Abstract
The application of edible coatings is suggested to protect the quality of dried fruits during storage. In this study, dried apricots were coated with edible coatings prepared by sodium alginate and pectin. The coated samples were kept at 25 ± 2 °C for four months. During storage, physicochemical, microbial, and sensory properties were evaluated. The results showed that edible coating effectively maintained the moisture content, color, and texture of dried apricots. Amount of vitamin C and carotenoid losses decreased in the coated samples. After four months of storage, dried apricot coated with sodium alginate and citric acid had the highest content of vitamin C (101 mg/kg) and carotenoid content (22.26 mg/kg). In addition, coating reduced the rate of the changes in pH, acidity, reducing sugars, and brix and delayed microbial growth. During storage, the microbial count of coated dried apricots was lower than the control. Sodium alginate-based edible coating was more able to maintain the quality of dried apricots. The results indicated that the addition of citric acid to the coating formulation enhanced its antimicrobial effect and decreased its brown color. Also, the results of sensory evaluation showed that coating had a significant impact on sensory properties. At the storage end, the highest overall acceptance (3.13) was observed in the sample coated with sodium alginate and citric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Apricot (Prunus armeniaca L.) is one of the species of Prunus genus and one of the most popular fruits in temperate regions of the world [1]. Polyphenolic compounds, carotenoids, minerals, and vitamins are among the high nutritional value compounds of apricots. Polyphenols have antioxidant, antimicrobial, anti-allergic, anti-mutagenic, anti-carcinogenic, and anti-inflammatory effects and reduce cardiovascular disease [2]. Apricot is a good source of minerals, especially calcium, potassium, and magnesium [3]. Fresh apricot is very perishable, and common preservation methods such as drying and canning are used to increase its shelf life [4]. Processed apricot is estimated at 40 to 45% of total world production [2]. Drying is one of the most common and inexpensive ways to increase the shelf life of fruits such as apricot. Moisture loss, texture hardening, and color changes are some of the undesirable changes that occur during the storage of dried apricot. Treating the dried apricot with sulfur dioxide (SO2) is a usual approach for preventing enzymatic browning reactions, non-enzymatic browning reactions, and microbial spoilage of this product during storage [5]. Excessive amounts of sulfur dioxide in dried fruits can expose consumers to some risks such as nausea, skin rashes, respiratory distress, and allergy [6]. Therefore, recently, the tendency of consumers to consume non-sulfur products has increased. The use of various pretreatments such as chemical compounds and ultrasound treatment before drying as alternatives for the addition of sulfur dioxide has been investigated in some researches [7]. In recent years, there is an increasing interest in using edible coating to increase the shelf life of fresh and dried fruits with the additional benefit of reducing the amount of non-biodegradable packaging materials [8]. Edible coatings act as physical barriers and decrease fruit surface permeability to gases such as O2, CO2, and water vapor. As a result, the rate of fruit respiration and transpiration decreases, leading to delay in the natural physiological ripening process [9]. Color changes were reduced in dried fruits coated by edible coatings due to the gas barrier properties of the coat [10].
Better color and high pro-vitamin A content was reported by Lago-Vanzela et al. [11] as the result of coating the dried pumpkin slices with natural and modified maize and cassava starch. Coating the dried apple slices with carboxymethylcellulose led to reduce in color changes [12]. Youssef and EL Kady [13] used four different edible coating materials, including starch, pectin, soy protein, and lipid, to coat raisin and prune. The results indicated that the performance of the lipid layer was better than other coats.
Baysal et al. [14] Used corn zein edible film for intermediate moisture apricot coating and observed this film reduced the color changes and moisture loss and delayed microbial growth during the storage period. The polymeric structure and composition of films affect their functional properties.
Based on previous reports, the application of edible coating containing Low methoxyl pectin (2%), glycerol, sunflower oil, and CaCl2 caused to protection of the quality attributes of melon [15]. Coating of the osmotically dehydrated pineapple with a pectin-based edible coat before hot air drying resulted in the preservation of vitamin C and reduction of the darkening of this product [10]. Also, the significant effect of pectin-based coating on maintaining the vitamin C content of air-dried papaya slices has been reported [16]. Also, Ghasemzadeh et al. [17] used an edible coating based on pectin to coat raisin. The effect of ultrasound-assisted osmotic dehydration and application of pectin coating on physical, textural, and microstructural properties of hot air-dried apricot has been investigated by Sakooei-Vayghan et al. [7]. Natural alginate polysaccharide, another compound used in the coating of fruits, is extracted from brown seaweed (Phaeophyceae). It is composed of two uronic acids: β-D-mannuronic acid and α-L-guluronic acid. Sodium alginate contains L-guluronate, D-manuronate, and alternating sequences of both sugars. Alginate is a hydrophilic biopolymer with coating function due to its unique colloidal properties. Thickening, suspension formation, gel formation, and emulsion stabilization are the unique colloidal properties of alginate [18]. According to literature, the coatings composed of sodium alginate and some active compounds such as beta-cyclodextrin, Ficus hirta fruit extract, calcium chloride, pectin, and trans-cinnamaldehyde could be delayed microbial spoilage of fruits. Coating of fruits with sodium alginate slows the browning by reducing enzymatic reactions. According to the literature, a semi-permeable boundary layer created by sodium alginate limits the water exchangeability of dried fruits [19]. The sodium alginate coating has been applied to maintain the postharvest quality of tomato [20] and peach [21]. The coating of plum with sodium alginate caused a decrease in ethylene production and a delay in weight loss, color changes, and acidity loss [22].
A few studies were conducted on the application of polysaccharide coatings for increasing the shelf life and maintaining the quality attributes of dried apricots, so in this study, the effect of edible polysaccharide-based coatings on nutritional value and quality properties of dried apricot were investigated during storage.
Material and methods
Fresh apricot was purchased from a local shop in Kerman, Iran, then cleaned and washed well. The fruits were cut in half and fruit’s pit was removed and fruits were dried with the open sun and shade method. All chemical materials and solvents used in chemical analyses were analytical grade and provided by Merck Chemical Company. Dried apricots were coated, and the studied treatments for coating dried apricots are listed below:
- T1: control (non-coated).
- T2: coated with pectin (2% w/w), glycerol (1.5% w/w) and Sunflower oil (0.05% w/w).
- T3: coated with pectin (2% w/w), glycerol (1.5% w/w), Sunflower oil (0.05% w/w) and citric acid (1% w/w).
- T4: coated with sodium alginate (2% w/w), glycerol (1.5% w/w) and Sunflower oil (0.05% w/w).
- T5: coated with sodium alginate (2% w/w), glycerol (1.5% w/w), Sunflower oil (0.05% w/w) and citric acid (1% w/w).
Distilled water was used for preparing all coating solutions. For preparing the coating solutions, after the addition of ingredients to water, the solutions were stirred and heated at 70 °C for 30 min on a hotplate with a magnetic stirrer (RH2, Exirhirad, Iran) to obtain uniform solutions. The solutions were cooled to ambient temperature, and dried apricots were immersed for 2 min. Then, to make cross-links in coatings, the samples were immersed in a 2% calcium chloride solution. The coated samples were placed on a basket to drip off residual coating solution and were dried at 25 °C. The control sample was treated with distilled water (T1). The samples were packaged in zip-kip polyethylene bags and stored at 25 °C for four months. During the storage, physicochemical properties (the moisture, total soluble solids, pH, total acidity, vitamin C, Carotenoid, reducing sugar, color, and hardness), microbial properties (total, mold, and yeast counts), and sensory attributes were evaluated.
Moisture
The moisture content of dried apricots was measured according to the AOAC method of 950.46 [23].
Total soluble solids
Total soluble solids were measured using a bench-top refractometer (2WAJ, Optima, Italy). To measure the Brix, 25 g of fine chopped sample was mixed with distilled water in a blender at low speed for 3 min, then reached to 125 ml by distillated water and stirred for 30 min, after filteration with filter paper, a few drops of the filtered solution were poured on the refractometer prism. Considering the ratio of water consumption, the measured Brix was multiplied by 5 and the percentage of total soluble solids was obtained [24].
pH and acidity
To measure the pH and titratable acidity (TA), the samples were homogenized using a manual blender, and 10 g of the homogeneous sample was rehydrated with 50 ml distilled water for 30 min at 25 °C. The mixture was blended in a blender for 5 min and filtered through a filter paper. The filtrate was used for pH and TA measurements. The pH content was determined using a digital pH meter (PTR79, Zagchemie, Iran), and The TA was measured by titration method using 0.1 N NaOH to the endpoint of pH 8.2. The amount of TA was calculated according to malic acid (by a factor of 0.0067), as the predominant organic acid of apricot, according to the following equation:
Vitamin C content
Total Ascorbic acid was measured according to the method described by Klein and Perry [25] after some modifications. For this purpose, 0.50 g of homogenized sample with a solution of 1% metaphosphoric acid was reached to the volume of 10 ml and stirred well using a shaker (KS 260 basic, IKA, Germany) at 200 rpm for 30 min and then centrifuged at 5000 g for 10 min (Sigma, 3-30 K, Germany). Finally, 1.2 ml 6,2-dichlorophenol-indophenol with a concentration of 50 μM was added to 133 μl of the supernatant and, the absorbance was measured using a spectrophotometer (UNICO, 2802, China) at 515 nm. Ascorbic acid, at the concentration ranges of 0–500 mg/kg, dissolved in 1% metaphosphoric acid was used as standard.
Carotenoid content
The determination of total carotenoids (TC) was measured by the spectrophotometric method. For this purpose, 0.50 g of dried sample was added to 10 ml petroleum ether containing 0.005% BHT, shacked (KS 260 basic, IKA, Germany) at 200 rpm for 30 min, and centrifuged at 5000 g for 10 min (Sigma, 3-30 K, Germany). The supernatant was separated, and its absorbance was measured at 450 nm [26, 27]. The amount of TC was calculated according to the following equation:
where A450, F, 548, and 135,310 are absorbance at 450 nm, dilution factor, the average molar mass of carotenoids, and average molar absorption coefficient, respectively, and d = 1 cm.
Reducing sugars
To determine the reducing sugars, 1 g of sample was chopped into small pieces, and added to 200 ml distilled water, shacked (KS 260 basic, IKA, Germany) at 200 rpm for 30 min, and centrifuged at 5000 g for 10 min (Sigma, 3-30 K, Germany). Then, 600 μl of supernatant was mixed with 600 μl 3,5-dinitrosalicylic acid (DNS) (1 g DNS and 1.6 g NaOH was reached to a volume of 100 ml by adding distilled water) and 200 μl 40% potassium sodium tartrate and kept in a water bath at 80 °C for 10 min. Finally, reading of the absorption was performed at 575 nm. The standard curve was prepared using glucose at different concentrations [28].
Color indices
The color indices of apricot samples were measured using a HunterLab colorimeter (TES-135A, Taiwan) standardized with white and black ceramic plates. The samples were scanned at three different locations, and the mean values of L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) were recorded. Also, the ratio of a*/b* was calculated for each sample.
Hardness
Apricot tissue assessment was performed using a texture analyzer (CT-3 10 kg, Brookfield, USA). The hardness was measured by compressing the samples using a TA39 probe (cylindrical probe with 2 mm diameter) at 0.5 mm/s. The maximum force (g) for compression of samples at a distance of 4 mm was considered hardness.
Microbial analysis
The microbial counts of samples (total count and mold and yeast counts) were determined during storage. For this purpose, 10 g of each homogeneous sample were separately diluted in 90 ml sterilized peptone water (pH = 7.2 ± 0.2) under a microbiological hood, and ten-fold serial dilution in the same diluent was performed till 10–4. For standard plate counts, one ml of each dilution was mixed with plate count agar (PCA) culture media (pour-plating method). The plates were incubated at 30 °C for 48 h (ISO 4833–1, 2013) [29]. For yeast and mold counts, surface-plating by spreading 0.1 ml of each dilution on culture media (YGC agar) and incubating at 25 °C for up to 5 days was accomplished (ISO 7954, 1987) [30]. All microbial analyses were carried out in duplicate. At the end of the incubation period, the colonies created in each plate were counted and multiplied by the dilution factor, and the results were expressed as colony forming unit per gram of sample (cfu/g).
Sensory evaluation
The sensory evaluation for color and appearance, taste and flavor, texture, and overall acceptability was conducted using a five-point hedonic scale (5 = Liked extremely; 4 = Liked moderately; 3 = Neither liked nor disliked; 2 = Disliked moderately; 1 = Disliked extremely) by 15 trained panelists.
Statistical analysis
A completely randomized factorial design was considered as experimental design. Analysis of variance (ANOVA) was conducted on data using MSTAT-C software, version 1.42 (Michigan State University). Differences of the mean values were also determined using Duncan’s multiple range test. A significant probability level of 0.05 was defined, and the experiments were carried out in 3 replications in 0, 1, 2, 3, and 4 months after storing.
Results and discussion
Moisture content
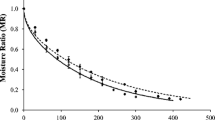
The dried apricots coated with different solutions are shown in Fig. 1. The effects of edible coating and storage time on the moisture content of dried apricots are shown in Fig. 2a. During the storage, the moisture content of dried apricot was gradually reduced. The rate of moisture reduction in coated apricots was slower than in the control. At all storage times, the samples coated with sodium alginate had more moisture content than those coated with pectin. The moisture content of food materials has a decisive effect on some of their essential properties, such as nutritional value, texture, and color. Controlling the moisture transfer of foods during storage is critical for protecting of the quality and safety of these materials [31]. Edible coating acts as a barrier to prevent the transfer of water vapor and reduces the moisture loss in the food products. Although polysaccharide coatings are good water vapor barriers, but it has been determined these coatings can act as agents that retard moisture loss in foods [32]. The ability of sodium alginate for the coating foods to decrease weight loss and moisture reduction by reducing the transpiration intensity of the coated product has been proven [19, 33]. Baysal et al. [11] observed that the moisture content of dried apricots was decreased during storage, and the zein edible coat reduced the amount of moisture loss. Similar results have been obtained by Sakooei-Vayghan et al. [7].
Total soluble solids
According to the results of total soluble solids measurement, the Brix of dried apricot increased during storage, while edible coating declined total soluble solids changes, especially in T4 and T5 treatments (Fig. 2b). The most increase in total soluble solids occurred in control. Data showed the total soluble solids content of the uncoated dried apricot were about 64% initially, which was increased to ≈ 83% at the storage end. It seems that a decrease in dried apricot moisture has led to an increase in Brix during storage, and since coating has reduced the amount of moisture loss, decreasing in the rate of soluble solids changes in the coated samples is not far from expectation. Total soluble solids are mainly represented by sugars, with acids and minerals contributing. According to the reports, the increase in total soluble solids during the storage is due to decrease in moisture content of dried fruits [34], and it has been reported that edible coating can limit the moisture loss of dried fruits during storage [13].
Texture analysis
The texture is one of the most essential characteristics of food acceptance by consumers. The texture of foods is affected by their composition and microscopic and macroscopic structures [35]. Non-physiological dehydration of tissues, starch degradation, and modification of cell wall caused to change in fruits texture [36]. The effects of edible coating and storage time on the hardness are shown in Fig. 2c. The results indicated that the texture of dried apricot gradually hardened during storage. Application of edible coatings, especially the coatings based on sodium alginate, was effective to delay in hardness progress. In a similar trend, previous observations showed the edible coating caused to retaining textural properties of semi-moist apricot cubes mixed with cornflakes during storage [7]. Sanjuán et al. [37] observed that the maximum force for compression and extrusion of dried apricot was increased during storage and found that rising the storage temperatures led to more hardening of the texture of this product. Also, hardening of raisin texture during storage has been reported in the research conducted by Cañellas et al. [38]. The amorphous to glassy state transition of sugars has been expressed as one of the reasons for the increasing the foodstuffs’ texture firmness during storage [38].
Changes in pH and TA
The results of the analysis of variance showed that the pH value of dried apricots significantly increased during storage, while the effect of edible coating on pH was not significant. Considering decrease in TA of dried apricot during the storage, an increase in pH is not unexpected. The effect of storage time on the pH of dried apricots is shown in Fig. 3a.
The perfect balance between organic acids and sugars, along with the rich aroma of apricot, is highly appreciated by consumers [1]. The organic acid content of the apricot, in particular, malic and citric acids have a significant influence on the quality and stability of the aromatic compounds and its acidic flavor [39]. As shown in Fig. 3b, the TA of dried apricots gradually decreased during storage. In addition, the use of edible coating has no significant effect on this property. It has been found that the TA of fruits is mainly correlated to the concentration of their organic acids, and the decrease in TA during storage has been attributed to metabolic changes in fruits or related to the consumption of organic acids in the respiratory process [40].
Vitamin C content
The results showed that the effect of storage time and edible coating on the vitamin C content of apricot was significant (Fig. 4a). The vitamin C content of apricot was decreased during storage, but the vitamin C content of coated samples was higher than the control. The effect of sodium alginate on the preservation of vitamin C was more than pectin, and the use of citric acid in the coating composition caused more protection of vitamin C. Similar results have been reported by Silva et al. [10]. The results of Garcia et al. [14] on the use of edible coatings on papaya before drying showed that the edible coating was effective in maintaining the vitamin C content of the product. Carotenoids, polyphenols, vitamin C, and minerals are among the bioactive compounds of apricot fruit [41]. Vitamin C in fruits and vegetables is crucial for human nutrition [42].
Carotenoid content
Evaluation of carotenoid content of dried apricot indicated a gradual decrease in these compounds during storage. The results of the analysis of variance confirmed the significant effect of edible coating on the protection of carotenoid compounds. The samples coated with sodium alginate had a higher carotenoid content than the other samples (Fig. 4b). The results agree with the results of the research conducted by Sakooei-Vayghan et al. [7]. Consumption of foods rich in carotenoids affects consumer health and reduces the risk of various diseases. On the other hand, postharvest treatments of plant products affect the preservation of their nutrients, such as carotenoids [43]. Apricot is a rich source of carotenoids, so consuming about 250 g of fresh apricot or 30 g of dried apricot can supply the daily body requirement for vitamin A. β-carotene, γ-carotene, lycopene, β-cryptoxanthin, lutein, phytoene, phytofluene, and zeaxanthin are the most common reported carotenoids in apricot fruit [44]. Beta-carotene is the major carotenoid in apricot, which makes up about 60 to 70% of the carotenoids in this fruit [45]. Due to the existence of double bonds in the structure of carotenoids, these compounds are highly reactive. They are degraded by some factors such as high temperature, light, and the presence of reactive oxygen species. Oxidation and geometrical isomerization are the main degradation mechanisms of carotenoids. Enzymatic oxidation of carotenoids is occurred in the presence of lipoxygenase and results in the bleaching of these compounds, but their non-enzymatic oxidation leads to the formation of various apocarotenals and epoxides [43].
Reducing sugars
The analysis of variance indicated there was no significant effect of edible coating on reducing sugar. The reducing sugars content of dried apricot was significantly affected by the storage time. According to observations, the reducing sugars of dried apricot gradually decreased during storage. The effect of storage time on reducing sugars is illustrated in Fig. 3c. Wani et al. [46] observed a continuous decrease in reducing sugars of dried apricot during storage and attributed this decline to the utilization of reducing sugar in browning reactions.
Color indices
The results of color indices (L*, a*, and b*) evaluation are given in Fig. 5a. As shown in Fig. 5a, the L* was decreased in all samples during storage. Although the effect of edible coating on L* was not significant, this factor in coated samples was higher than the control at all storage times. These results are in agreement with the results observed by Baysal et al. [14], Garcia et al. [16], and Sakooei-Vayghan et al. [7]. Changes in color indices are reduced in dried fruits coated by edible coating due to the gas barrier properties of coating compounds [10]. According to the observation of Özkan et al. [47], with the increase in the moisture content of dried apricots, L* and b* increased, but a* value decreased. Therefore, it can be concluded that changes in moisture content has also been influential on the color indices of dried apricots.
According to observations, a* was increased in dried apricot during storage. At all times, the redness of coated samples was less than control. However, based on the results of the analysis of variance, the effect of edible coating on a* was not significant. Increasing a* values and the impact of edible coating on reducing the intensity of color changes in coated semi-moist apricot cubes during storage has been reported [7].
Based on the results obtained in the present study, although coating had no significant effect on b*, the impact of storage time on this color parameter was substantial. In all treatments, b* increased initially and then decreased, while the coated samples showed more b* than the control at the end of storage time.
Evaluation of color changes of dried apricot during storage was also performed using the ratio of a*/b*. The results showed that the a*/b* ratio was slightly decreased at the beginning of storage and then increased. At the end of storage, the lowest a*/b* ratio was related to T5 treatment (Fig. 5b). Baysal et al. [14] reported that the amount of redness and consequently a*/b* ratio in control was higher than in dried apricot coated with corn zein because of more browning reactions. Enzymatic browning is the most crucial reaction that results in the formation of brown color in dried apricot during the drying process. The presence of oxygen is essential for enzymatic browning reactions and the formation of brown pigments such as melanins. On the other hand, non-enzymatic browning reactions, especially the millard reaction, are more critical in producing brown color during the storage of dried fruits, and the storage temperature and time, and sugar and amino acids compositions of fruit are the main influential factors in the rate of these reactions [5]. The edible coatings can reduce the intensity of browning reactions by reducing the oxygen penetration into the dried fruit tissue. The effect of using pectin in coating ingredients applicated in hot air-dried apricot pretreated by ultrasound-assisted osmotic dehydration on reducing browning reactions has been reported by Sakooei-Vayghan et al. [7].
Microbial properties
Evaluation of microbial properties of dried apricot indicated the significant effects of storage time and edible coating on total count and mold and yeast count (Fig. 6). During the storage period, mold and yeast and total counts showed an increasing trend in control, but in the coated samples, the microbial growth did not follow a specific pattern, although in most coated samples the microbial count initially decreased and then gradually increased. The highest microbial count at the end of the storage period was related to the control, and the lowest mold and yeast, and total counts were observed in the samples coated with pectin and citric acid. Baysal et al. [14] observed that the microbial load of dried apricot coated with zein decreased in the first four months of storage, and after that, the microbial count was increased in these samples. It has been proved that edible coatings modify the internal atmosphere of coated fruit, by reducing the fruit peel permeability [14]. Also, the antimicrobial compounds of edible films and coatings can reduce the growth of spoilage and pathogenic microorganisms. Sorbic acid, propionic acid, potassium sorbate, benzoic acid, sodium benzoate, and citric acid are the common antimicrobial agents used in edible coatings [48].
Sensorial properties
Edible coatings protect the nutritional and organoleptic properties of foods, and extend their shelf-life by reducing some adverse reactions such as enzymatic browning, textural breakdown, and off-flavors development [49]. The results of the sensory evaluation showed that coating, as well as storage time, had crucial effects on the organoleptic properties of dried apricot. Increasing storage time led to reducing in color and appearance, taste and flavor, texture, and overall acceptability scores of dried apricots. This was while the panellists almost had given higher sensory scores to coated samples at all times of study. As shown in Table 1, at the end of the storage period, T5 obtained the most scores for sensory attributes. Similar results have been observed for the effect of edible coatings based on pectin and sodium alginate on the preservation of fruit product’s organoleptic properties [15, 22, 50,51,52,53,54,55,56].
Conclusions
Processing and storage change the nutrient compounds and physiochemical properties of fruits. In addition, other quality characteristics of fruit, such as color, texture, and microbial stability, are affected by these factors. Edible coatings by reducing the gas exchange and decrease in moisture and solute migration, as well as by reducing the respiration and oxidation reactions, lead to a decline in the risk of growth of pathogenic and spoilage microorganisms on the foods surface during storage. In the present study, dried apricot was coated with edible coatings based on sodium alginate, and pectin, and its quality attributes were evaluated during four months of storage at 25 ± 2 °C. The results showed that storage led to undesirable changes in the quality of dried apricot, and edible coating delayed these changes. Coating reduced the moisture loss, microbial growth, and chemical, textural, and color changes, and had a positive effect on maintaining vitamin C, carotenoids, and sensory acceptability. The addition of citric acid to edible coating ingredients enhanced its protective effect, and sodium alginate, due to its unique colloidal properties, was more effective than pectin in maintaining the quality attributes of dried apricot during storage.
References
O. Caliskan, S. Bayazit, A. Sumbul, Fruit quality and phytochemical attributes of some apricot (Prunus armeniaca L.) cultivars as affected by genotypes and seasons. Not Bot Horti Agrobo. 40(2), 284–294 (2012). https://doi.org/10.15835/nbha4028044
M.A. Madrau, A. Piscopo, A.M. Sanguinetti, A. Del Caro, M. Poiana, F.V. Romeo, A. Piga, Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur Food Res Technol. 228, 441–448 (2009). https://doi.org/10.1007/s00217-008-0951-6
B. İncedayi, C.E. Tamer, G.Ö. Sinir, S. Suna, Ö.U. Çopur OPUR, Impact of different drying parameters on color, β-carotene, antioxidant activity and minerals of apricot (Prunus armeniaca L.) J. Food Sci. Technol. (Campinas). 36(1), 171–178 (2016). DOI: https://doi.org/10.1590/1678-457X.0086
E. García-Martínez, M. Igual, M.E. Martín-Esparza, N. Martínez-Navarrete, Assessment of the bioactive compounds, color, and mechanical properties of apricots as affected by drying treatment. Food Bioprocess Technol. 6(11), 3247–3255 (2013). https://doi.org/10.1007/s11947-012-0988-1
M. Türkyılmaz, S. Tağı, M. Özkan, Changes in chemical and microbial qualities of dried apricots containing sulphur dioxide at different levels during storage. Food Bioprocess Technol. 6, 1526–1538 (2013). https://doi.org/10.1007/s11947-012-0884-8
T. Lou, W. Huang, X. Wu, M. Wang, L. Zhou, B. Lu, L. Zheng, Y. Hu, Monitoring, exposure and risk assessment of sulfur dioxide residues in fresh or dried fruits and vegetables in China. Food Additives Contaminants: Part A. 34(6), 918–927 (2017). https://doi.org/10.1080/19440049.2017.1313458
R. Sakooei-Vayghan, S.H. Peighambardoust, J. Hesari, M. Soltanzadeh, D. Peressini, Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol Biotech. 58(3), 1–25 (2020)
C.A. Campos, L.N. Gerschenson, S.K. Flores, Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol. 4, 849–875 (2011). https://doi.org/10.1007/S11947-010-0434-1
M. Huertas, D. Mula, M. Serrano, D. Valero, Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Technol. 5, 2990–2997 (2012). https://doi.org/10.1007/s11947-011-0599-2
K.S. Silva, C.C. Garcia, L.R. Amado, M.A. Mauro, Effects of edible coatings on convective drying and characteristics of the dried pineapple. Food Bioprocess Technol. 8, 1465–1475 (2015). https://doi.org/10.1007/s11947-015-1495-y
E.S. Lago-Vanzela, P. Nascimento, E.A.F. Fontes, M.A. Mauro, M. Kimura, Edible coatings from native and modified starches retain carotenoids in pumpkin during drying. LWT—Food Sci. Technol. 50, 420–425 (2013). https://doi.org/10.1016/j.lwt.2012.09.003
E. Hossein, P. Farzaneh, H. Fatemian, H. Asadi, Influence of edible coating and drying methods on quality and thermal properties of apple slices. World Appl. Sci. J. 28(12), 2182–2187 (2013). https://doi.org/10.5829/idosi.wasj.2013.28.12.745
M.A. Youssef, A.A. EL Kady, Evaluation the activity of edible coating for maintenance the shelf life of raisins and prunes. Curr. Sci. Int. 5(1), 103–110 (2016)
T. Baysal, S.E. Bilek, E. Apaydın, The effect of corn Zein edible film coating on intermediate moisture apricot (Prunus armenica L.) quality. GIDA—J Food. 35(4), 245–250 (2010)
G. Oms-Oliu, R. Soliva-Fortuny, O. Martín-Belloso, Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT Food Sci. Technol. 41, 1862–1870 (2008). https://doi.org/10.1016/j.lwt.2008.01.007
C.C. Garcia, L.C. Caetano, K. de Silva Souza, M.A. Mauro, Influence of edible coating on the drying and quality of papaya (Carica papaya). Food Bioprocess Technol. 7, 2828–2839 (2014). https://doi.org/10.1007/s11947-014-1350-6
R. Ghasemzadeh, A. Karbassi, H.B. Ghoddousi, Application of edible coating for improvement of quality and shelf-life of raisins. World Appl Sci J. 3, 82–87 (2008)
C.A. Acevedo, D.A. López, M.J. Tapia, J. Enrione, O. Skurtys, F. Pedreschi, D.I. Brown, W. Creixell, F. Osorio, Using RGB image processing for designating an alginate edible film. Food Bioprocess Technol. 5, 1511–1520 (2010). https://doi.org/10.1007/s11947-010-0453-y
A. Wang, B. Siddique, L. Wu, I. Ahmad, X. Liu, Sodium alginate edible coating augmented with essential oils maintains fruits postharvest physiology during preservation: a review. Int J Multidiscip Res Dev. 7(8), 135–140 (2020)
P.J. Zapata, F. Guillén, D. Martínez-Romero, S. Castillo, D. Valero, M. Serrano, Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J Sci Food Agric. 88, 1287–1293 (2008). https://doi.org/10.1002/jsfa.3220
N. Maftoonazad, H.S. Ramaswamy, M. Marcotte, Shelf life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int J Food Sci Technol. 43, 951–957 (2008). https://doi.org/10.1111/j.1365-2621.2006.01444.x
D. Valero, H.M. Díaz-Mulaa, P.J. Zapataa, F. Guilléna, D.M. Romeroa, S. Castilloa, M. Serranob, Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol Technol. 77, 1–6 (2013). https://doi.org/10.1016/j.postharvbio.2012.10.011
AOAC, Official methods of analysis (Association of Official Analytical Chemists, Washington, DC, 2002)
S. McCoy, J.W. Chang, K.T. McNamara, H.F. Oliver, A.J. Deering, Quality and safety attributes of afghan raisins before and after processing. Food Sci. Nutr. 3(1), 56–64 (2015). https://doi.org/10.1002/fsn3.190
B. Klein, A. Perry, Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J Food Sci. 47, 941–945 (1982). https://doi.org/10.1111/j.1365-2621.1982.tb12750.x
E. Biehler, F. Mayer, L. Hoffmann, E. Krause, T. Bohn, Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J Food Sci. 75(1), C55–C61 (2010). https://doi.org/10.1111/j.1750-3841.2009.01417.x
K.D. Sharma, R. Kumar, B.B. Kaushal, Effect of packaging on quality and shelf-life of osmo-air dried apricot. J Sci Ind Res. 59, 49–954 (2000)
G. Miller, Modified DNS method for reducing sugars. Anal. Chem. 31(3), 426–428 (1959)
ISO standard 4833–1 Microbiology of the food chain—Horizontal method for the enumeration of microorganisms–Part 1: Colony count at 30 degrees C by the pour plate technique (2013).
ISO standard 7954 Microbiology—General guidance for enumeration of yeasts and moulds colony count technique at 25 degrees C (1987).
Z.G. Weinberg, Y. Yan, Y. Chen, S. Finkelman, G. Ashbell, S. Navarro, The effect of moisture level on high-moisture maize (Zea mays L.) under hermetic storage conditions-in vitro studies. J Stored Prod Res. 44(2), 136–144 (2008). https://doi.org/10.1016/j.jspr.2007.08.006
T. Bourtoom, Edible films and coatings: characteristics and properties. Int. Food Res. J. 15(3), 237–248 (2008)
A. Venkatram, A.S. Padmavathamma, B.S. Rao, A.S. Sankar, K. Manorama, D. Vijaya, Influence of storage temperature on sugars, total soluble solids and acidity of raisins prepared from seedless varieties of grape (Vitis vinifera L). Int. J. Curr. Microbiol. App. Sci. 6(8), 2095–2102 (2017). https://doi.org/10.20546/ijcmas.2017.603.249
N.E. Morsy, A.M. Rayan, Efect of diferent edible coatings on biochemical quality and shelf life of apricots (Prunus armenica L cv Canino). J Food Meas Charact. (2019). https://doi.org/10.1007/s11694-019-00240-2
E. Alvarez, A. Cancela, R. Maceiras, Rheological behavior of powdered baby foods. Int J Food Prop. 8(1), 79–88 (2005). https://doi.org/10.1081/JFP-200048082
C. Martel, J. Giovannoni, Fruit ripening, in Plant cell separation and adhesion. ed. by J.A. Roberts, Z. Gonzalez-Carranza (Blackwell Publishing, Oxford, UK, 2007)
N. Sanjuán, J. Bon, M.V. Bermejo, J. Tarrazó, A. Mulet, Influencia de las condiciones de almacenamiento en la calidad de orejones de albaricoques deshidratados, in Anales Del I Congreso Iberoamericano De Ingeniería De Alimentos, Tomo II (Servei de Publicacions de la Universitat Politècnica de València. ed. by E. Ortega, E. Parada, P. Fito (València, Spain), 1996), pp. 312–318
J. Cañellas, C. Rosselló, S. Simal, L. Soler, A. Mulet, Storage conditions affect quality of raisins. J Food Sci. 58(4), 805–809 (1993). https://doi.org/10.1111/j.1365-2621.1993.tb09363.x
X. Fan, H. Zhao, X. Wang, J. Cao, W. Jiang, Sugar and organic acid composition of apricot and their contribution to sensory quality and consumer satisfaction. Sci Hortic. 225, 553–560 (2017). https://doi.org/10.1016/j.scienta.2017.07.016
E. Echeverria, J. Valich, Enzymes of sugar and acid metabolism in stored Valencia organs. J Amer Soc Hort Sci. 114, 445–449 (1989)
E.A. Pop, A. Bunea, F. Copaciu, C. Socaciu, A. Pintea, Stability of carotenoids in dried apricots (Prunus Armeniaca L.) during storage. Bulletin UASVM Food Sci Technol. 73(2), 93–98 (2016). https://doi.org/10.15835/buasvmcn-fst:12263
S.K. Lee, A.A. Kader, Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 20, 207–220 (2000). https://doi.org/10.1016/S0925-5214(00)00133-2
L. Ngamwonglumlert, S. Devahastin, N. Chiewchan, V. Raghavan, Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr Rev Food Sci Food Saf. 19, 1561–1604 (2020). https://doi.org/10.1111/1541-4337.12564
D. Ruiz, M. Reich, S. Bureau, C.M.G.C. Renard, J. Audergon, Application of reflectance colorimeter measurements and infrared spectroscopy methods to rapid and nondestructive evaluation of carotenoids content in apricot (Prunus armeniaca L.). J Agric Food Chem. 56, 4916–4922 (2008). https://doi.org/10.1021/jf7036032
P. Drogoudi, S. Vemmos, G. Pantelidis, E. Petri, C. Tzoutzoukou, I. Karayiannis, Physical characters and antioxidant, sugar, and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L) cultivars and hybrids. J Agric Food Chem. 56(22), 10754–10760 (2008). https://doi.org/10.1021/jf801995x
S.M. Wani, F.A. Masoodi, M. Ahmad, S. Ahmad Mir, Processing and storage of apricots: effect on physicochemical and antioxidant properties. J Food Sci Technol. 55(11), 4505–4514 (2018). https://doi.org/10.1007/s13197-018-3381-x
M. Özkan, A. Kirca, B. Cemeroğlu, Effect of moisture content on CIE color values in dried apricots. Eur Food Res Techno. 216, 217–219 (2003). https://doi.org/10.1007/s00217-002-0627-6
S. Quintavalla, L. Vicini, Antimicrobial food packaging in meat industry. Meat Sci. 62, 373–380 (2002). https://doi.org/10.1016/s0309-1740(02)00121-3
A. Valdés, L. Vidal, A. Beltrán, A. Canals, M.C. Garrigós, Microwave-assisted extraction of phenolic compounds from almond skin byproducts (prunus amygdalus): a multivariate analysis approach. J Agric Food Chem 63, 5395–5402 (2015). https://doi.org/10.1021/acs.jafc.5b01011
J.A.Q. Gallo, M.R. Diaz Amaro, D.M.B. Gutiérrez Cabrera, M.A. Castañeda Álvarez, F. Debeaufort, A. Voilley, Application of edible coatings to improve shelf-life of mexican Guava. Acta Hortic. 599, 589–594 (2003). https://doi.org/10.17660/ActaHortic.2003.599.76
M.A. Rojas-Grauä, M.S. Tapia, O. Martı´nmartı´n-Belloso, Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT–Food Sci Technol. 41, 139–147 (2008). DOI: https://doi.org/10.1016/j.lwt.2007.01.009
I.M. Brasil, C. Gomes, A. Puerta-Gomez, M.E. Castell-Perez, R.G. Moreira, Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT–Food Sci Technol. 47, 39–45 (2012). https://doi.org/10.1016/j.lwt.2012.01.005
C.C. Ferrari, C.I.G.L. Sarantópoulos, S.M. Carmello-Guerreiro, M.D. Hubinger, Effect of osmotic dehydration and pectin edible coatings on quality and shelf life of fresh-cut melon. Food Bioprocess Technol. 6, 80–91 (2013). https://doi.org/10.1007/s11947-011-0704-6
R.E. Sipahi, M.E. Castell-Perez, R.G. Moreira, C. Gomes, A. Castillo, Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (citrullus lanatus). LWT Food Sci Technol. 51, 9–15 (2013). https://doi.org/10.1016/j.lwt.2012.11.013
M.Z. Treviño-Garza, S. García, M.S. Flores-González, K. Arévalo-Niño, Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria ananassa). J Food Sci 80, M1823–M1830 (2015). https://doi.org/10.1111/1750-3841.12938
A.C. Guerreiro, C.M.L. Gago, M.L. Faleiro, M.G.C. Miguel, M.D.C. Antunes, The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol Technol. 110, 51–60 (2015). https://doi.org/10.1016/j.postharvbio.2015.06.019
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayoubi, A., Balvardi, M. & Mahmoudi-kordi, F. Maintaining the nutritional quality and increasing the shelf life of dried apricot using sodium alginate and pectin as edible coating. Food Measure 16, 4025–4035 (2022). https://doi.org/10.1007/s11694-022-01508-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01508-w