Abstract
Quercetin (Qu) is a most active biological flavonoid and it has a very wide spectrum of potential applications. Herein, we have synthesized ionic liquid assisted Co3O4 nanostructures through an aqueous chemical growth method and fabricated a Co3O4 modified GCE as an electrochemical sensor for the sensitive detection and determination of Qu. The proposed electrochemical sensor was not only prepared with a very easy, simple and cheap method but it was also found to be very selective, sensitive and highly stable for the detection of Qu in standard solutions as well as in real food samples like onion, honey and green tea. The prepared electrochemical sensor has shown an excellent electrochemical response for Qu with a wide range of detection from 0.01 to 3 µM. The oxidation current response of Qu on Co3O4 modified GCE was found 4 times higher than the response of bare GCE which is due to the high conductivity, tremendous catalytic ability and large surface area of Co3O4 nanostructures. The limit of detection (LOD) and the limit of quantification (LOQ) for Co3O4/GCE sensor was calculated and found to be 0.0002 µM and 0.0007 µM respectively. While, the amount of Qu in real samples was found to be 5.367 µg/mL in honey, 15.58 µg/g in onion and 3.473 mg/g in green tea respectively. In comparison to the previously reported sensors, the prepared Co3O4/GCE sensor has shown a higher electrocatalytic capability, remarkable stability, super sensitivity and adequate selectivity for the determination of Qu in standard solutions as well as in real samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, polyphenols have been considered as the fifth important nutrient after vitamins carbohydrates and proteins [1]. Flavonoids are the naturally occurring subclass of polyphenolic compounds that are extensively present in plants, roots, seeds, stems, vegetables, fruits and flowers [1, 2]. Based on phenolic rings, the position of hydroxyl groups and the presence of double bonds, the flavonoids are classified into flavonols, flavones, flavanones, isoflavones, flavan-3-ols and anthocyanin categories [3]. Among these classes, flavonols have strong antioxidant [4], anti-inflammatory [5], antiulcer [6], antiviral [7, 8] and many other pharmacological and biological activities. In flavonols, quercetin (3,3′,4′,5,7-penthydroxyflavone, Qu) is considered as a most biologically active and abundant dietary flavonoid which shows high antioxidant activity and can protect human DNA from the oxidative attack in vitro by its free radical scavenging property [9]. Besides antioxidant properties, Qu also possesses anti-inflammatory, antiviral, anti-allergic, anti-tumor and anti-pathogenic activities and may use to treat cardiovascular and cerebrovascular illness [9, 10]. However, its excessive amount can also cause kidney cancer [11]. Based on these facts, the simple, accurate and sensitive determination of Qu has attracted great interest in many fields of science and chemistry.
Several analytical methods have been developed for the quantification and detection of Qu including HPLC [12], spectrophotometry [13], capillary electrophoresis [14] and gas chromatography [15]. Although these methods are effective and sensitive but at the same time, they are costly, time-consuming and require expensive reagents, pretreatment of samples and complex instrumental handling. As Qu is an electroactive compound [16], therefore it can be detected and quantified by electrochemical methods, while the electrochemical methods have been proven less expansive, simple and fast with super sensitivity and remarkable stability [17,18,19,20,21,22,23,24,25] so they can be used for exact and sensitive recognition of Qu [7]. However, in electrochemical sensing the conventional GCE (glassy carbon electrode) or CPE (carbon paste electrode) suffers from electrode fouling and high over-voltage problems because of slow electron transfer kinetics, therefore, supplies high detection limits [26, 27]. In order to enhance the performance and the sensitivity of the electrode for the quantification and detection of Qu and other biomolecules, it is compulsory to modify the traditional GCE or CPE with different modifier materials [28,29,30]. Nowadays, a variety of nanomaterials has been used for the modification of electrodes [31] such as noble metals [32], polymers [33], metal oxides [25, 34] and carbon nanomaterials [35]. Among these nanomaterials, metal oxides have attracted great interest from the scientific community because of their large surface area, excellent working ability and good catalytic performance [36,37,38,39]. To date, different metal and metal oxide-based nanomaterials or nanocomposites have been synthesized and used for the determination of Qu as Sepideh Tajyani et al. [40] have synthesized Fe3O4@NiO magnetic nanoparticles for the detection of Qu. Vilian et al. [41] have fabricated a sensor of Au NPs on a porous aromatic framework (Au-PAF-6) for Qu detection and M.L. Yola et al. [42] have developed a graphene oxide/silver NPs based sensor for the inspection of Qu. But recently cobalt oxide nanostructures have gained huge attention of the scientists because of their inimitable properties and three different polymorphs, for example, cobaltous oxide (CoO), cobaltic oxide (Co2O3) and cobaltosic oxide (Co3O4) [25]. In Co3O4, Co2+/Co3+ ion pairs coexist at the same time in which Co2+ ions engage tetrahedral sites and Co3+ ions engage octahedral sites with oxygen and it crystallizes in spinal structure [43]. Co3O4 spinal nanostructures have great potential application in many fields of science and technology such as in electrochemical sensors [44, 45] lithium-ion batteries [46] supercapacitor [47] and energy storage devices [48]. Whereas, ionic liquids (ILs) are the class of low temperature organic molten salts that possess a wide range of temperatures. Due to their high polarity, negligible vapor pressure, good dissolving ability, tailorable structures, high thermal stability and high ionic conductivity [49] ILs have been widely used in catalysis [50], as an inert solvent in electrochemistry [51] and in some chemical processes can be used to replace water due to their unique properties. In material synthesis, they have been used as solvents, morphological templates, additives and reactants for the synthesis of inorganic nanomaterial with enhanced properties [52, 53]. An important feature of using ILs in the synthesis of nanostructures involves the growth and nucleation of nanoparticles [54].
In this study, we have successfully synthesized the IL assisted Co3O4 nanostructures through a low temperature aqueous chemical growth method in which IL was used as a morphological template. This synthesized material was then used to fabricate a nanosensor to investigate the electrochemical behavior of Qu. The electrochemical investigation on Co3O4/GCE sensor was carried out by using the cyclic voltammetry (CV) technique and differential pulse voltammetry (DPV) technique on optimized parameters. Because of the large surface area, prodigious catalytic activity and highly conductive nature of Co3O4 nanostructures, the modified Co3O4/GCE shown excellent performance for the determination of Qu in real samples of onions, honey and green tea.
Experimental
Chemical reagents and solutions
Cobalt chloride hexahydrate and urea were purchased from Sigma-Aldrich (UK and Sweden). Quercetin dihydrate and 1-butyl-3-methyl imidazolium hexafluorophosphate [BMIM][PF6] IL was purchased from Fluka (Switzerland). All the reagents were of analytical grade and used without any pretreatment process. Deionized (D.I) water was used throughout all experiments. A 0.01 M stock solution of Qu was prepared by dissolving the appropriate amount of Qu in 1:1 v/v of water and ethanol. The phosphate buffer of 0.1 M concentration (pH 4) was prepared by dissolving the measured quantities of NaH2PO4, KH2PO4, Na2HPO4, NaCl and KCl in one litter volume of D.I water, and 0.1 M NaOH or HCl was then used to adjust the pH of the buffer solution.
Synthesis of Co3O4 nanostructures
Co3O4 nanostructures were prepared through a low-temperature aqueous chemical growth method by following our previously reported method [25]. For this purpose, three separate solutions containing 0.1 M cobalt chloride hexahydrate salt, and 0.1 M urea were prepared in 100 mL of D.I. water followed by the addition of variant volumes (25, 50, and 75 µL s) of IL [BMIM]+[PF6]− in each solution. The amount of precursor salt and urea were kept the same in all solutions only the volumes of IL were varied. The solutions containing the chemical mixture were kept on magnetic stirring for several minutes in order to homogenize the mixture. After that, the beakers containing the growth solutions were covered with aluminum foil and kept in an oven at 90 °C for 4 h. As the time was completed, the synthesized nanostructures were filtered through Whatman filter paper and washed several times with D.I water to remove all the impurities. The filtered precipitates were then dried in an oven for 30 min at about 80 °C and then the dried materials were kept in an electric furnace for 4 h at 500 °C for annealing to remove their hydroxide phase and to achieve pure cobalt oxide nanostructures.
Modification of electrode with synthesized Co3O4 nanostructures
For the modification of GCE, 10 mg of Co3O4 nanostructures were dissolved in 2.5 mL of D.I water (containing 50 µL of 5% Nafion®) and sonicated for 30 min as reported [25, 39]. Before the modification of GCE, the electrode was thoroughly polished with 0.5 µ pore alumina powder and washed with D.I water after that the modification was done by drop-casting method, in which 10 µL of prepared Co3O4 solution was carefully dropped on the surface of GCE and left at room temperature until the excess water evaporates and the electrode surface gets dried.
Structural and electrochemical characterization of nanomaterial
The functionalities present in the material were studied by using Fourier transform infrared spectrophotometer (FTIR) (Thermo Nicolet 5700). While the purity and the crystallinity of Co3O4 nanostructures were checked through X-ray powder diffraction (XRD) by utilizing Phillips PW 1729 powder diffractometer. The elemental analysis and composition of the materials were confirmed by energy dispersive spectroscopy (EDS) and the shape of the synthesized materials was evaluated by field emission scanning electron microscopy (FESEM) by using LEO 1550 Gemini worked at 20 kV.
CV, DPV and electrochemical impedance spectroscopy (EIS) measurements were carried out on CHI-760 electrochemical work station (USA) and a three-electrode containing conventional assembly was established in which GCE was used as working electrode, Ag/AgCl electrode (0.1 M KCl) used as reference electrode and the Pt wire was used as a counter reference electrode. A phosphate buffer of 0.1 M concentration (pH 4) was used as a supporting electrolyte. The CV studies were carried out in the potential range of − 0.2 V to + 0.5 V at the scan rate of 50 mVs− 1. While the DPV was performed on the optimized parameters in the potential range of 0.1 to 0.4 V with the amplitude of 50 mV and the pulse width and pulse period of 0.03 and 0.1 s respectively. However, EIS was carried out in the frequency range of 100 kHz to 1 Hz at the initial potential of 1.5 V. All the experiments were done at room temperature.
Sample preparation for onion, honey and green tea
Different onion samples were cut into small pieces and 25 g of them were ground in a mortar till the onion paste was obtained, then the paste was added to 50 mL of methanol and sonicated up to 30 min at 40 °C. After that, the obtained extract was filtered twice with Whatman filter paper and then the appropriate amount of the onion extract was further diluted in the electrolyte solution and the measurements were carried out through the standard addition method by using the DPV technique.
Same as, 1 g of dried green tea leaves were ground and added in 10 mL of methanol while for honey sample, 5 mL of honey was diluted in 10 mL of methanol and the extract of both samples was obtained by following the same procedure and the measurements were carried out in the same way as mentioned in the above statement.
Results and discussion
Structural characterization of Co3O4 nanostructures
The functionalities present in the prepared Co3O4 nanostructures were checked through FTIR analysis in the wavelength range of 400 to 2750 cm−1. The two infrared bands appeared at 497.5 and 640.2 which are the specific vibration modes of (Co-O) confirming the successful preparation of spinal Co3O4 nanostructures. While the infrared band that appeared at 1643.1 is the OH bending vibration of water absorbed from the environment.
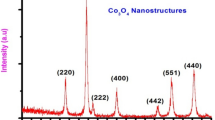
The crystalline nature and the phase purity of the synthesized nanostructures were inspected through the XRD technique and the results are shown in Fig. 1. The diffraction peaks in the spectrum of all three materials at 2θ represent the crystal planes of (111), (220), (311), (400), (511), and (440) cubic phase of Co3O4. Meanwhile, the absence of the other phase peaks assures the purity of the prepared material while the high sharpness of the peaks indicates the high crystalline nature of the prepared nanostructures. The average crystalline size for all Co3O4 nanostructures (prepared with variant volumes of IL) was calculated from the major XRD diffraction peaks by using the Scherrer formula (τ = kλ/(β cos θ) and it was observed to be 22.9 nm for the material which was synthesized with 25 µL volume of IL while 24.7 nm and 27.5 nm for the materials which were synthesized with 50 and 75 µL volumes of IL respectively.
Furthermore, to ensure the elemental composition of the synthesized nanostructures, the EDX analysis was carried out and the results confirm that the synthesized material is only composed of Co and O elements with a total weight % of 67.7%, 31.4% (in 25 µL IL), 67.9 %, 31.3 % (in 50 µL IL) and 69.8 %, 31.1 % (in 75 µL IL) respectively.
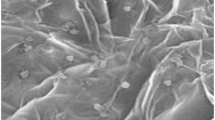
The structural characteristics or morphological characteristics of the prepared Co3O4 nanostructures were studied through FE-SEM analysis and the obtained results are displayed in Fig. 2a and b which contains low- and high-resolution images of the material which have shown good catalytic activity in sensing application and have been prepared with less volume (25 µL) of IL. The FE-SEM images of the other two materials that have been prepared with 50 and 75 µL of IL have been reported previously [25]. The surface morphology of the nanostructures plays a crucial role in designing or fabricating the electrochemical sensors. Herein, the IL was utilized as a morphological templet and different volumes of IL were examined to study their effect on the morphology and properties of the material. Here we observed from the FE-SEM images that all the Co3O4 nanostructures produced with different volumes of IL were showing almost the same morphological texture of Co3O4 as nanorods like surface with little balls on the nanorods. The Co3O4 nanorods produced with 25 µL of IL are well dispersed but due to the magnetic nature of Co3O4, the rods are seemed to be little accumulated. The other Co3O4 nanostructures produced with the 50 and 75 µL volumes of the IL were also alike in texture as produced with the 25 µL (Co3O4 nanorods) but those nanorods are accumulated more closely due to the higher volumes of IL. Though, it was observed that the higher volume of the IL did not alter the morphology of the Co3O4 nanorods but somehow aggregate them. The dispersed and well-defined shape of Co3O4 nanorods was produced with 25 µL of IL so it could be the better candidate in the sensing applications.
Electrochemical characterization using EIS and CV
Initially, the electrochemical characterization of the material was done through EIS and CV techniques to check the efficiency of the prepared material in electrochemical sensing. The measurements were carried out in 5 mM [Fe (CN)6]−3/−4 and 0.1 M KCl solutions (used as a redox probe), both solutions were taken in the same ratio.
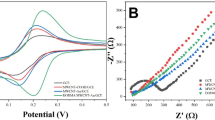
EIS is a powerful technique used to study the charge transfer efficiency of fabricated electrochemical sensors. It calculates the charge transfer resistance (Rct) by fitting the data in an equivalent electrical circuit. In EIS the semicircle part (in the low-frequency region) of the resultant Nyquist plot corresponds to the charge transferring activity of the modified sensors, and the diameter of that semicircle curve is proportional to their electron transferring efficiency which means that as smaller the diameter of the semicircle curve as lower the Rct of the sensor and higher its electron transferring ability. Meanwhile, the linear part in the high-frequency region corresponds to the diffusion process [11, 55]. The Rct values of bare and different Co3O4 modified electrodes were obtained by fitting the data in a Randles equivalent circuit diagram and the resultant plot is shown in Fig. 3a. The largest semicircle diameter and highest Rct value were obtained with bare GCE (1475 Ω) which is indicating that the bare GCE has less conductivity and very poor electron transferring efficiency. However, with different Co3O4 modified electrodes the smallest semicircle diameter and lowest Rct value of 202.7 Ω were obtained from the electrode that was modified with the material in which 25 µL of IL was used which indicates that this material has high electron transferring efficiency and providing more active surface area due to the small size of the particles as compared to the other two materials that have the Rct values of 356 Ω and 568.4 Ω (for 50 and 75 µL IL) respectively. The active electrode surface area of bare and different Co3O4 modified electrodes was also calculated by the Randles–Sevcík equation. The active surface area obtained for the bare GCE was 6.12 mm2 and for the Co3O4 modified electrodes, it was obtained to be 9.76 mm2, 8.45 mm2, and 7.24 mm2 for the materials in which 25, 50, and 75 µL volumes of IL were used respectively.
Figure 3B shows the CV results of bare GCE and various Co3O4 modified GCE obtained in the same redox probe solution. These results also justifying the EIS results, as can be seen from the voltammogram that as compared to the bare GCE and other two modified GCEs (50, 75 µL IL) a well-resolved peak with high peak current was obtained from the electrode that was modified with the material in which 25 µL of IL were used.
The electrochemical response of Qu
CV has been performed to investigate the response of 0.05 mM Qu in PBS (pH 4) at modified GCE and bare GCE and the resultant voltammogram is presented in Fig. 4A. As can be clearly seen from this figure that, in the absence of Qu both modified and bare GCE given no response which defines that no redox activity has been taken place on the surface of both electrodes. While, a high redox current of 1.25 µA (Ipa) and 0.95 µA (Ipc) has been achieved with the modified electrode at a less positive potential in comparison to the bare GCE in presence of 0.05 mM Qu indicating that, the fast electron transfer kinetics took place on the surface of modified GCE which is owing to the high catalytic property and large surface area of the Co3O4 nanorods. The oxidation of the Qu initiates in the sequential order of five hydroxyl groups connected with 3 aromatic rings and the resultant oxidation associated with the 3,4-dihydroxy electron-donating group on ring B (which is more electroactive and oxidize at lower potentials) shown in Scheme 1 resulting in 2 electrons and 2 protons transfer reaction [7].
The cyclic voltammograms of different Co3O4 modified GCEs and bare GCE are displayed in Fig. 4B and as can be observed from these results, all three Co3O4 modified electrodes have shown excellent redox features at higher currents (compared to the bare GCE) for 0.05 mM of Qu ensuring their good catalytic activity. In these three modified electrodes, the electrode modified with the material synthesized with 25 µL of IL has shown a relatively higher oxidation current of 1.25 µA which is 0.49, 0.39 and 0.26 µA higher than the bare GCE and other two Co3O4 modified electrodes so all the other parameters were optimized on the material which was synthesized with 25 µL of IL.
Effect of pH
The pH study was carried out at Co3O4/GCE by using the DPV method in 0.1 M PBS of various pH (from 4 to 6.5) containing 0.05 mM Qu and the results are summarized in Fig. 5 A. As can be observed from this figure that when the pH increases from 4 to 6.5 the oxidation peak potential of the 0.05 mM Qu shifted negatively and the peak current also decreases with the gradual increase of pH showing that, in the redox reaction of Qu not only the electrons but protons are also involved in the reaction process. Moreover, Fig. 5B shows the linear co-relation plot of oxidation peak potential vs. pH and the corresponding linear equation can be stated as Epa= − 0.061 pH + 0.48 (R2 = 0.998).
The observed value of slope 0.061 V/pH is very near to the theoretical value which is 0.059 V/pH. Suggesting that, an equal number of protons and electrons are taking part in the redox reaction of Qu [11]. The possible electrochemical reaction mechanism is given in Scheme 2.
Effect of scan rate
The effect of scan rate on the redox behavior of 0.05 mM Qu at modified Co3O4/GCE was studied by CV in the range of 10 mVs− 1 to 70 mVs− 1. In Fig. 6A the results show that as the scan rate increased gradually from 10 to 70 mVs− 1 the redox current of Qu has also increased simultaneously which indicates that the scan rate has a linear relationship with the current of Qu. The plot of Ipa and Ipc vs. the square root scan rate is revealed in Fig. 6B and two linear regression equations have been achieved Ipa (µA) = 1.9246 (mVs− 1) – 3.5044 (R2 = 995) and Ipc (µA) = 1.4002 (mVs− 2) – 3.0180 (R2 = 987) which indicates that an adsorption controlled process is taking place on the modified electrode surface.
Analytical parameters of Qu
To investigate the sensitivity of the proposed sensor for Qu in 0.1 M PBS of pH 4, the DPV method was explored under the optimized conditions (mentioned in the “Experimental” section) and the results are presented in Fig. 7A. As the concentration of Qu increased gradually from 0.01 to 3 µM the oxidation peak current response of Qu was also increased linearly. The relationship of the peak current with concentration is shown in Fig. 7B and their corresponding regression equation is stated as Ipa = 2.94544E− 6 C (µM) + 7.95248E− 7 (R2 = 0.999).
The LOD and LOQ were calculated from the signal-to-noise ratio (3*(S/M) for LOD and 10*(S/M) for LOQ) and the values were come to be 0.0002 µM and 0.0007 µM respectively. Keeping these results in view and compared to the previously reported works shown in Table 1, we can say that our proposed sensor has shown a spectacular electrocatalytic performance for the determination of Qu with a broad dynamic range of detection and a very low LOD (0.0002 µM).
Repeatability, reproducibility, selectivity and stability
The repeatability is an important parameter for a modified electrode sensor in sensing applications for long term detection. To evaluate this parameter on the proposed sensor we have taken thirteen repetitive runs on the same electrode in the presence of 0.05 mM Qu and the proposed Co3O4 modified GC electrode sensor has shown marvelous stability of the current in Qu detection with the relatively small%RSD of 1.32 % (Fig. 8A), hence we can say that our proposed sensor can be used for long term sensing applications.
Moreover, the reproducibility of the proposed sensor was also investigated by comparing the results of 5 separately modified electrodes studied in 0.05 mM solution of Qu and their%RSD of the currents was calculated. The%RSD values of the currents obtained for different modified electrodes were less than 4 %. The calculated%RSD values depict that the fabricated sensor is highly reproducible for the proposed analyte.
The selectivity of the proposed sensor has been elucidated in 0.05 mM Qu solution by adding 10 µL from the equimolar concentration of different organic and inorganic interfering analytes for example lactic acid, gallic acid, ascorbic acid, ethanol and NaCl into the test solution (Fig. 8B, C). The results have shown that these interfering species have a negligible effect on the anodic current response of Qu. Additionally, the stability of the Co3O4/GCE sensor was investigated by taking one run every day for 28 consecutive days and the sensor did not show any noticeable change in the redox response of Qu and has maintained its sensitivity of about 97%. But after the 28th day, the redox response of the sensor for Qu was seemed to be much decreased. By looking at these results thus, we can say that our proposed sensor is just not selective for Qu, but it is highly stable too and can be used for practical applications.
Detection of Qu in real samples
To find the practical applicability of the prepared Co3O4/GCE sensor for the determination of Qu from honey, onion and green tea samples, the real samples were prepared first as described above then the appropriate amount of the extract was diluted in 10 mL PBS (pH 4) and the measurements were performed by DPV technique. The amount of Qu in real samples was calculated to be 5.367 µg/mL in honey, 15.58 µg/g in onion and 3.473 mg/g in green tea respectively. In order to assure the accuracy of the results, a standard addition method has been used and the recovery results are summarized in Table 2 which indicates that our proposed sensor has shown accurate results for the determination of Qu with good % recoveries.
Conclusions
In this report, IL assisted Co3O4 nanostructures have been successfully synthesized and used to fabricate a GCE for a very selective and highly sensitive electrochemical determination of Qu in standard solutions as well as in real samples like honey, green tea and onion. The fabricated electrode was found highly stable, extremely sensitive and an excellent electrochemical sensor for the detection of Qu in a broad dynamic range of detection from 0.01 µM to 3 µM with a very low limit of detection which is 0.0002 µM. This outstanding performance of the electrode is due to the high conductivity, best catalytic ability and large surface area of Co3O4 nanostructures which are the reason for the adsorption of Qu at the electrode surface. Based on our results we can recommend that our proposed sensor is very easy, cheap, highly sensitive, selective and stable that can be used for the determination of Qu in any real application.
References
S. Takahashi, H. Muguruma, N. Osakabe, H. Inoue, T. Ohsawa, Electrochemical determination with a long-length carbon nanotube electrode of quercetin glucosides in onion, apple peel, and tartary buckwheat. Food Chem. 300, 125189 (2019)
A. Saljooqi, T. Shamspur, A. Mostafavi, Fe3O4@ SiO2-PANI-Au nanocomposite prepared for electrochemical determination of quercetin in food samples and biological fluids. Electroanalysis (2019). https://doi.org/10.1002/elan.201900386
M. Kawser Hossain, A. Abdal Dayem, J. Han, Y. Yin, K. Kim, S. Kumar Saha, G.-M. Yang, H.Y. Choi, S.-G. Cho, Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 17(4), 569 (2016)
M. Skerget, P. Kotnik:, A.R. Hras, M. Simonic, M. Ha dolin. Z. Knez. Food Chem 89, 191 (2005)
A. Mondal, D. Rajalingam, T.K. Maity, Anti-inflammatory effect of O-methylated flavonol 2-(3, 4-dihydroxy-phenyl)-3, 5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. J. Ethnopharmacol. 147(2), 525–529 (2013)
M. Saito, H. Hosoyama, T. Ariga, S. Kataoka, N. Yamaji, Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 46(4), 1460–1464 (1998)
D. Saritha, A. Koirala, M. Venu, G.D. Reddy, A.V.B. Reddy, B. Sitaram, G. Madhavi, K. Aruna, A simple, highly sensitive and stable electrochemical sensor for the detection of quercetin in solution, onion and honey buckwheat using zinc oxide supported on carbon nanosheet (ZnO/CNS/MCPE) modified carbon paste electrode. Electrochim. Acta 313, 523–531 (2019)
F.C. Bekkering, A.U. Neumann, J.T. Brouwer, R.S. Levi-Drummer, S.W. Schalm, Changes in anti-viral effectiveness of interferon after dose reduction in chronic hepatitis C patients: a case control study. BMC Gastroenterol. 1(1), 14 (2001)
R. Khani, R. Sheykhi, G. Bagherzade, An environmentally friendly method based on micro-cloud point extraction for determination of trace amount of quercetin in food and fruit juice samples. Food Chem. 293, 220–225 (2019)
Z. Zhou, C. Gu, C. Chen, P. Zhao, Y. Xie, J. Fei, An ultrasensitive electrochemical sensor for quercetin based on 1-pyrenebutyrate functionalized reduced oxide graphene/mercapto-β-cyclodextrin/Au nanoparticles composite film. Sens. Actuators B 288, 88–95 (2019)
P. Zhao, M. Ni, Y. Xu, C. Wang, C. Chen, X. Zhang, C. Li, Y. Xie, J. Fei, A novel ultrasensitive electrochemical quercetin sensor based on MoS2-carbon nanotube@ graphene oxide nanoribbons/HS-cyclodextrin/graphene quantum dots composite film. Sens. Actuators B 299, 126997 (2019)
S. Kumar, V. Lather, D. Pandita, Stability indicating simplified HPLC method for simultaneous analysis of resveratrol and quercetin in nanoparticles and human plasma. Food Chem. 197, 959–964 (2016)
R. Ravichandran, M. Rajendran, D. Devapiriam, Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 146, 472–478 (2014)
Y. Sun, T. Guo, Y. Sui, F. Li, Quantitative determination of rutin, quercetin, and adenosine in Flos Carthami by capillary electrophoresis. J. Sep. Sci. 26(12-13), 1203–1206 (2003)
C. Wang, Y. Zuo, Ultrasound-assisted hydrolysis and gas chromatography–mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 128(2), 562–568 (2011)
J.B. Raoof, R. Ojani, M. Amiri-Aref, M. Baghayeri, Electrodeposition of quercetin at a multi-walled carbon nanotubes modified glassy carbon electrode as a novel and efficient voltammetric sensor for simultaneous determination of levodopa, uric acid and tyramine. Sens. Actuators B 166, 508–518 (2012)
M. Ghanei-Motlagh, M. Baghayeri, Determination of trace Tl (I) by differential pulse anodic stripping voltammetry using a novel modified carbon paste electrode. J. Electrochem. Soc. 167(6), 066508 (2020)
M. Nodehi, M. Baghayeri, R. Ansari, H. Veisi, Electrochemical quantification of 17α-ethinylestradiol in biological samples using a Au/Fe3O4@ TA/MWNT/GCE sensor. Mater. Chem. Phys. 244, 122687 (2020)
M. Nodehi, M. Baghayeri, R. Behazin, H. Veisi, Electrochemical aptasensor of bisphenol A constructed based on 3D mesoporous structural SBA-15-Met with a thin layer of gold nanoparticles. Microchem. J. 162, 105825 (2021)
M. Ghanei-Motlagh, M.A. Taher, M. Fayazi, M. Baghayeri, A. Hosseinifar, Non-enzymatic amperometric sensing of hydrogen peroxide based on vanadium pentoxide nanostructures. Journal of The Electrochemical Society 166(6), B367 (2019)
M. Baghayeri, R. Ansari, M. Nodehi, I. Razavipanah, H. Veisi, Label-free electrochemical bisphenol A aptasensor based on designing and fabrication of a magnetic gold nanocomposite. Electroanalysis 30(9), 2160–2166 (2018)
M. Baghayeri, R. Ansari, M. Nodehi, I. Razavipanah, H. Veisi, Voltammetric aptasensor for bisphenol A based on the use of a MWCNT/Fe3O4@ gold nanocomposite. Microchim. Acta 185(7), 1–9 (2018)
M. Baghayeri, H. Beitollahi, A. Akbari, S. Farhadi, Highly sensitive nanostructured electrochemical sensor based on carbon nanotubes-Pt nanoparticles paste electrode for simultaneous determination of levodopa and tyramine. Russ. J. Electrochem. 54(3), 292–301 (2018)
M. Baghayeri, A. Sedrpoushan, A. Mohammadi, M. Heidari, A non-enzymatic glucose sensor based on NiO nanoparticles/functionalized SBA 15/MWCNT-modified carbon paste electrode. Ionics 23(6), 1553–1562 (2017)
N.H. Khand, I.M. Palabiyik, J.A. Buledi, S. Ameen, A.F. Memon, T. Ghumro, A.R. Solangi, Functional Co3O4 nanostructure-based electrochemical sensor for direct determination of ascorbic acid in pharmaceutical samples. J. Nanostruct. Chem. (2021). https://doi.org/10.1007/s40097-020-00380-8
S. Kiranmai, Y.V.M. Reddy, M. Venu, C. Madhuri, K. Anitha, G. Madhavi, A.V. Reddy, Determination of Terazosin by using poly (Congo red) modified carbon paste electrode. Anal. Bioanal. Electrochem. 9(2), 154–163 (2017)
H. Karimi-Maleh, M. Alizadeh, Y. Orooji, F. Karimi, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V.K. Gupta, Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: a docking/experimental investigation. Ind. Eng. Chem. Res. 60(2), 816–823 (2021)
M. Khadem, F. Faridbod, P. Norouzi, A. Rahimi Foroushani, M.R. Ganjali, S.J. Shahtaheri, R. Yarahmadi, Modification of carbon paste electrode based on molecularly imprinted polymer for electrochemical determination of diazinon in biological and environmental samples. Electroanalysis 29(3), 708–715 (2017)
H. Karimi-Maleh, F. Karimi, Y. Orooji, G. Mansouri, A. Razmjou, A. Aygun, F. Sen, A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 10(1), 11699 (2020)
H. Karimi-Maleh, K. Cellat, K. Arıkan, A. Savk, F. Karimi, F. Şen, Palladium–nickel nanoparticles decorated on functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 250, 123042 (2020)
J.A. Buledi, A.M. Zia-ul-Hassan Shah, A.R. Solangi, Current perspective and developments in electrochemical sensors modified with nanomaterials for environmental and pharmaceutical analysis. Curr. Anal. Chem. 17, 1–4 (2021)
F.A. Harraz, M. Faisal, A. Al-Salami, A.M. El-Toni, A. Almadiy, S. Al-Sayari, M. Al-Assiri, Silver nanoparticles decorated stain-etched mesoporous silicon for sensitive, selective detection of ascorbic acid. Mater. Lett. 234, 96–100 (2019)
K. Saksena, A. Shrivastava, R. Kant, Chiral analysis of ascorbic acid in bovine serum using ultrathin molecular imprinted polyaniline/graphite electrode. J. Electroanal. Chem. 795, 103–109 (2017)
X. Wu, Y. Xing, D. Pierce, J.X. Zhao, One-pot synthesis of reduced graphene oxide/metal (oxide) composites. ACS Appl. Mater. Interfaces 9(43), 37962–37971 (2017)
Y. Hei, X. Li, X. Zhou, J. Liu, M. Hassan, S. Zhang, Y. Yang, X. Bo, H.-L. Wang, M. Zhou, Cost-effective synthesis of three-dimensional nitrogen-doped nanostructured carbons with hierarchical architectures from the biomass of sea-tangle for the amperometric determination of ascorbic acid. Anal. Chim. Acta 1029, 15–23 (2018)
M.R. Ganjali, H. Salimi, S. Tajik, H. Beitollahi, M. Rezapour, B. Larijani, Application of Fe3O4@ SiO2/MWCNT film on glassy carbon electrode for the sensitive electroanalysis of levodopa. Int. J. Electrochem. Sci. 12, 5243–5253 (2017)
M.R. Ganjali, H. Beitollahi, R. Zaimbashi, S. Tajik, M. Rezapour, B. Larijani, Voltammetric determination of dopamine using glassy carbon electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 13(3), 2519–2529 (2018)
H. Baksh, J.A. Buledi, N.H. Khand, A.R. Solangi, A. Mallah, S.T. Sherazi, M.I. Abro, Ultra-selective determination of carbofuran by electrochemical sensor based on nickel oxide nanoparticles stabilized by ionic liquid. Monatsh. Chem. 151(11), 1689–1696 (2020)
J.A. Buledi, S. Ameen, N.H. Khand, A.R. Solangi, I.H. Taqvi, M.H. Agheem, Z. Wajdan, CuO nanostructures based electrochemical sensor for simultaneous determination of hydroquinone and ascorbic acid. Electroanalysis 32(7), 1600–1607 (2020)
S. Tajyani, A. Babaei, A new sensing platform based on magnetic Fe3O4@ NiO core/shell nanoparticles modified carbon paste electrode for simultaneous voltammetric determination of Quercetin and Tryptophan. J. Electroanal. Chem. 808, 50–58 (2018)
A.E. Vilian, P. Puthiaraj, C.H. Kwak, S.R. Choe, Y.S. Huh, W.-S. Ahn, Y.-K. Han, Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim. Acta 216, 181–187 (2016)
M.L. Yola, V.K. Gupta, T. Eren, A.E. Şen, N. Atar, A novel electro analytical nanosensor based on graphene oxide/silver nanoparticles for simultaneous determination of quercetin and morin. Electrochim. Acta 120, 204–211 (2014)
S. Vladimirova, V. Krivetskiy, M. Rumyantseva, A. Gaskov, N. Mordvinova, O. Lebedev, M. Martyshov, P. Forsh, Co3O4 as p-type material for CO sensing in humid air. Sensors 17(10), 2216 (2017)
A. Numan, M.M. Shahid, F.S. Omar, K. Ramesh, S. Ramesh, Facile fabrication of cobalt oxide nanograin-decorated reduced graphene oxide composite as ultrasensitive platform for dopamine detection. Sens. Actuators B 238, 1043–1051 (2017)
S.A. Memon, D. Hassan, J.A. Buledi, A.R. Solangi, S.Q. Memon, I.M. Palabiyik, Plant material protected cobalt oxide nanoparticles: sensitive electro-catalyst for tramadol detection. Microchem. J. 159, 105480 (2020)
G. Wang, F. Zhu, J. Xia, L. Wang, Y. Meng, Y. Zhang, Preparation of Co3O4/carbon derived from ionic liquid and its application in lithium-ion batteries. Electrochim. Acta 257, 138–145 (2017)
Z. Song, Y. Zhang, W. Liu, S. Zhang, G. Liu, H. Chen, J. Qiu, Hydrothermal synthesis and electrochemical performance of Co3O4/reduced graphene oxide nanosheet composites for supercapacitors. Electrochim. Acta 112, 120–126 (2013)
R.K. Das, A.K. Golder, Co3O4 spinel nanoparticles decorated graphite electrode: Bio-mediated synthesis and electrochemical H2O2 sensing. Electrochim. Acta 251, 415–426 (2017)
M. Antonietti, D. Kuang, B. Smarsly, Y. Zhou, Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew. Chem. Int. Ed. 43(38), 4988–4992 (2004)
R. Sheldon, Catalytic reactions in ionic liquids. Chem. Commun. 23, 2399–2407 (2001)
J. Fuller, R.T. Carlin, R.A. Osteryoung, The room temperature ionic liquid 1-ethyl‐3‐methylimidazolium tetrafluoroborate: electrochemical couples and physical properties. J. Electrochem. Soc. 144(11), 3881 (1997)
W. Zheng, X. Liu, Z. Yan, L. Zhu, Ionic liquid-assisted synthesis of large-scale TiO2 nanoparticles with controllable phase by hydrolysis of TiCl4. ACS Nano 3(1), 115–122 (2009)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis (Wiley, New York, 2008).
J. Shen, B. Yan, M. Shi, H. Ma, N. Li, M. Ye, One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 21(10), 3415–3421 (2011)
V. Vinothkumar, A. Sangili, S.M. Chen, P. Veerakumar, K.-C. Lin, Sr-doped NiO3 nanorods synthesized by simple sonochemical method as excellent materials for voltammetric determination of quercetin. New J. Chem. 44, 2821–2832 (2020)
W. Zhang, L. Zong, G. Geng, Y. Li, Y. Zhang, Enhancing determination of quercetin in honey samples through electrochemical sensors based on highly porous polypyrrole coupled with nanohybrid modified GCE. Sens. Actuators B 257, 1099–1109 (2018)
B. Xu, L. Yang, F. Zhao, B. Zeng, A novel electrochemical quercetin sensor based on Pd/MoS2-ionic liquid functionalized ordered mesoporous carbon. Electrochim. Acta 247, 657–665 (2017)
Funding
The authors are highly thankful to the Higher Education Commission of Pakistan for providing funds under the project “6714/Sindh/NRPU/R&D/HEC/HEC/2015”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khand, N.H., Solangi, A.R., Ameen, S. et al. A new electrochemical method for the detection of quercetin in onion, honey and green tea using Co3O4 modified GCE. Food Measure 15, 3720–3730 (2021). https://doi.org/10.1007/s11694-021-00956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00956-0