gu, J. Food Sci. 76, C1125–C1135 (2011)
Y. Yin, Y. Zhao, J. Food Meas. Charact. 13(3), 2406–2416 (2019)
H. Zhu, J. Zhu, L.L. Wang, Z.G. Li, J. Food Sci. Tech. 53, 171–183 (2016)
D. Rojas-Valverde, J. Pino-Ortega, C.D. Gómez-Carmona, M. Rico-González, Int. J. Env. Res. Pub. He. 17(23), 8712 (2020)
Acknowledgements
This work was financially supported by Innovation fund of advanced analysis and testing center of nanjing forestry university and supported by ‘Analysis and testing of new methods and new research independent subject’ Program supported by the Jiangsu Scientific Instrument and Equipment Association.
Funding
Funding was supported by Collaborative Innovation Center of Audit Information Engineering and Technology (Grant No. 2016-020) and Analysis and testing of new methods and new research independent subject (Grant NO. 6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no a conflict of interest in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, X., Xu, L. Comparison and analysis of the volatile compounds in solid-state and liquid-state fermented vinegars. Food Measure 16, 4914–4922 (2022). https://doi.org/10.1007/s11694-022-01590-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01590-0