Abstract
Olive oils produced in different years from different varieties were studied with UV–Vis spectroscopy for classification and prediction. Multivariate models were created with second derivative spectral data, and tested with external validation sets. For varietal classification, orthogonal partial least square discriminant analysis resolved oil samples into various classes with correct classification rate more than 89% for validation set (n = 20). A sample of fresh and stored oils were also classified with a correct classification rate more than 90% for validation set (n = 20). In the predictions of chemical parameters (70 for calibration, 30 for validation), the combination of UV–Vis spectroscopy with orthogonal partial least square regression models showed potential for simultaneous quantification of chlorophylls (0.6–5.6 mg/kg; \({\text{R}}_{\text{val}}^{2}\), 0.79; RPD, 1.97); carotenoids (0.6–3.3 mg/kg; \({\text{R}}_{\text{val}}^{2}\), 0.80; RPD, 2.38); ratio of mono to polyunsaturated fatty acids (3.6–8.8; \({\text{R}}_{\text{val}}^{2}\), 0.77; RPD, 1.90), oleuropein derivatives (1.2–62.3 mg/kg; \({\text{R}}_{\text{val}}^{2}\), 0.66; RPD, 1.77), and total phenol content (62.2–505 mg/kg; \({\text{R}}_{\text{val}}^{2}\), 0.67; RPD, 1.74), although showed poor to moderate results for the quantification of free fatty acid (0.3–5.4%; \({\text{R}}_{\text{val}}^{2}\), 0.67; RPD, 1.64); monounsaturated fatty acids (66-76.5%; \({\text{R}}_{\text{val}}^{2}\), 0.71; RPD, 1.67); polyunsaturated fatty acids (8.6–18.2%; \({\text{R}}_{\text{val}}^{2}\), 0.73; RPD, 1.65). The models were unable to estimate oxidative stability, saturated fatty acids, and individual phenolics such as hydroxytyrosol, pinoresinol, luteolin, total phenolic acids (\({\text{R}}_{\text{val}}^{2}\), 0.26–0.64; RPD, 0.60–1.52). Results showed the capacities of UV–Vis spectroscopy for classification of olive oils, and prediction of total pigments and phenol content and ratio of mono to polyunsaturated fatty acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Olive oil has been widely investigated for its characterization and authentication. There are common inherent attributes among oils obtained from different varieties, harvest time and years of olives. As the demands for a monovarietal olive oil and product origins continue to increase, some efforts by international organizations, such as European Union and International Olive Council, have been made to establish the guidelines for olive oil certification; stipulating quality reference limits for different classes and categories [1]. However, when it comes to dealing with large number of samples, rapid and less laborious methods of analyzing properties peculiar to oils of different sources like variety, harvest time, geographical origin and method of processing become necessary.
Applications of certain regions of electromagnetic spectrum as an easy-to-use tool for quality of food products have gained prominence. Consistent and practically reproducible fingerprints relating spectra responses to the underlined properties of olive oil have been accomplished using these spectroscopic methods [2]. These technologies have economically contributed to the industry in terms of low cost, minimum waste and highly repeatable analysis of olive oil. The most commonly applied regions are within mid and near infrared and quite a number of studies have shown some models developed in olive oil characterizations [3, 4]. One of its many advantages is the rapid classification of olive oils into different grades and categories with as much as low amount of chemicals spent. Usually, the spectroscopic classification of olive oils is applied in the areas of varietal and harvest time differentiation, production or geographical origin distinction, typicality in terms of chemical composition, adulteration detection and quantification [5,6,7]. Spectroscopy offers additional benefits of optimizing olive oil production process for better product control and real-time monitoring.
UV–Vis spectroscopy was shown as a practical technique for different uses in characterization, classification, and determination of adulteration in various kinds of foods such as oils [8,9,10], tea [11], ketchup [12], wines and spirits [13, 14]. The technique has been satisfactorily applied to quantify color pigments in fruits and vegetables [15, 16]. Similarly, characterization and prediction of one or more chemical properties in other food products using UV–Vis spectroscopy have also been profiled in recent years. The works of Cifuni et al. and Nguyen et al. show that lipid oxidation and myoglobin in meats can be successfully predicted [17, 18]. Comparatively, UV–Vis pattern analysis has been the least exploited technique in olive oil research. The intensity of the energy measured as absorbance against wavelength is capable of creating a unique profile containing information that can be expounded with chemometric tools. Data fusion strategies of UV–Vis with other rapid, non-invasive technologies were also reported [19, 20]. The importance of inclusion of multivariate modeling in the evaluation of UV–Vis spectra in food analysis was also expressed in a recent review [21].
Olive oil is a very characteristics food article, as it does not necessarily need any pretreatment prior to UV–Vis spectrum acquisition. For this reason, it is important to show more effective usage of UV–Vis spectroscopy in oil analysis as a classification and prediction tool. A comprehensive study was aimed for the use of UV–Vis spectral data of olive oils for their classification and prediction of major chemical parameters by using samples of different years and different varieties. Simultaneous changes in spectra intensities with olive oil quality were elaborated and chemical properties such as phenolics, fatty acids and quality indices were predicted using orthogonal projection to latent structure regression models. In order to confirm this hypothesis, the multivariate models were validated with independent data sets for evaluation of the method to monitor variations in the properties of olive oil as a function of variety and year.
Materials and methods
Olive oil samples
A hundred olive oil samples obtained between 2012 and 2016 were evaluated. All the samples belonged to Aegean region (west coast of Turkey), more specifically around 150–200 km distance to north, east and south from the city of Izmir. The olives from different harvest times (early, mid and late) and different varieties over different harvest years were included in the sampling to cover a wide range of olive oil samples. The oils were mainly from Ayvalik (A), Memecik (M) and Erkence (E) olive varieties with few others from local varieties. In the harvest year of 2012, thirty-six olive oil samples (eighteen from each of Ayvalik and Memecik varieties) were collected. These 36 olives belong to early, mid, and late harvest times. Four Ayvalik oil samples were obtained in 2014 harvest year. In 2015, twelve oils of Erkence variety, 10 oils of local varieties, and 8 oils of Ayvalik and Memecik varieties from mid to late harvest times were analyzed; then they were stored in amber bottles in dark at room temperature for 12 months, and re-evaluated in 2016 (Table 1). Taris Olive and Olive Oil Cooperatives Union provided the oil samples of Ayvalik and Memecik, while others were provided by known and trusted local producers. Maturation degree for Ayvalik and Memecik olives were varied from early to late, while Erkence oils were obtained from olives at late maturation. Other local oils were obtained at the mid maturation degree. The chemical and spectroscopic analysis were conducted in an order without a specific time interval on the same year the samples were collected. 2016 oils are the stored oils of year 2015, so they have undergone the same treatment after one year of storage. The oil samples collected yearly were carefully preserved in clean airtight bottles and kept at refrigeration temperature (4oC) until analyzed. A constant replacement of headspace of the sample with nitrogen was ensured throughout the course of the analyses.
Chemicals
The standards for phenolic acids such as caffeic, 2,3-dihydroxylbenoiz, 4-hydroxyphenylacetic, p-coumaric, 3-hydroxyphenylacetic acid ferulic acids that are profiled in the oil were products of Extrasynthese (France), while phenolic alcohols (hydroxytyrosol and tyrosol), luteolin and pinoresinol were sourced from Fluka (Germany). The Fatty Acid Methyl Ester (FAME) mixture used in the percentage quantification of individual fatty acids was a brand of Supelco37 (C4–C24 Supelco #47,885-U). All the reagents and HPLC grade solvents (methanol, acetic acid, ethanol, ethyl ether, cyclohexane, hexane) were the products of Sigma-Aldrich and Fluka (Germany). Ultrapure water used for general cleaning and reagents’ solvent was produced in Sartorius Arium 611 VF system (Sartorius AG, Gottingen, Germany).
UV–Vis spectra acquisition
Spectra of oils were collected using UV–Vis spectrophotometer (Shimadzu UV-2450 Kyoto, Japan) equipped with deuterium-discharge lamp as a source of ultraviolet wavelength range and a tungsten lamp for the visible range, and a resolution of 2.0 nm, was used. Absorption spectra of oils were acquired in UV and visible ranges (200–800 nm) at 1 cm path length, thus resulting in 301 wavelengths (spectral variables) in data matrix. Spectra were collected in replicates and averaged out, then, they were pretreated in SIMCA (v.16, Umetrics, Umea, Sweden) prior to multivariate analysis.
Oxidative stability index (OSI)
An automated Rancimat system (Model 873 Biodiesel, Metrohm, Switzerland) was used to estimate the oxidative stability of the oil samples [22]. The system contains reaction vessels into which 3.0 g of olive oil was measured. The vessels were covered and connected to conductivity cells containing ultrapure water as volatile adsorbent. Airflow at 20 L/h and 120 °C facilitates rapid oxidation of the oil and serves as a carrier of the primary oxidative products from the vessel to the absorbent, while the rate of oxidation is monitored by thermal conductivity. Oxidative stability in Rancimat method is reported in terms of time (induction point) needed to fully oxidize the oil samples in this accelerated process.
Total phenol content (TPC)
Phenolic extract of olive oil was quantified according to the method of Montedoro et al. [23], using Folin Ciocalteau spectrometric method. A total of 10 mL of the reaction mixture (consisting of 1 mL of phenolic extract, 6 mL of dionized water, 0.5 mL of Folin reagent and 2 mL of 15% g/mL Na2CO3 solution) was mixed and incubated for 2 h in the dark, and then absorbance values at 765 nm were taken. TPC was expressed as equivalent of gallic acid (mg GA/kg oil).
Free fatty acid (FFA)
European Official Methods of Analysis [24] was used to determine FFA, which is expressed as oleic acid %. It was determined by neutralizing oil solution (ethanol: ethyl ether, 1:1) with 0.1 N KOH in the presence of phenolphthalein.
Total pigments
The pigment content of the oil as chlorophyll and carotenoids were estimated according to a procedure by Mínguez-Mosquera et al. [25]. Olive oil sample (7.5 g) was mixed and made up to 25 mL with cyclohexane. The absorbance representing chlorophyll (A670) and carotenoids (A470) were measured with a UV spectrophotometer (Shimadzu UV-2450 Kyoto, Japan), having optical path length (d) of 1 cm. Chlorophyll and carotenoid contents were expressed as mg/kg of oil using Eqs. 1 and 2:
Phenolic profile by HPLC
Phenolic extraction was done according to the method described by Alkan et al. [26]. HPLC system (Agilent 1200, Santa Clara, CA, USA) equipped with a quaternary pump, an auto-sampler and a DAD photodiode array detector was used to resolve individual phenolic compounds composed in the sample. A C18 column (250 mm, 4mm, 5 μm, SGE 8211, Australia), maintained at 35 °C, with injection volume of 20 µL was used for the analyses. Initially, the mobile phase was 90% of water/acetic acid (99.8:0.2 v/v) and 10% methanol and the concentrations were adjusted according to a predetermined gradient. Each phenolic compound was quantified by comparing its response to that of commercial standard at two wavelengths, 280 and 320 nm. Gallic acid was used as internal standard, five-point calibration curves were prepared for each phenol standard and the results were expressed in mg/kg oil. Oleoropein derivative (O-der) is the summation of hydroxytyrosol (Hyt) and tyrosol (Tyr). Total phenolic acids (TPA) represent the summation of phenolic acids (caffeic, 2,3-dihydroxylbenoiz, 3 and 4-hydroxyphenylacetic, p-coumaric, and ferulic acids) determined in the sample.
Fatty acid profile by GC
GC-FID system (Agilent 6890, USA) equipped with split/split-less (1:50) injector, HP 88 capillary column (100 m × 0.25 mm × 0.20 mm, Agilent, USA) was used to profile fatty acid methyl ester contents of the oils in accordance to European Official Methods of Analysis [24]. Helium at 2 mL/min flow rate was used as the carrier gas. Injection temperature and volume was 250 °C and 1 mL, respectively. Initially, the oven temperature was set at 140 °C, maintained for 10 min and increased to 220 °C at 3 °C/min rate. The detector temperature was kept at 280 °C. Individual fatty acids were quantified in percentages by comparing samples chromatograms with FAME standard (C4–C24 Supelco #47,885-U). Results were given as total saturated (SFA), monounsaturated (MUFA), polyunsaturated fatty acids (PUFA), as well as MUFA/PUFA and oleic acid to linoleic acid (C18:1/C18:2) ratios.
Data matrices and multivariate analysis
Mean, standard deviation, minimum and maximum values of each of the parameters in classification prediction models are given in Tables 2, 3 and 4. As a result of prior comparisons of the results, the best models in classification and prediction were created with standard normal variate filtering (SNV) treated second derivative spectra (SNV-2d). In addition to the whole spectral (200–800 nm) data, reduced spectral range (270–700 nm) was also considered in variable prediction. The UV–Vis spectra and chemical parameters were designed in three different ways to apply multivariate techniques:
-
a.
Data for classification with respect to variety: the matrix consists of UV–Vis spectra of 60 samples of known varieties (Ayvalik, Memecik and Erkence) from 2012, 2014 and 2015 harvest years; calibration and external validation sets were selected as 40 and 20 observations, respectively (samples in calibration set: 2012 (25); 2014 (2); 2015 (13)).
-
b.
Data for classification with respect to storage for over a year: the matrix consists of UV–Vis spectra of 30 samples obtained in 2015 and those stored for one-year (2016); calibration and external validation sets were selected as 40 and 20 observations, respectively (samples in calibration set: 2015 (20); 2016 (20)).
-
c.
Data for prediction of chemical parameters: the matrix consists of UV–Vis spectra of 100 samples (70 calibration and 30 validation) with quality parameters (FFA, OSI, chlorophylls, and carotenoids), fatty acids (SFA, MUFA, PUFA, MUFA/PUFA and C18:1/C18:2 ratios) and phenolic compounds (hydroxytyrosol, tyrosol, oleuropein derivatives, pinoresinol, luteolin, total phenolic acids, and total phenol content).
Partial least square discriminant analysis and orthogonal form of partial least square analysis (OPLS-DA) were tried for classification, as the latter produced models with higher cross validation. OPLS-DA evaluates the relationship between X (spectra) and Y (user-defined class allocation matrix): class 1 (Ayvalik), class 2 (Memecik), class3 (Erkence) for the varietal differences, and 5 (2015) and 6 (2016) in the case of discrimination with respect to year. The variations in X matrix are partitioned into two: a predictive part correlated to Y variable (class information in this case) and an orthogonal part uncorrelated to Y. OPLS-DA models were validated using leave-one-out cross validation (internal validation) and external validation. Class modelling performances were measured by considering percentage of correctly classified samples (confusion matrices) for both calibration and prediction. OPLS regression was applied to model the predictive capacity of the spectra over chemical parameters of oxidative stability, nutritional and quality characteristics. Output of the models were given in terms of root mean square error of calibration (RMSEcal), cross-validation (RMSECV), external validation (RMSEval), regression coefficients for calibration, \({\text{R}}_{\text{cal}}^{2}\), cross-validation \({\text{R}}_{\text{cv}}^{2}\), and validation \({\text{R}}_{\text{val}}^{2}\). The ratio of performance to deviation (RPD) for external validation were presented [27]. RPD values for validation sets were calculated as the ratio of standard deviation of the data to the root mean squared errors. The number of significant principal components of OPLS models were recorded as PCp + PCo, where p and o stand for predictive and orthogonal components, respectively. Multivariate data analyses were carried out using SIMCA 14 (Umetrics, Umea Sweden). VIP (variable importance) values summarize the overall contribution of X variables (UV–Vis wavelengths) to the PLS models of classification and prediction. The significant wavelength ranges were given based on the VIP lists of SIMCA.
Results and discussion
Chemical parameters of olive oils samples
Olive fruit shows variation in chemical parameters with respect to variety, harvest year and time of harvest, geography, and agricultural applications. The variation in quality variables, fatty acid and phenolic profiles of tested olive oil samples are the result of these factors and the oil production phase, as well (Tables 2, 3 and 4). FFA is the basic parameter used in the classification of olive oils with respect to quality. FFA is mainly affected by the olive harvest, oil production, and storage practices [24]. The oils with FFA less than 0.8% are defined as extra virgin olive oil. The oils with low acidity have high OSI (h). The samples with FFA less than 1.0% belongs to A and M oils, and they consequently had high OSI values (such as 7.5 h). Most of the E oils had high free fatty acid (> 3.0%), which resulted in low OSI (such as 2.7 h). The samples have a wide range of pigment concentration (1.0 to 5.4 mg/kg for chlorophyll; 0.8 to 3.3 mg/kg for carotenoids). It was observed that E oils had lower pigment concentrations compared to A and M oils. The main mono-unsaturated fatty acid in olive oil is oleic acid (C18:1). The range of oleic acid was observed within 66 and 76.5%. M and A oils have higher oleic acid content than E oils. The other important fatty acid group are poly-unsaturated fatty acids. Two significant poly-unsaturated fatty acids in olive oils are linoleic (C18:2) and linolenic (C18:3) acids, which adversely affect the oxidative stability [28]. The range of this group among fatty acids was determined as [8.6–18.2%]. The E oils had higher amount of these fatty acid groups, which explain their low OSI. Among all chemical parameters, the phenolic compounds showed larger standard deviations due to their high variability with respect to variety, maturity, harvest year, pre and post extraction processes (Table 4). A and M oils had higher concentrations of hydroxytyrosol (Hyt) and tyrosol (Tyr) than E oils did. These two derivatives of secoiridoids are known to contribute antioxidant capacity of oils, and positively affect OSI values. TPC of oils changed between 62.2 and 505 mg/kg, not necessarily contributing to high OSI [28].
Spectra characterization
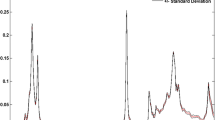
Representative spectra for oils of different varieties, and oils as fresh and stored are shown in Fig. 1. The information embedded in the bands of the spectrum can reveal both qualitative and quantitative differences among oils. Spectra differ particularly in the visible range between 380 and 720 nm, which constitutes the most informative bands, alongside a broad band at near ultraviolent region between 300 and 390 nm. In olive oils, characteristic absorbance between 300 and 500 nm have been linked to different classes of phenolic compounds and other minor constituents in general [29]. The range of 440–500 nm corresponds to carotenoid pigments such as lutein and beta-carotene. Chlorophylls as pheophytin a and pheophytin b compounds give maximum absorbance between 415 and 440 nm and also 655–670 nm [20]. There are three small peaks along the spectrum at 540 nm, 610 nm and 670 nm. Only high quality olive oils possess these three peaks as in the case of Ayvalik and Memecik oils, which had lower free fatty acid content (less than 1.0%). These peaks tend to disappear in the low quality oils (Erkence oils with free fatty acid content greater than 1.0%), as also observed by other researchers [30]. The weak peak around 540 nm can be attributed to anthocyanins [29]. The main peaks at 670 nm and other at 610 nm are caused by electronic transitions between grounds and first excited state of chlorophylls. It can be stated that transitions to higher energy state corresponds with the absorptions in these shorter wavelengths [31, 32]. The appearance of the peaks in terms of height and width indicate broadening of those states. Strong absorption bands at 400–500 nm range were observed in both high and low quality olive oils. However, the shape of the spectra differed from those containing significant amount of chlorophyll (Memecik and Ayvalik) as supported by Tarakowski et al. [30]. A detailed information in another study supports the explanation of observed peaks between 400 and 500 (mainly carotenoids and derivatives, and some derivatives of chlorophylls such as pheophytin a), 530–565 (anthocyanins), 610–670 (chlorophylls) [33].
The differences in the spectra of 2015 and 2016 oils (stored) was observed in the range of 440–510 nm (Fig. 1b). The increase in concentration of more stable pigment derivatives during storage was reported as a result of pheophytinization, pyropheophytinization, and isomeration reactions [33, 34]. Similarly, there are some amount of increase in hydroxytyrosol and tyrosol concentrations during 12-month storage as reported elsewhere [35]. The total effects of these transformations can be explained as the increase of the absorbance between 440 and 500 nm at the end of the storage.
Classification with respect to variety and storage effect
In the classification of olive oil samples with UV–Vis spectra, OPLS-DA models were created by untreated, SNV-2d treated and reduced-SNV-2d (270–700 nm) spectra. Details of multivariate models in terms of number of principal components, R2 for calibration and cross validation, and confusion matrix containing the percentage of correctly classified observations (CC%) in each group are given in Table 5. For varietal classification of olive oils, OPLS-DA models were built with classes 1, 2 and 3 ascribed to oils of Ayvalik (A), Erkence (E) and Memecik (M) varieties, respectively, as shown in the score plot (Fig. 2a). It was seen that models were successful in terms of correct classification for calibration data (100%). Reduced SNV-2d filtered data gave the best results (greater than 89%) for validation data with a 4-component model. According to VIP results of the model, the important contributors are in the ranges of 282–350 nm, 392–426 nm, and 442–546 nm. In Table 6, the significant UV–Vis wavelength ranges are given for each chemical parameter. It can be deduced that all chemical parameters had their effects on the varietal discrimination. With validation set, the classification capacity of UV–Vis spectroscopy showed similar or better performances than those observed in case of combined data of UV–Vis and headspace mass spectroscopy data [31, 36]. The results are comparable to another report about classification techniques applied on selected wavelengths of UV–Vis spectra and sensory data of larger olive oil samples [37]. The spectra of olive oils of 2015 were modeled with the spectra of the same oils after they were stored for a year (2016) to show the capacity of UV–Vis spectroscopy in discriminating fresh and stored oils. The score plot of OPLS-DA model with two classes is shown in Fig. 2b. Models for calibration produced 100% correct classification for calibration and 90–100% for validation data. Untreated and reduced SNV-2d spectra gave similar results. VIP plots of OPLS-DA model showed that the most significant wavelength ranges were 282–328 nm and 426–546 nm. These UV–Vis ranges match up with phenolic compounds and pigment content of oils (Table 6). Chemical analyses showed that hydroxytyrosol, tyrosol, pinoresinol contents of stored oils increased even though total phenolic contents decreased up to 25%. The chlorophyll and carotenoid contents of stored oils decreased by 40 and 32%, respectively (data not shown). No significant changes observed in fatty acid profiles, while free fatty acid values increased and oxidative stability values decreased, in general. A recent study indicated fluctuations in some chemical components of olive oils, especially the “fluorophores” such as pigments, phenolic compounds, tocopherols and its derivatives, during a long time storage [38]. UV–Vis spectroscopic evaluation of olive oil with respect to its freshness has a potential of being used in authentication problems to verify its quality consistency over time.
Prediction of olive oil quality and chemical parameters
The set of predicted variables consist of quality parameters (FFA, OSI, total chlorophylls and carotenoids), fatty acids (SFA, MUFA, PUFA, ratios of MUFA/PUFA and oleic/linoleic acid), and phenolic compounds (individual phenols including hydroxytyrosol, tyrosol, pinoresinol, luteolin, total phenolic acids, and total phenol content). The best OPLS models were obtained with pretreated data, and results are given in terms of calibration and validation in Table 6. The characteristics of models were discussed in terms of parameters of external validation.
Quality parameters
The acidity of olive oils was predicted with a 5-component model, \({\text{R}}_{\text{val}}^{2}\) of 0.67 and RPD of 1.64. The prediction of OSI with 5 latent variables generated \({\text{R}}_{\text{val}}^{2}\) of 0.66 and RPD of 1.15. The most informative wavelength ranges (VIP from Simca) were determined as 300–410 nm for FFA and 390–680 nm for OSI. The indicative wavelengths for oxidative stability index overlapped with those for phenolic compounds and pigments, which are the main protective components against oxidation in olive oils. However, the performance of the UV–Vis spectra model on induction values was found lower compared to mid-IR spectra [39]. The prediction of total chlorophylls and carotenoids were resulted in \({\text{R}}_{\text{val}}^{2}\) of 0.79 and 0.80; RPD of 1.97 and 2.38, respectively. Borello and Domenici [40] showed the use of UV–Vis spectra over visible range to determine olive oil pigment concentration, and discussed better estimation of pigments by the whole range of UV–Vis as oppose to the absorbance readings at single wavelengths. The significant wavelength range for pigments in the prediction models were determined as the band of 412–698 nm. There are sources reporting that RPD values of 2.0 or less are considered as poor models [27]. According to classifications of RPD values in other studies, pigment models can be considered as good, FFA model as moderate, OSI model as poor [41, 42].
Lower RMSE values, and higher R2 and RPD values for chlorophyll and carotenoids (compared to those for other parameters) support that the responsiveness of UV–Vis spectroscopy to strong chromophore-containing or chromogen-like chemical substances (pigments in olive oil) is higher than that to other chemical compounds. In a study, the absorbance at these wavelengths corresponding to the pigments was found useful for adulteration detection in EVOO in which the concentration of the adulterant is inversely proportional to the absorption intensities [30].
Fatty acids
The predictions of oleic acid and MUFA, and predictions of linoleic acid and PUFA with UV–Vis spectra gave very similar results. MUFA and PUFA have been reported to contribute positively and negatively to the oxidative stability of olive oil, respectively [28]. The 6-component models of the ratio of MUFA/PUFA and C18:1/C18:2, and 5-component models of MUFA and PUFA were found similar in terms of validation R2 and RPD values. These observations are slightly lower than those obtained by mid-IR spectra [43]. Yet, the present results appear more promising as opposed to the other reports claiming that UV–Vis spectra have limitations in oleic and linoleic acid predictions [44]. The most informative bands for the ratio of mono and poly-unsaturated fatty acids were determined as [340–500]. The prediction of SFA was not found successful with UV–Vis spectra (RPD = 0.6).
Phenolics
In the case of phenolic compounds, validation measures of models are found lower compared to those of quality indices and fatty acids. Among phenolic compounds, total phenolic content (TPC) modeled by SNV-2d pretreated, reduced spectra between 270 and 700 nm produced \({\text{R}}_{\text{val}}^{2}\) of 0.67 and RPD of 1.74. The concentration of oleuropein derivatives (summation of hydroxytyrosol and tyrosol) was estimated by a 6-component model with \({\text{R}}_{\text{val}}^{2}\) of 0.66 and RPD of 1.77. As for the other phenolics, models produced validation results in terms of R2 and RPD values in the ranges of [0.26–0.65] and [0.84–1.52], respectively. The uncertainty in the spectral band responsible for phenols is due to the low selectivity of UV–Vis spectroscopy for olive oil minor compounds; a view that was also shared by Gonçalves et al. [45]. The most informative wavelengths for TPC model were determined as 276–500 nm, which covers all the significant ranges observed for individual phenolic compounds (between 282 and 500 nm).
Conclusions
UV–Vis spectroscopy and chemometrics was found applicable in classification with respect to variety, and classification with respect to freshness. SNV filtered 2nd derivative spectra produced models that classified oils of three varieties with over 89% correct classification rate in validation data. Similarly, validation data of fresh and stored olive oils were classified with over 90% correct classification rates. Multivariate regression models produced promising results for the estimation of some chemical parameters (total chlorophyll and carotenoids, ratio of mono-unsaturated to poly-unsaturated fatty acids, total phenol content), with RPD values between 1.74 and 2.38. The improvements in classification and prediction models can be achieved by using higher number of observations from different varieties, harvest time, harvest year, and quality.
References
P. Dais, E. Hatzakis, Anal. Chim. Acta 765, 1 (2013)
A.M. Gomez-Caravaca, R.M. Maggio, L. Cerretani, Anal. Chim. Acta 913, 1 (2016)
N. Sinelli, L. Cerretani, V. Di Egidio, A. Bendini, E. Casiraghi, Food Res. Int. 43, 369 (2010)
G. Squeo, S. Grassi, V.M. Paradiso, C. Alamprese, F. Caponio, Food Control 102, 149 (2019)
K. Georgouli, J. Martinez, A. Del Rincon, Koidis, Food Chem. 217, 735 (2017)
A. Hirri, M. Bassbasi, S. Platikanov, R. Tauler, A. Oussama, Food Anal. Method 9, 974 (2016)
O.S. Jolayemi, F. Tokatli, S. Buratti, C. Alamprese, Eur. Food Res. Technol. 243, 2035 (2017)
F.C. Alves, A. Coqueiro, P.H. Março, P. Valderrama, Food Chem. 273, 124 (2019)
K.D.T.M. Milanez, T.C.A. Nobrega, D.S. Nascimento, M. Insausti, B.S.F. Band, M.J.C. Pontes, LWT-Food Sci. Technol. 85, 9 (2017)
J.S. Torrecilla, J.C. Cancilla, G. Matute, P. Díaz-Rodríguez, A.I. Flores, J. Food Eng. 118, 400 (2013)
A. Palacios-Morillo, Á. Alcázar, F. de Pablos, J.M. Jurado, Spectrochim. Acta A 103, 79 (2013)
C.V. Di Anibal, S. Rodriguez, L. Albertengo, M.S. Rodriguez, Food Anal. Method 9, 3117 (2016)
R. Rios-Reina, S.M. Accurate, J. Camiña, R.M. Callejón, J.M. Amigo, Chemometr. Intell. Lab. 191, 42 (2019)
I. Sen, F. Tokatli, J. Food Compos. Anal. 45, 101 (2016)
J.S. Torrecilla, V. Fernández-Ruiz, M. Cámara, S. Mata, Talanta 85, 2479 (2011)
M. Zude-Sasse, I. Truppel, B. Herold, Postharvest. Biol. Technol. 25, 123 (2002)
G.F. Cifuni, M. Contò, S. Failla, J. Food Eng. 169, 85 (2016)
T. Nguyen, S. Kim, J.G. Kim, Food Chem. 275, 369 (2019)
E. Borràs, J. Ferré, R. Boqué, M. Mestres, L. Aceña, A. Calvo, O. Busto, Food Chem. 203, 314 (2016)
O. Uncu, B. Ozen, F. Tokatli, Talanta 201, 65 (2019)
A.C. Power, J. Chapman, S. Chandra, in Cozzolino in Evaluation Technologies for Food Quality. ed. by B.J. Zhong, X. Wang (Woodhead, Duxford, 2019), pp. 91–104
R. Farhoosh, S. Einafshar, P. Sharayei, Food Chem. 115, 933 (2009)
G.F. Montedoro, M. Servilli, M. Baldioli, R. Selvagini, E. Miniati, J. Agric. Food Chem. 40, 1577 (1992)
EEC Commission Regulation (EEC), J. Eur. Communities L 248 (1991)
I. Mínguez-Mosquera, L. Rejano-Navarro, B. Gandul-Rojas, A.H. Sanchez-Gomez, J. Garrido-Fernandez, J. Am. Oil Chem. Soc. 68, 332 (1991)
D. Alkan, F. Tokatli, B. Ozen, J. Am. Oil Chem. Soc. 89, 261 (2012)
Y. Huang, T.M. Rogers, M.A. Wenz, A.G. Cavinato, D.M. Mayes, G.E. Bledsoe, B.A. Rasco, J. Agric. Food Chem. 49, 4161 (2001)
O.S. Jolayemi, F. Tokatli, B. Ozen, Food Chem. 211, 776 (2016)
D. Casoni, I.M. Simion, C. Sarbu, Spectrochim. Acta A 213, 204 (2019)
R. Tarakowski, A. Malanowski, R. Kościesza, R.M. Siegoczyński, J. Food Eng. 122, 28 (2014)
M. Casale, C. Armanino, C. Casolino, M. Forina, Anal. Chim. Acta 589, 89 (2007)
E. Giannakopoulos, E. Isari, K. Bourikas, H.K. Karapanagioti, G. Psarras, G. Oron, I.K. Kalavrouziotis, J. Environ. Manage. 195, 186 (2017)
M.J. Moyano, F.J. Heredia, A.J. Meléndez-Martínez, Compr. Rev. Food Sci. Food Saf. 9, 278 (2010)
L. Gallardo-Guerrero, B. Gandul-Rojas, M. Roca, M.I. Mínguez-Mosquera, J. Am. Oil Chem. Soc. 82, 33 (2005)
J.R. Morelló, M.J. Motilva, M.J. Tovar, M.P. Romero, Food Chem. 85, 357 (2004)
C. Pizarro, S. Rodríguez-Tecedor, N. Pérez-del-Notario, I. Esteban-Díez, González- Sáiz. Food Chem. 138, 915 (2013)
D. Kružlicová, J. Mocák, E. Katsoyannos, E. Lankmayr, J. Food Nutr. Res. 47, 181 (2008)
J.A. Snouber, I. Abdelraziq, M. Abu-Jafar, A. Zyoud, H. Hilal, A. Pasqualone, J. Sci. Food Agric. 99, 2757 (2019)
O. Uncu, B. Ozen, LWT Food Sci. Technol. 63, 978 (2015)
E. Borello, V. Domenici, Foods 8, 18 (2019)
C.W. Chang, D.A. Laird, M.J. Mausbach, C.R. Hurburgh, Soil Sci. Soc. Am. J. 65, 480 (2001)
R.V. Rossel, R.N. McGlynn, A.B. McBratney, Geoderma 137, 70 (2006)
A.M. Inarejos-García, S. Gómez-Alonso, G. Fregapane, Food Res. Int. 50, 250 (2013)
M. Casale, P. Oliveri, N. Sinelli, P. Zunin, C. Armanino, M. Forina, S. Lanteri, Anal. Chim. Acta 712, 56 (2012)
T.R. Gonçalves, L.N. Rosa, R.P. Gonçalves, A.S. Torquato, P.H. Março, S.T.M. Gomes, M. Matsushita, P. Valderrama, Food Anal. Method 11, 1936 (2018)
Acknowledgements
The authors wish to thank Environmental Research Center and Biotechnology and Bioengineering Application and Research Center of Izmir Institute of Technology (IZTECH) for their technical support in chromatography analysis.
Funding
This work was supported by Izmir Institute of Technology (2014IYTE03, 2015IYTE15 and 2016IYTE39).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jolayemi, O.S., Tokatli, F. & Ozen, B. UV–Vis spectroscopy for the estimation of variety and chemical parameters of olive oils. Food Measure 15, 4138–4149 (2021). https://doi.org/10.1007/s11694-021-00986-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00986-8