Abstract
Fortification of foods including yogurt is one of the most important processes to compensate for the deficiency in some minerals and vitamins. This study aimed to determine the effect of fortification of low-fat synbiotic yogurt samples with iron (25 mg Fe/kg) and vitamin D3 (1000IU) on physicochemical (pH, acidity, viscosity, and syneresis) and sensory properties of prepared yogurts during 21 days of storage at 4 °C. As well, the viability of probiotic bacteria and changes in vitamin D3 and iron content of prepared yogurt samples were evaluated during the storage time. According to the results, the counts of probiotic bacteria in fortified yogurts were significantly higher compared to the control sample during the storage time. As found, fortification of yogurts with iron and vitamin D3 in combination had led to increase acidity and decrease pH values of the samples significantly. However, no remarkable changes were observed in the viscosity and syneresis values of the fortified yogurts compared to the control sample at the end of the storage time. Although iron and vitamin D3 content of fortified yogurts decreased over time, their content was efficient in experimental yogurts at the end of the expected shelf life. As well, the sensory analysis showed no significant differences in fortified samples in comparison to the unfortified yogurt sample which was indicated that it was a suitable vehicle for iron and vitamin D3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumers’ demand for high-quality and healthy food products has paid more attention to developing functional foods which are identified as modified foods that offer health benefits beyond satisfying traditional nutrient requirements [1,2,3]. Accordingly, food fortification is considered as one of the main processes to develop functional properties of food products by improvement of their nutrient quantity and quality [4]. Yogurt is one of the nutritious fermented milk products which is the source of calcium, protein, phosphorus, vitamin B12, riboflavin, thiamin, niacin, zinc, and magnesium and is known for its therapeutic effects [5, 6]. However, yogurt is a generally insufficient source for trace elements such as iron and vitamin D3 that makes it impossible to meet these nutrients Recommended Daily Allowance (RDA) [7].
Vitamin D3 is a fat-soluble vitamin with hormonal function and its deficiency has become one of the most prevalent health concerns in almost every region of the world [8,9,10]. Vitamin D3 has many extra-skeletal effects such as immunomodulatory and anti-inflammatory functions as well as many therapeutic effects against several diseases such as metabolic syndrome, obesity, autoimmune disorders, cardiovascular disease, diabetes, and cancer [11, 12]. However, it has been estimated that people cannot earn more than 2 µg/day of vitamin D3 from dietary intake which indicates that fortification of food products with vitamin D3 is inevitable [11].
As well, iron is one of the essential and trace elements in human nutrition that is naturally present in the structure of cytochromes, enzymes, hemoglobin, and myoglobin and is stored in the liver in the form of ferritin and hemosiderin. Iron plays a vital role in osmotic pressure, maintaining acid–base balance as well as transport, storage, and utilization of oxygen [13]. Accordingly, iron deficiency induces anemia, lower working capacity, and alters mental development in adults [13]. In this regard, low absorption of iron as well as interacting between non-hem iron of vegetable origin and food substances such as phytates, tannins, and phenols have led to its low availability and insufficient dietary intake of iron [13]. In this respect, due to the high consumption of dairy products such as yogurt, fortifying these products are logical and considered a cost-effective and practical solution [7]. Previous studies had investigated the production of iron-fortified lean and low-fat yogurt [14], iron and calcium-fortified soybean yogurt [15], vitamin D3 fortified ice cream and cheddar cheese [16], vitamin D3, and iron-fortified pasteurized cheese [17] and concluded that fortified food products could provide additional health benefits beyond nutrient provision due to the containing bioactive compounds and live and active cultures [7, 8]. Furthermore, much attention has been focused on probiotic products and food containing dietary fiber [18]. Probiotics are living microorganisms that have proved beneficial effects on the health of the host when demonstrated in an adequate amount [2, 6, 19]. As well, the nutritional role of high-fat products has been challenged due to the high level of saturated fatty acids and cholesterol which can replace with a suitable fat replacer such as inulin and lead to a decrease in the adverse effects of reducing fat content on dairy products [20, 21]. Inulin as a prebiotic compound is water-soluble and has the capacity of forming a gel with water as well as compensating for the deficiency caused by fat reduction [22]. In this regard, many studies had proved the desirable effect of inulin as a fat replacer in dairy products with no significant changes in physicochemical and sensory properties compared to full-fat products [18, 20, 23]. However, to the best of our knowledge, no studies have been conducted to fortify low-fat synbiotic yogurt samples with iron and vitamin D3. Therefore, considering the deficiency of nutrients such as iron and vitamin D3 in yogurt, this study aimed to fortify low-fat synbiotic yogurt with an appropriate amount of vitamin D3 and iron to produce a functional food and investigate the effect of iron and vitamin D3 on the physicochemical, the viability of probiotics, and sensory properties of low-fat synbiotic yogurt.
Materials and methods
Materials
Raw cow milk (non-fat solid 8.52 ± 0.21%, protein 3.54 ± 0.11%, fat 1.50 ± 0.10%) was purchased from Pegah Co. (Tabriz, Iran). Yogurt starter culture (ABY-10) containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophiles, as well as probiotic bacteria including Lactobacillus acidophilus (LA-5) and Bifidobacterium lactis (BB-12), procured from Chr. Hansen (Roskilde, Denmark). Long-chain inulin (TEX with a purity of 99.5%) as a prebiotic compound was purchased from Sensus Co. (Roosendaal, Netherland). Vitamin D3 was purchased from Dana Pharma Co, (Tabriz, Iran). FeCl3, NaOH, Phenolphthalein, methanol, acetonitrile, MRS-Bile-Agar culture medium, and Ringer solution were purchased from Merck Chemicals Co. (Darm-Stadt, Germany).
Preparation of synbiotic yogurt samples
The synbiotic yogurt samples were prepared by addition of the 3% inulin into the cold milk and mixed thoroughly with a mixer. The prepared milk was pasteurized at 90 °C for 5 min and cooled to 50 °C for the addition of the vitamin D3 (1000IU) as well as FeCl3 (25 mg Fe/1 kg). Subsequently, fortified milk samples were cooled up to 42 °C. Then, 2% lyophilized yogurt starter culture (ABY-10) was added (initial probiotics counts of 10 Log CFU/g) to the heat-treated milk through the DVS method as recommended by the producer. Fermentation was performed until pH 4.4 and then samples were stored in the refrigerator condition for 24 h before further analysis. Control yogurt was produced using raw milk with the same type of starter culture and inulin. The samples were taken from each experimental treatment after 1, 7, 14, and 21 days from production for physicochemical and microbial tests and 21 days after production for the sensory analysis [8, 13] (Table 1).
Physicochemical properties
The pH of the yogurt samples was determined using a calibrated pH meter (Mettler Toledo, Five Easy F20, Swiss). The titrable acidity was measured by the titration of the diluted yogurt sample (1 yogurt:9 water) with 0.1 N NaOH in the presence of phenolphethalein and expressed as a percentage of lactic acid [5]. The iron content of the samples was measured by atomic absorption spectroscopy (Perkin Elmer A-Analyst 100-USA flame model). Accordingly, 0.4 ± 0.1 g yogurt samples were treated with nitric acid overnight following the digestion in the microwave oven at 160 °C with a ramp time of 30 min and a hold time of 10 min. Then 2 ml of H2O2 (8%) was incorporated into the samples to stop the ashing process. Subsequently, the iron content of yogurt samples was determined based on standard curves achieved for iron analysis using atomic absorption spectrophotometer at λ = 248.3 and 422.7 nm, respectively [24]. For evaluating the Vitamin D3 content of yogurt samples, preparation was done according to the method of Kazmi et al. [16]. In this regard, 1 g of homogenized yogurt sample with water in the proportion of 1:2 (yogurt:water) were weighed into 10 ml test tubes and aliquot with 0.5 ml of aqueous potassium hydroxide (KOH) (60% w/v). Then to reduce vitamin D3 oxidation, all samples were nitrogen-flushed and the tubes were capped, shaken, and transferred to a water bath at 70 °C for 30 min as well as shaking the tubes once after 5 min. Samples were cooled in an ice-water bath for 10 min and treated with 3.75 ml of methanol:chloroform mixture (2:1) followed by vortexing. Subsequently, a further 1.25 ml of chloroform was added to each tube, which was again vortexed. Samples were centrifuged at 1500×g for 10 min at 4 °C and the clear chloroform layer at the bottom of each test tube was removed using a glass syringe and transferred to an evaporation vial. The chloroform extract was dried under a flow of nitrogen gas, reconstituted in 2 ml of the high-performance liquid chromatography (HPLC) mobile phase, and left undisturbed for at least 15 min. Finally, the reconstituted extract was filtered through a 0.45 mm filter and kept in a sealed vial until HPLC analysis. Vitamin D3 was quantified using an HPLC system (X-Agilent technologies, USA) equipped with a UV detector at 228 and 254 nm. Operating conditions were: mobile phase was methanol:acetonitrile:water (49.5:49.5:1, v/v); at ambient temperature (25 °C); the injection volume was 100 ml, and the flow rate was 1 ml/min. To identify vitamin D3, a C18 column (5 mm particles, 4.6 mm ID, and 30 mm length) was used. The viscosity of yogurt samples was evaluated with a viscometer (RV-DV II, Brookfield, USA) using a spindle No. 5 at a shear rate of 60 rpm at 5 °C [25]. The syneresis of yogurt samples was calculated using the method of Dönmez et al. [26] with some modifications. In this regard, 10 ml of mixed yogurt samples were placed in the test tube and centrifuged at 1500×g for 15 min at 25 °C. The volume of separated serum was measured and expressed as %.
Viability of probiotic bacteria
Probiotic culture (Lactobacillus acidophilus and Bifidobacterium lactis) of yogurt samples fortified with iron and vitamin D3 were evaluated by homogenizing 1 g of yogurt with 9 ml sterile ringer serum using Stomacher (Brinkmann, NY, USA). Subsequently, decimal dilutions were performed and inoculated in MRS-bile agar. Plates were incubated in anaerobic conditions for 72 h at 37 °C and the results are expressed as Log CFU/g [27].
Sensory evaluation
The sensory evaluation of fortified synbiotic yogurt samples with iron and vitamin D3 as well as control samples was performed 21 days after production. The sensory analysis consisted of the texture and appearance (whey off, weakness, mouthfeel), taste and odor (too low and high acid, bitter taste, unusual odor), and overall acceptability. The analysis was performed based on a 5-point hedonic scale (1: dislike extremely; 5: like extremely) with 50 semi-trained panelists. The samples were blindly encoded with three-digit random numbers and the sensory properties were explained beforehand to the panelists [5].
Statistical analysis
Effect of vitamin D3 and iron fortification of the synbiotic yogurt samples as well as storage time on physicochemical, nutritional, the viability of probiotics, and sensorial parameters were analyzed based on One-way analysis of variance (ANOVA) and Dunkan’s mean comparison test at a significant level of p ≤ 0.05 using Minitab 16 (IBM Corporation, Armonk, NY, USA) software. Additionally, the General Linear Model (GLM) was used to analyze of variance of two factors’ effects (treatment and storage time). All the analyses were performed in three replicates and data were presented as mean ± SD.
Results and discussion
Changes in pH and acidity values of low-fat synbiotic yogurt samples
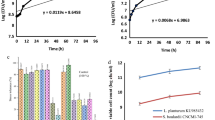
The pH and acidity of yogurt samples during storage time are presented in Fig. 1. As shown, a decrease in pH, as well as an increase in acidity of all tested samples, were observed due to the residual of lactic acid produced by the activity of probiotic bacteria during storage time at 4 °C [2, 28]. Accordingly, the highest and the lowest pH values of yogurt samples were associated with T4 (4.21 ± 0.02) and T3 (4.07 ± 0.02) samples, respectively after 21-days storage time. Lactic acid production in yogurt is vital, due to its effect on the generation of specific taste, instability of casein micelles, conversion of phosphate complex and colloidal calcium to calcium phosphate solution, excretion of calcium from micelles, coagulation of casein, and formation of yogurt gel [29]. In this regard, El-kholy et al. [30] reported the same trend about the effect of iron fortification of yogurt samples and storage time on reducing the pH value of the samples. According to the results, the acidity values of the fortified yogurt samples with vitamin D3 and iron were increased significantly (p < 0.05) during the storage time. The findings were in line with the results of Ziena and Nasser [13], according to the high acidity value of the iron-fortified yogurt samples compared to the control sample after a 7-day storage time. The results demonstrated that the rate of pH reduction of the fortified samples was severe compared to the control sample, which was due to the effect of mico-nutrients on elevating the activity of probiotic bacteria that led to decomposition of lactose and the production of the higher amount of lactic acid [13, 30]. As found, samples fortified with iron and vitamin D3 in combination had the highest acidity at the end of the storage time (1.40 ± 0.07%), which was in line with pH results. However, Cavalini and Rossi [15] reported no significant changes in the acidity of the iron-fortified yogurt samples compared to the control sample which was inconsistent with the present study.
Changes in the pH and acidity of low-fat synbiotic yogurt samples during the 21-days storage at 4 °C. Data are presented as mean ± standard deviation (n = 3), and different letters indicate significant differences at the 5% level in Duncan’s test (p < 0.05). Capital letters indicate storage time effect and small letters indicate treatment effect T1: yogurt fortified with 1000IU vitamin D3; T2: yogurt fortified with 25 mg Fe/kg; T3: yogurt fortified with 1000IU vitamin D3 and 25 mg Fe/kg in combination; T4: control yogurt (un-fortified)
Changes in syneresis value of low-fat synbiotic yogurt samples
Syneresis is defined as serum release and related to the ability of dietary fibers and proteins to retain water within the structure of yogurt and is considered as the most important factor to evaluate the quality of the yogurt during storage time [26]. Figure 2 shows the changes in the syneresis of the yogurt samples containing vitamin D3 and iron separately and in combination. As found, the tendency to exhibit syneresis increased significantly (p < 0.05) during 7-days of storage time. This phenomenon could be due to the reduction of pH during the storage time which affected the casein micelles and increased the serum release. Furthermore, acidification of yogurt samples could lead to dissolving the calcium as well as inorganic phosphate and consequently reducing the net negative electric charge of the casein micelles, which weakens colloidal stability and enhance serum releasing from the gel matrix [5]. In this regard, Dönmez et al. [26], reported that the gel structure of yogurt samples was dependent on the changes in pH value of the samples and demonstrated that production of lactic acid as the result of lactic acid bacteria’s growth was responsible for the reduction of the pH as well as elevating the syneresis value of the yogurt samples during the storage time. Although the highest syneresis of yogurt samples after 21-days storage time was associated with the T3 sample (60.49 ± 4.11%), no significant changes were observed in the syneresis value of the fortified yogurts compared to the control sample at the end of the storage time. El-kholy et al. [30] reported the same results about the non-significant changes in syneresis value of iron-fortified yogurt samples compared to the control sample during the 10-days storage time.
Changes in syneresis of low-fat synbiotic yogurt samples during the 21-days storage at 4 °C. Data are presented as mean ± standard deviation (n = 3), and different letters indicate significant differences at the 5% level in Duncan’s test (p < 0.05). Capital letters indicate storage time effect and small letters indicate treatment effect. T1: yogurt fortified with 1000IU vitamin D3; T2: yogurt fortified with 25 mg Fe/kg; T3: yogurt fortified with 1000IU vitamin D3 and 25 mg Fe/Kg in combination; T4: control yogurt (un-fortified)
Changes in viscosity value of low-fat synbiotic yogurt samples
The viscosity of the yogurt samples could be affected by various factors including heat treatment, type of starter culture, the composition of the yogurt, and processing method [5]. According to Fig. 3, the viscosity of all tested samples decreased remarkably over the storage time. However, no significant changes were observed in the viscosity of fortified yogurt samples compared to the control sample at the end of the storage time. Yogurt is a gel system of casein micelles with entrapped water and its viscosity was affected by the strength and number of bonds between casein micelles as well as their distribution and structure [26]. As seen, the highest and the lowest viscosity of yogurt samples were associated with the T4 (1525 ± 79.23 cP) and T3 (1391.54 ± 80.63 cP) samples, respectively. Although the viscosity of iron and vitamin D3 fortified synbiotic yogurt was lower than the control sample during all the storage period, the control and fortified yogurt samples presented a stable gel with the characteristic of natural set yogurt. This could also be the result of the capability of yogurt starter culture to produce extracellular polysaccharides [31]. Additionally, probiotic bacteria (L. acidophilus and B. lactis) could also produce exopolysaccharides known as viscosifying, emulsifying, stabilizing, sweetening, gelling, and water-binding compounds [32,33,34]. In this regard, Amiri et al. [34] demonstrated that Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12 can produce exopolysaccharides. As well, Cavallini and Rossi [15], reported that no significant changes were observed in the viscosity of iron and calcium-fortified soy yogurts compared to the control sample.
Changes in viscosity of low-fat synbiotic yogurt samples during the 21-days storage at 4 °C. Data are presented as mean ± standard deviation (n = 3), and different letters indicate significant differences at the 5% level in Duncan’s test (p < 0.05). Capital letters indicate storage time effect and small letters indicate treatment effect. T1: yogurt fortified with 1000IU vitamin D3; T2: yogurt fortified with 25 mg Fe/kg; T3: yogurt fortified with 1000IU vitamin D3 and 25 mg Fe/kg in combination; T4: control yogurt (un-fortified)
Changes in iron and vitamin D3 concentrations of low-fat synbiotic yogurt samples
Table 2 shows the stability of the iron and vitamin D3 in yogurt samples during 21-days storage at 4 °C. Accordingly, the recoveries of vitamin D3 in the yogurts compared to the initially added concentration to the milk were 78.31% and 67.94% for T1 and T3 samples, respectively at the end of the storage time. As seen, a small loss of vitamin D3 during storage time could be associated with utilization of micro-nutrients by probiotic bacteria which accelerated their growth and resulted in a decrease in pH and increase in acidity as well as a small effect of acid production on the stability of vitamin D3 [16]. The same trend was observed according to the samples fortified with iron. As found, iron recoveries from T2 and T3 samples were 84.44% and 82.68% respectively. In this regard, Kazmi et al. [16] reported the same results according to the effect of post acidification on the stability of the vitamin D in the fortified yogurt sample. Findings were also in line with the results of Cavallini and Rossi [15] who reported the high recovery of iron from iron and calcium-fortified soy yogurt samples. In this regard, Gilliard Nkhata et al. [35] revealed that the presence of iron in fortified yogurt samples had led to increasing some interactions in samples such as lipid oxidation. Additionally, Sanni [36] also reported that the iron content of fortified gari during storage time decreased slightly. Moreover, Martinez-Navarrete et al. [37] reported that ferrous oxidation increased at a higher moisture level which increased oxidation of iron as a result of moisture absorption from the environment.
Viability of probiotic bacteria in low-fat synbiotic yogurt samples
The viability of Lactobacillus acidophilus and Bifidobacterium lactis as the probiotic bacteria in the yogurt samples during the 21-days storage time under refrigerated conditions are illustrated in Fig. 4. As seen, fortification of synbiotic yogurt samples with vitamin D3 and iron in combination had a remarkable (p < 0.05) effect on the viability of probiotics. As a result, the highest and the lowest viability of probiotics after 21 days storage time attributed to the T3 (8.83 ± 024 Log CFU/g) and control (7.84 ± 0.52 Log CFU/g) samples, respectively. This phenomenon could be due to the factors affecting the fermentation and survival of probiotics such as acidification and fortification [1]. As found, the count of probiotic bacteria in synbiotic yogurts significantly decreased (p < 0.05) during the storage time, which was probably associated with the higher acid production that led to making the environment undesirable for growth and survival of probiotics [2, 21]. Therefore, differences in the viability of probiotic bacteria of fortified yogurt sampled could be due to the changes, caused by metabolites derived from probiotic bacteria [38]. In this regard, Falah et al. [39] investigated the effect of using different concentrations of inulin as a prebiotic ingredient on the viability of Lactobacillus brevis as a probiotic bacteria and revealed that inulin could successfully retain the viability of the probiotics after 14 days of storage; however, production of organic acids by probiotic bacteria makes the condition more unfavorable for the growth of bacteria over time. Furthermore, Rodrigues et al. [40] reported that decreasing the growth of probiotic bacteria referred to the accumulation of lactic acid, diacetyl, and acetaldehyde produced by probiotic bacteria and decreased their viability during storage time. As well, these results were in line with the findings of Mahmood et al. [41] who reported that the viability of probiotic bacteria declined over time. The results suggested that the fortification of yogurt samples with iron and vitamin D3 enhanced the viability of probiotic bacteria in the synbiotic yogurt samples. In the present study, although the viability of probiotic bacteria decreased (p < 0.05) during the storage time, all samples could be considered as a probiotic (> 7 Log CFU/g). The same results were reported by Kahraman and Ustunol [42], according to the effect of zinc fortification of cheddar cheese on elevating the growth and activity of starter culture in cheese samples.
Changes in the viability of probiotics bacteria of low-fat synbiotic yogurt samples during the 21-days storage at 4 °C. Data are presented as mean ± standard deviation (n = 3), and different letters indicate significant differences at the 5% level in Duncan’s test (p < 0.05). Capital letters indicate storage time effect and small letters indicate treatment effect. T1: yogurt fortified with 1000IU vitamin D3; T2: yogurt fortified with 25 mg Fe/kg; T3: yogurt fortified with 1000IU vitamin D3 and 25 mg Fe/kg in combination; T4: control yogurt (un-fortified)
Sensory evaluation of low-fat synbiotic yogurt samples
The effect of iron and vitamin D3 fortifications on sensory parameters of yogurt samples are presented in Table 3. As found, no remarkable differences were shown in the texture and flavor of yogurt samples by incorporating iron and vitamin D3 into the synbiotic yogurt samples. As found, all yogurts were rated above the average on the hedonic scale and were liked by the panelists, showing that the small increase in oxidized and metallic flavor that resulted from iron fortification had a negligible effect on how well the yogurts were liked [14]. According to the results, although the overall acceptability of the T3 sample (3.52 ± 0.43) was slightly lower than other samples, no significant differences were observed between the treated and control sample. This phenomenon could also be attributed to the slightly acidic flavor of the T3 sample as the result of acid production due to the effect of micronutrients on the improvement of probiotics growth in fortified yogurt samples [1, 28]. A similar observation was reported by El-Kholy et al. [30] according to the no significant differences in sensory properties of yogurt samples fortified with iron compared to the control sample. As well, Gaur et al. [4] reported no significant sensory differences in fortified chhash (sour cultured buttermilk) samples with micro-nutrients compared to control samples. Moreover, the same results were reported in previous studies [11, 15]. However, Yeh et al. [43] reported that the inclusion of vitamin A concentrate into the fluid milk had led to detectable off-flavor particularly in low fat and skim milk products which were inconsistent with the findings of this study.
Conclusions
The addition of iron and vitamin D3 to low-fat synbiotic yogurt samples demonstrated no significant effect on syneresis, viscosity, and sensory attributes of the fortified samples compared to the control sample, despite the observed changes in the pH, titratable acidity, and viable probiotic bacteria count cells. The fortification of yogurt with vitamin D3 and iron in combination had led to an increase in the acidity and decreased pH of treated yogurt samples during the storage time. Although some vitamin D3 and iron degradation had occurred in yogurt samples during storage, the nutrient amount of fortified yogurt samples was also efficient after 21-days storage at 4 °C. Additionally, the survival of probiotics in all samples was higher than 107 cfu/mL at the end of the storage period. In conclusion, the present study resulted in the development of an iron and vitamin D3 fortified yogurt which was stable during 21 days at 4 °C and demonstrate that fortified yogurt may serve as viable vehicles for vitamin D3 and iron that could be used in the prevention and control of mineral deficiencies in the general population.
References
C.V. Bergamini, E.R. Hynes, A. Quiberoni, V.B. Suárez, C.A. Zalazar, Food Res. Int. 38, 597 (2005)
A. Alizadeh, N. Aghayi, M. Soofi, L. Roufegarinejad, Acta Aliment. 50, 299 (2021)
M. Soofi, A. Alizadeh, H. Hamishehkar, H. Almasi, L. Roufegarinejad, Int. J. Biol. Macromol. 169, 352 (2021)
S. Gaur, A.W. Waller, J.E. Andrade, Foods 8, 5 (2019)
M. Ardabilchi, S. Amjadi, M. Ardabilchi, L. Roufegarinejad, S.M. Jafari, Powder Technol. 359, 76–84 (2019)
Madhu, S. Neetu, Eur. J. Mol. Clin. Med. 7, 5064 (2020)
H.H. Gahruie, M.H. Eskandari, G. Mesbahi, M.A. Hanifpour, Food Sci. Hum. Wellness 4, 1 (2015)
S. Hajipoor, A. Hekmatdoost, M. Rezaei, S.M. Nachvak, M. Alipour, S. Eskandari, R. Mostafai, M.R. Sobhiyeh, R. Mohammadi, Y. Pasdar, Food Sci. Nutr. 9, 303 (2021)
E. Rajwar, S.S. Parsekar, B.T. Venkatesh, Z. Sharma, Syst. Rev. 9, 1 (2020)
R. Sakhaei, N. Talenezhad, M. Mohammadi, N. Ramezani-Jolfaie, J. Nutr. Food Secur. 4, 126 (2019)
T. Jafari, G. Askari, M. Mirlohi, S. Javanmard, E. Faghihimani, A. Fallah, Adv. Biomed. Res. 5, 52 (2016)
M. Morvaridzadeh, S.M. Nachvak, R. Mohammadi, S. Moradi, R. Mostafai, A.B. Pizarro, H. Abdollahzad, Clin. Nutr. Res. 10, 36 (2021)
H. Ziena, S.A. Nasser, Adv. Dairy Res. 7, 223 (2019)
S. Hekmat, D.J. McMahon, J. Dairy Sci. 80, 3114 (1997)
D.C.U. Cavallini, E.A. Rossi, Aliment. E Nutr. Araraquara 20, 7 (2009)
S.A. Kazmi, R. Vieth, D. Rousseau, Int. Dairy J. 17, 753 (2007)
P. Upreti, V.V. Mistry, J.J. Warthesen, J. Dairy Sci. 85, 3173 (2002)
R.P.D.S. Oliveira, P. Perego, M.N. De Oliveira, A. Converti, J. Food Eng. 107, 36 (2011)
S. Sarkar, Nutr. Food Sci. 49, 182 (2019)
M. Soofi, A. Alizadeh, S.E. Mousavi Kalajahi, Iran. J. Food Sci. Technol. 16, 109 (2019)
M. Delavari, R. Pourahmad, R. Sokutifar, Adv. Environ. Biol. 8, 17 (2014)
D. Meyer, S. Bayarri, A. Tárrega, E. Costell, Food Hydrocoll. 25, 1881 (2011)
P. Chand, M.D. Kumar, A.K. Singh, G.K. Deshwal, P.S. Rao, S.K. Tomar, H. Sharma, J. Food Process. Preserv. 45, e15322 (2021)
E. Poitevin, J. AOAC Int. 99, 42 (2016)
K. Achanta, K.J. Aryana, C.A. Boeneke, LWT-Food Sci. Technol. 40, 424 (2007)
Ö. Dönmez, B.A. Mogol, V. Gökmen, J. Dairy Sci. 100, 901 (2017)
J. Ehsani, M. Mohsenzadeh, M. Khomeiri, N. Resources, A. Ghasemnezhad, Nat. Resour. 37, 219 (2018)
N. Askary, M. Bolandi, J. Chem. Health Risk 3, 1 (2013)
K. Szajnar, A. Znamirowska, D. Kalicka, P. Kuźniar, J. Elem. 22, 559 (2017)
A.M. El-Kholy, M. Osman, A. Gouda, W.A. Ghareeb, World J. Dairy Food Sci. 6, 159 (2011)
F. Bouzar, J. Cerning, M. Desmazeaud, J. Dairy Sci. 80, 2310 (1997)
S.H. Hong, R.T. Marshall, J. Dairy Sci. 84, 1367 (2001)
J. Nishimura, Adv. Microbiol. 4, 1017 (2014)
S. Amiri, R. Rezaei Mokarram, M. Sowti Khiabani, M. Rezazadeh, Bari, M. Alizadeh Khaledabad, Int. J. Biol. Macromol. 123, 752 (2019)
S. Gilliard Nkhata, Z. Ustunol, A. Menevseoglu, J. Nutr. Health Food Sci. 3, 1 (2015)
S.A. Sanni, J. Food Process. Preserv. 36, 207 (2012)
N. Martínez-Navarrete, M.M. Camacho, J. Martínez-Lahuerta, J. Martínez-Monzó, P. Fito, Food Res. Int. 35, 225 (2002)
W.K. Ding, N.P. Shah, Int. Food Res. J. 15, 219 (2008)
F. Falah, A. Vasiee, F.T. Yazdi, B.A. Behbahani, Biomed. Res. Int. 2021, 1057531 (2021)
S. Rodrigues, N. Narain, W.S.C. Feitosa, V.K.G. Abreu, T.O. de Lemos, W.F. Gomes, A.L.F. Pereira, Food Res. Int. 100, 603 (2017)
T. Mahmood, T. Masud, A. Qayyum, A. Mehmood, W. Ahmed, M. Liaquat, M.J. Tareen, S.U. Khan, S. Ali, Food Sci. Technol. 39, 267 (2019)
O. Kahraman, Z. Ustunol, J. Dairy Sci. 95, 2840 (2012)
E.B. Yeh, D.M. Barbano, M.A. Drake, J. Food Sci. 82, 856 (2017)
Acknowledgements
The authors gratefully thank the supports of the Islamic Azad University, Tabriz Branch, Tabriz, Iran. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jalal Aghdasian, A., Alizadeh, A. & Soofi, M. Development of iron and vitamin D3 fortified low-fat synbiotic yogurt as a potential substrate for Lactobacillus acidophilus and Bifidobacterium lactis: evaluation of physicochemical and sensory Properties during the storage time. Food Measure 16, 2718–2725 (2022). https://doi.org/10.1007/s11694-022-01377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01377-3