Abstract
The aim of the present study was to investigate the effects of microwave-assisted extraction parameters on total phenolic content (TPC), total flavonoid content (TFC), condensed tannins content (CTC), DPPH-scavenging activity, and colour attributes of aqueous green tea extracts and to optimise the microwaving conditions using response surface methodology. Microwave power (120–360–600 W) and irradiation time (1–3–5 min) were selected as independent variables. Statistical analysis revealed that all responses were significantly affected by extraction parameters. The optimum microwave-assisted extraction conditions were 350.65 W microwave power and 5 min irradiation time to maximise TPC, TFC, CTC, DPPH-scavenging activity, and L* values and to minimise a* and b* values of aqueous green tea extracts. The predicted TPC was 116.58 mg gallic acid equivalents/g (GAE/g), TFC was 49.33 mg catechin equivalents/g (CE/g), CTC was 9.89 mg catechin equivalents/g (CE/g), DPPH-scavenging activity (IC50) was 294.46 µg/mL, L* value was 12.31, a* value was 2.61 and b* value was 7.02 under optimum microwave-assisted extraction conditions. This study revealed that microwave extraction conditions should be controlled to obtain aqueous green tea extracts with both high bioactivity and acceptable colour quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green tea, a non-fermented tea type, is basically composed of leaf and gemma of the plant Camellia sinensis (L.) O. Kuntze. Dry green tea includes about 36% polyphenols, 25% carbohydrates, 15% protein, 6.5% lignin, 4% amino acids, 3.5% methyl xanthines, 2% lipids and the rest are organic acid, carotenoids and volatiles [1].
Green tea is rich in three main bioactive compounds which have impact on human health; xanthic bases (caffeine and theophylline), polyphenolic compounds and essential oils [2]. It is an excellent source of caffeine (3–6%) and polyphenols (25–40%) particularly flavanones, flavonols, flavanols, and phenolic acids [3]. More than 80% of green tea polyphenols are catechin-type flavanols. These catechins are the main contributors of excellent antioxidant activity of green tea. (−)-epigallocatechingallate (EGCG) is the most abundant catechin in green tea, which is other common ones such as (−)-epigallocatechin (EGC), (−)-epicatechingallate (ECG), (−)-epicatechin (EC) [1, 2, 4]. Consumption of green tea has been linked to reduced risk of chronic or cardiovascular diseases, well-controlled body weight, and improved oral and bone health [5]. Green tea also possesses antihypertensive, antioxidative, hypolipidemic properties as well as anticarcinogenic, antitumorigenic, antiarteriosclerotic and antimicrobial effects. These health benefits of green tea are mostly associated with polyphenolic compounds consisting mainly of flavonols and catechins, including also their gallate derivatives [2, 6, 7].
The selection of adequate method for extraction of phenolics and other antioxidative compounds is crucial. The extraction of polyphenolic compounds in green tea was previously studied by different techniques such as solvent [8], ultrasonic [9], carbon dioxide [10], high hydrostatic pressure [11], microwave-assisted [12,13,14] and far-infrared [15] extractions. In comparison to conventional methods, microwave-assisted extraction is more efficient technique with enhanced product integrity, well-preserved volatiles, improved bioactive recovery and reduced environmental burden, process time, energy and cost. In this method, sample exposed to microwaves is heated up by duo mechanism involving ionic conduction of electromagnetic waves and dipole rotation [16, 17].
Previous studies focused on microwave-assisted extraction of green tea bioactive compounds with different solvents such as ethanol and/or ethanol–water mixture, citric acid–water mixture [13, 18,19,20,21]. Until now, few studies have been carried out to determine optimum microwave-assisted extraction conditions for maximization of green tea phenolics extraction by water [7, 22, 23]. To the best of our knowledge, this research will be the first study which deals with the colour quality of aqueous green tea extracts as well as TPC, TFC, CTC and antioxidative characteristics. The objectives of the present study are (a) to investigate the effects of extraction conditions (microwave power and irradiation time) on TPC, TFC, CTC, DPPH-scavenging activity and colour characteristics of aqueous green tea extracts, and (b) to determine optimum microwave-assisted extraction conditions to obtain extracts with maximum TPC, TFC, CTC yield and antioxidative activity, and minimum colour alterations.
Material and methods
Materials
A single lot of commercially available green tea bags were purchased from local market. Distilled water was used for extractions. Folin-Ciocalteu’s reagent, AlCl3, NaNO2, and methanol were purchased from Merck. Gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) reagent and Na2CO3 were purchased from Fluka. (+)-catechin hydrate and vanillin were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals used were of analytical reagent grade.
Methods
Microwave-assisted extraction
Each green tea bag (1.5 g) was placed in 200 mL distilled water in a 250 mL glass beaker and located in a household microwave oven (1200 W max power, 2450 MHz frequency; Beko-1505, Turkey). Then, beakers were irradiated at 3 different powers (120–360–600 W) for three different lengths of irradiation time (1–3–5 min). After irradiation, each teabag was removed from the brewed infusion and squeezed. Irradiated infusions were cooled to room temperature before analysing for their TPC, TFC, CTC, DPPH-scavenging activity, and colour parameters.
Total phenolic content (TPC)
TPC was determined using Folin-Ciocalteu assay previously developed by Singleton and Rossi [24] and modified by Li et al. [25]. 2.5 mL of Folin-Ciocalteu solution (10%, v/v) was added onto 100 µL of irradiated extracts and final mixture vortexed for 15 s, then allowed to stand for 5 min at room temperature in the dark. 5 mL of sodium carbonate solution (7.5%, w/v) was added. Next, mixture was incubated in the dark at room temperature for 60 min. Finally, the absorbance was read at 760 nm by spectrophotometer (Shimadzu UV-1601, Japan) against blank. TPC was calculated using linear regression equation obtained from the gallic acid standard curve (r2 = 0.9844). Results were expressed as mg gallic acid equivalents (GAE) per gram of sample.
Total flavonoid content (TFC)
TFC of green tea extracts was determined spectrophotometrically according to the method suggested by Castiglioni et al. [26]. Briefly, 50 µL of tea extract was mixed with 1.35 mL of distilled water. Then, 50 µL of 5% NaNO2 and 50 µL of 10% AlCl3 was added, respectively and shaken. Mixtures were incubated for 10 min in the dark and then absorbance was read at 415 nm. Results were expressed as mg catechin equivalents per gram of sample (mg CE/g) using a (+)-catechin calibration curve (r2 = 0.9991).
Condensed tannin content (CTC)
CTC in aqueous tea extracts was determined according to vanillin/HCl method described by Broadhurst and Jones [27]. Briefly, 0.5 mL of aqueous extract was weighed into aluminium foil-covered tube. Then, 3 mL of vanillin/methanol solution (4%, w/v) was added. After shaking, 1.5 mL of concentrated HCl was added and mixture was vortexed. Following 15-min incubation at room temperature in the dark, absorbance was measured at 500 nm. Results were expressed as mg catechin equivalents per gram of sample (mg CE/g) using a (+)-catechin calibration curve (r2 = 0.9956).
DPPH-radical scavenging activity
The antioxidant activity of the extracts was determined by DPPH-radical scavenging method [28, 29] with slight modifications. 3.8 mL of diluted DPPH solution was added onto 200 µL sample containing different concentrations of extract. Next, the mixture was vortexed for 15 s. Then, it was incubated in the dark at room temperature for 60 min. Absorbance was read at 515 nm against blank and DPPH-radical scavenging activity was calculated using Eq. 1.
The sample concentration required to scavenge DPPH-radical by 50% (IC50 value) was calculated by using linear regression equation obtained from the graph plotting inhibition percentage against sample concentration [30]. The DPPH-radical scavenging activity results were expressed as IC50 values (µg extract/mL).
Colour analysis
The International Commission on Illumination (CIE) colour parameters L*, a*, b* of irradiated extracts were measured with a colorimeter (Minolta CR5, Japan). Corresponding L* value (100 = lightness, 0 = darkness), a* value (+ a = redness, − a = greenness) and b* value (+ b = yellowness, − b = blueness) were measured for all extracts.
Experimental design and statistical analysis
In the present study, face-centered central composite design (CCD) was employed to determine the best combination of process parameters for optimisation of microwave-assisted aqueous extraction conditions of green tea. Independent variables were microwave power (120–360–600 W) (X1) and time (1–3–5 min) (X2), while TPC, TFC, CTC, IC50, L*, a* and b* values were selected as dependent variables. Table 1 shows the face-centered CCD consisting of 13 experimental runs with five center points. The results of the analyses were fitted using linear, 2FI, reduced quadratic, and quadratic models. The adequacy of the proposed model for each response was checked by statistical analysis. It is suggested the models to have low standard deviation, prediction error sum of squares (PRESS), and high predicted and adjusted coefficient of determination (R2) values [31]. Experimental data was analysed using Design-Expert software (Version 11, Stat-Ease Inc., Minneapolis, MN, USA). The fitted model should have p value lower than 0.05, insignificant lack of fit (p > 0.05), adequate precision value larger than 4, and (adjusted R2-predicted R2) < 0.2 [32]. Desirability function was used for optimisation. Predictable responses without target like maximising or minimising were not considered throughout optimisation process.
Results and discussion
Fitting the model using response surface methodology
The experimental and predicted values obtained for TPC, DPPH-scavenging activity, L*, a*, and b* were shown in Table 2. Microwave-assisted extraction conditions were optimised using response surface methodology. After removal of insignificant terms (p > 0.05), the obtained linear, 2FI, and reduced quadratic equations in terms of coded values for TPC, TFC, CTC, IC50, L*, a*, and b* were given in Eqs. 2, 3, 4, 5, 6, 7 and 8, respectively.
The results of the analysis of variance (ANOVA) for the fitted models were summarized in Table 3. The regression models with insignificant (p > 0.05) lack-of-fit values for all responses were highly significant (p < 0.01). The high R2 values ranging from 0.8608 to 0.9865 indicated that 86.08–98.65% of the variability in the responses could be explained by the fitted models. Coefficients of variation (C.V.) lower than 10% show that the experimental data obtained through each run were around the mean value of each response [33]. Models with higher Adeq-Precision values than 4 is reliable for prediction [34]. The ANOVA results confirmed that each model was satisfactory for prediction of each response.
Effects of independent variables on TPC of aqueous green tea extracts
Phenolic content of green tea is mainly comprised of flavanols such as epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin gallate (EGCG) [35]. TPC of irradiated green tea extracts ranged from 96.23 to 117.84 mg GAE/g (Table 2). The lowest TPC was obtained in the extract irradiated at 120 W for 1 min, while the highest TPC was detected at 360 W for 5 min treatment.
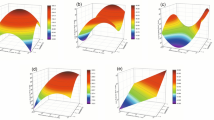
The single factor analysis revealed that microwave power drastically affected TPC of aqueous green tea extracts (Online Resource, Fig. S1a). TPC of the extracts increased with the increase of microwave power from 120 W to around 440 W. Further increase of microwave power led to a steady decrease. The remarkable quadratic effect of microwave power on TPC was also confirmed by the highest coefficient of this term in the Eq. 2. On the other side, at a fixed microwave power, irradiation time shorter than 4 min did not greatly influence TPC of the extracts. In general, a considerable increase in TPC occurred after a 4-min microwave application (Online Resource, Fig. S1b). Both linear and quadratic terms of irradiation time and linear term of microwave power had significant (p < 0.05) positive effects on TPC of the aqueous green tea extracts, while quadratic term of microwave power significantly (p < 0.01) influenced in a negative way. Response surface and contour plots (Fig. 1a) show that application of microwave power lower than 440 W at a constant irradiation time promoted release of phenolic compounds by means of cell wall matrix disruption and increased solubility. However, microwave power higher than 440 W led to degradation of green tea phenolics due to superheating effect of water as a result of its high dielectric constant and lower dissipation factor as previously suggested by Proestos and Komaitis [36]. Hence, the use of moderate microwave power-short irradiation time combination was recommended for extraction of grape seed and skin phenolics without deterioration of heat-sensitive ones [37]. The underlying mechanism of microwave-assisted extraction of bioactives is the increased extraction of these components by means of high temperatures. High microwave power means more electromagnetic energy is transferred to extraction system faster [38]. Therefore, higher microwave power may increase the temperature of extraction solvent in a very short time compared to application of lower microwave power for longer time. Based on our findings, we can deduce that increase of microwave power could lead to more remarkable changes in extraction temperature than that of caused by irradiation time. Some changes in chemical structures of phenolics are induced by heat treatment. Thermal processing of tea catechins may lead to epimerization and hydrolysis of these compounds. Furthermore, long-term heating may degrade phenolics of green tea through oxidation/condensation reactions [39]. Similarly, Chen et al. [40] reported that catechins were degraded by 20% as a result of heating of aqueous green tea catechin solution at 98 °C for 7 h, while heating at 37 °C for 7 h did not cause a remarkable change. Hence, extraction temperature is considered an essential parameter to be controlled for maximizing phenolic extraction by some researchers [41,42,43].
Effects of independent variables on TFC of aqueous green tea extracts
Flavonoids, mainly flavan-3-ols (catechins) derivatives, are the dominant polyphenolic compounds in green tea [44, 45]. A strong correlation (r = 0.80) was found between TFC and TPC in our study as previously reported by Ho et al. [46]. TFC of irradiated green tea extracts varied from 11.71 to 71.18 mg CE/g (Table 2). The lowest TFC was obtained in the extract irradiated at 120 W for 1 min, while aqueous extract that was subjected to 600 W for 5 min had the highest TFC.
The linear terms of both microwave power and irradiation time, and interaction between these two independent variables had significant (p < 0.0001) effects on TFC of aqueous green tea extracts (Table 3). As can be seen in Eq. 3, closer coefficient estimates of linear terms suggest almost equally positive contribution of microwave power and irradiation time on extraction of green tea flavonoids. At a fixed irradiation time, TFC extraction yield consistently increased as the microwave power increased from 120 to 600 W (Online Resource, Fig. S1c). Similarly, increase of irradiation time promoted the release of flavonoids (Online Resource, Fig. S1d). The positive effects of both microwave power and irradiation time on extraction of total flavonoids can be also seen from response surface and contour plots (Fig. 1b). Microwave application may affect the extraction yield of flavonoids in the same way as polyphenolics. However, differently from TPC, TFC of aqueous green tea extracts increased under all experimental conditions used in our study. This difference may be attributed to epimerization of catechins, dominant flavonoids of green tea, rather than degradation reactions at high temperatures [47, 48]. Due to degradation of phenolics other than flavonoids under excessive microwave exposure, TPC of aqueous green tea extracts started to decrease after a certain point. Here, conversion of catechins to epi- or non-epi structures may maintain TFC of the extracts to a great extent. A similar phenomenon in green tea infusions was previously suggested by Jin et al. [49]. Improved release of flavonoid compounds from cell walls as a result of high temperatures may be the responsible of this steady increase depending on microwave application at higher power for longer time. Higher release rate of total flavonoids in green tea infusions at high temperature was also confirmed by Kılıç et al. [50]. Our findings indicate that selected microwave power levels (120–360–600 W) and length of irradiation time (1–3–5 min) did not trigger degradation reactions of green tea flavonoids. Therefore, microwave-assisted extraction under these conditions can be considered a suitable way for promotion of aqueous extraction of green tea flavonoids. Similarly, Xiao et al. [38] reported increased flavonoid yields in aqueous ethanolic (90%) extracts of Radix astragali when power was raised from 200 to 1000 W for short-term microwave applications (5–10 min). Moreover, our results are in accordance with the findings of Shao et al. [51], who obtained aqueous extracts of Perilla frutescens leaves with improved flavonoid contents when microwave-assisted extraction was carried out up to 600 W for 10 or 20 min.
Effects of independent variables on CTC of aqueous green tea extracts
CTs (non-hydrolysable tannins) are olygomers or polymerised forms of flavonoids and also known as proanthocyanidins or catechin tannins [52, 53]. They are beneficial for human health due to their antioxidant, cardioprotective, neuroprotective, anti-obesity, antidiabetic, anticancer, anti-inflammatory and antimicrobial activities [54, 55]. CTC of aqueous green tea extracts varied between 2.25 mg CE/g and 11.08 mg CE/g (Table 2). The extract exposed to 120 W for 1 min had the lowest CT yield, while that of subjected to microwave application at 600 W for 5 min had the highest CTC. Our results are accordance with the findings of Ramirez-Aristizabal et al. [56], who reported higher CTC in hot green tea infusions (5.43–8.41 mg CE/g) than those of cold green tea infusions (2.67–7.08 mg CE/g).
One-factor graph of microwave power shows its dominant linear effect along with a slight negative influence of its quadratic term (Online Resource, Fig. S2a). The significant (p < 0.0001) positive effect of linear term of microwave power was verified by the highest coefficient estimate in Eq. 4, whereas its significant (p < 0.05) quadratic term had the lowest coefficient estimate. CT extraction rate increased sharply up to about 400 W. Then, the increase became slighter depending on the increase of microwave power. This finding indicates that use of microwave power higher than 600 W may cause degradation of green tea CTs. Unlike microwave power, irradiation time caused a linear increase in this parameter for all microwave power levels used in this study (Online Resource, Fig. S2b). This phenomenon was also confirmed by significant (p < 0.0001) linear term of irradiation time in Eq. 4. Figure 2a depicts the interaction between microwave power and irradiation time on the extraction yield of CT. Synergistic effects of heat and mass transfer mechanisms that are working in the same direction lead to more efficient and faster extraction process by using microwaves [57]. As explained in TPC and TFC, microwave application heats both in situ water and extraction solvent. Excess rise of water vapour pressure in plant cell promotes cell disruption. Then, extraction solvent with lower viscosity due to high temperature can easily penetrate into cell and dissolve the target compound [58]. In our study, main determinant of microwave-assisted aqueous extraction is temperature. Both microwave power and irradiation time provide better extraction yield by increasing extraction temperature. However, the significance of quadratic term of microwave power in a negative way (Table 3) suggests that microwave power leads to more considerable increase in extraction temperature compared to irradiation time. Therefore, application of microwave energy at extreme power level may trigger thermal deterioration of CTs in green tea. Similarly, Rhazi et al. [59] observed an increase in CTC of aqueous extracts of Moroccan Acacia mollissima barks when microwave power raised from 150 to 300 W. However, they reported a decrease in CT yield due to liquefaction of CTs when microwave powers higher than 450 W were applied [59]. In conclusion, application of 600 W up to 5 min promoted extraction yield of green tea CTs in this study. Nonetheless, it should be kept in mind that optimisation of irradiation time may be required if microwave powers higher than 600 W are used to extract CTs.
Effects of independent variables on IC50 values of aqueous green tea extracts
Certain oxidants including DPPH, ABTS and ferric ions are readily degraded by plant-based antioxidants, particularly polyphenolics [60]. The main contributors of antioxidative activity are epigallocatechin gallate (EGCG), epicatechin gallate (ECG), epicatechin (EC), epigallo catechin (EGC), catechin, and gallic acid in Camellia sinensis [61]. The lower IC50 values obtained through DPPH assay indicate the higher DPPH-radical inhibition ability, in other words higher antioxidant capacity, of material. Therefore, as expected, a strong negative correlation between TPC and IC50 values of the extracts was detected (r = − 0.819) in the present study. The IC50 values of microwave-assisted aqueous extracts of green tea ranged from 292.96 µg/mL to 305.78 µg/mL (Table 2).
The change in IC50 values of aqueous extracts as a function of microwave power exhibited a parabolic curve (Online Resource, Fig. S2c). On the other side, irradiation time linearly contributed to the decrease of IC50 values at all microwave power levels used in this study (Online Resource, Fig. S2d). The interactive effects of independent variables on IC50 values were shown in Fig. 2b. IC50 values of the extracts remarkably decreased as the microwave power increased up to 440 and 360 W for 1 and 3-min treatments, respectively. On the other hand, in 5-min treatments, as the microwave power increased, the decrease rate of IC50 values became slighter in particular at microwave powers higher than 280 W. This may be attributed to the sudden and extreme rise of aqueous extracts’ temperatures due to longer microwave irradiation times. Heating of extraction solvent, here water, may damage the heat-sensitive bioactive constituents of green tea that have DPPH-scavenging ability. Hence, increase of microwave power at longer irradiation times led to a modest decrease IC50 values of the extracts. A similar trend in IC50 values was recently reported by by Azimi Mahalleh et al. [62], who investigated the effects of ultrasonic-assisted extraction conditions on some functional characteristics of Nepeta binaludensis.
Terms with positive coefficient have synergistic, while those with negative coefficient have antagonistic effect on response [63]. Linear terms of both independent variables had significant (p < 0.01) negative effects, whereas quadratic term of microwave power and interaction between these two independent variables had significant (p < 0.05) positive impact on IC50 values (Eq. 5). In the presence of water in microwave-assisted extraction, superheating occurs due to very high dielectric constant of water. Hence, temperature within the sample originating from in situ water extremely increases and this phenomenon promotes disruption of cell walls and tissues [57]. Correspondingly, microscopic observations obtained in another study [64] confirmed that microwave treatment led to breakage in mesocarp and exocarp layers of the cells due to absorption of microwave energy by the water found in cells. Moreover, high temperatures generated during microwave treatment may stimulate the release of bioactives by boosting solubility of them and reducing viscosity of solvent [65]. On the other hand, epimerization and accordingly degradation of green tea phenolics may occur at temperatures above 80 °C as previously suggested by Vuong et al. [23]. Conversion of epi-isomers to their non-epi-counterparts having lower anti-radical activities eventually decreases antioxidative capacity of tea extracts [66, 67]. Consequently, higher microwave power till a certain extent and longer irriadiation time improved antioxidant activities of aqueous green tea extracts by the mechanisms discussed above. Accordingly, due to better antioxidant activities of these extracts, lower amounts of extracts (IC50) were required to inhibit 50% of DPPH-radical. Similar results were previously obtained in studies, where antioxidant activity against DPPH-radical increased by increasing microwave power and irradiation time for Kinnow fruit (Citrus reticulata Blanco cv Kinnow) peel extracts [68] and grape seed extracts [69].
Effects of independent variables on colour attributes of aqueous green tea extracts
The colour of green tea infusions is determined by presence and amounts of chlorophyll, pheophytin, neoxanthin, lutein, β-carotene, and some flavonols such as quercetin and kaempferol [45, 70]. On the other side, while catechins, the dominant phenolics of green tea, are colourless, their degradation products originating from epimerization and oxidation reactions impair the colour quality of green tea infusions [4]. CIE L*, a*, and b* values of aqueous green tea extracts ranged between 11.06 and 14.10, 1.53–3.55, and 6.48–8.29, respectively (Table 2). The L* values of extracts decreased, whereas a* and b* values increased with the increase of both microwave power and irradiation time (Online Resource, Fig. S3). These results suggest that the extracts became darker accompanied with more intense red and yellow colour formation due to microwave treatment.
L* value
The linear terms of microwave power and irradiation time, interaction between these two independent factors, and quadratic term of irradiation time exhibited significant (p < 0.05) negative effect on L* values of aqueous green tea extracts. At a fixed irradiation time, L* value (lightness) gradually decreased as the microwave power increased (Online Resource, Fig. S3a). On the other side, the decline in L* value exhibited a parabolic curve as a function of irradiation time. After a 2-min microwave application, reduction in L* value became apparent (Online Resource, Fig. S3b). Figure 3a illustrates the response surface graph and contour plot of interactive effects microwave power and irradiation time on L* values. For short irradiation times, slight changes in L* values occurred depending on the increase of microwave power. However, considerable differences in L* values were observed between the extracts exposed to different microwave powers for longer irradiation times (5 min). Decrease of L* value as a result of microwave application may be attributed to formation of catechin oxidation products (theaflavins, theabrownins, thearubigins) at high extraction temperatures [4]. Additionally, catechin oligomers, mainly dehydrodicatechins A, originating from non-enzymatic oligomerization of epi-type catechins and catechin may be responsible for the browning of aqueous green tea extracts [39]. In an earlier research, it was reported that browning of green tea infusions due to longer brewing times led to lower colour scores assigned by sensory panellists [71]. Hence, in our study, colour of aqueous green tea extract is considered an essential parameter to be controlled for consumer acceptability.
a* value
As given in Eq. 7, microwave power is the predominant factor (p < 0.0001) followed by irradiation time (p < 0.0001) and interaction of microwave power × irradiation time (p < 0.01) affecting a* value positively. However, quadratic term of irradiation time (p < 0.05) negatively influenced a* value. a* values of the aqueous green tea extracts increased linearly as microwave power increased at a fixed irradiation time (5 min) (Online Resource, Fig. S3c). On the other hand, for a fixed microwave power (600 W), microwave application longer than 3 min led to slight increase in rising of a* values (Online Resource, Fig. S3d). The lowest and highest a* values were obtained in the extracts exposed to 120 W–1 min and 600 W–5 min microwave applications, respectively. These results were also confirmed by 3D surface graph and contour plot (Fig. 3b). Increase of a* values depending on microwave exposure suggests that aqueous green tea extracts had less green and more intense red colour. Accordingly, previous researchers recorded increased a* values for green tea infusions subjected to heat treatment [72,73,74]. Increase of a* values may be mainly explained by oxidation of green tea flavanols induced by high temperature generated during microwave application. Especially oxidation of EGCG leads to dramatic alterations in colour [75]. Autoxidation products of EGCG are EGCG quinones, EGCG dimers such as theasinensin A, EGCG dimer quinones, and other dimers like P2, respectively [76]. Moreover, thermal degradation of chlorophyll, a water-insoluble green pigment, may contribute to this increase to a certain extent as its presence in minor amounts in hot water green tea infusion was earlier confirmed by Wang et al. [45]. However, it should be kept in mind that lipophilic pigments such as chlorophyll, neoxanthin, and pheophytin a have limited effect on colour of green tea infusions because their concentrations are extremely low in aqueous infusions [70]. Recently, Donlao and Ogawa [77] correlated the colour changes occurred in green tea infusions with thermal degradation of quercetin, a flavonol with bright green-yellow colour, whereas Dai et al. [78] ascribed this to the formation of coloured complexes as a result of EGCG and green tea flavonols interaction. As it is seen, browning mechanism of green tea infusions/extracts is dominated by various components and interactions formed between them.
b* value
As seen in Eq. 8, b* values of aqueous green tea extracts were significantly (p < 0.01) influenced by only linear terms of microwave power and irradiation time. As microwave power or irradiation time increased, b* values also increased. This linear increase was verified by one-factor graphs (Online Resource, Fig. S3e-f). Similarly, Fig. 3c illustrates that the highest b* value (8.29) was obtained in the green tea extracts exposed to highest microwave power (600 W) for longest irradiation time (5 min), while lowest microwave power-shortest irradiation time combination (120 W–1 min) yielded extracts with lowest b* value (6.48). The increase of b* values indicates that microwave application resulted in more intense yellow coloured aqueous green tea extracts. As previously mentioned, and explained for L* values, the b* values of the extracts may increase due to heat-induced formation of catechin oligomers, in particular A-type dehydrodicatechins that have intense yellow colour [39]. In addition to oxidation/condensation reactions, deposition of gallic acid through epimerization of tea catechins promoted by high temperatures may be partly responsible for perception of yellow colour in the extracts [4].
In summary, microwave treatment leads to increase of extracts’ temperature and hence, promotes oxidation, condensation, and epimerization reactions of native phenolic compounds of green tea. Consequently, new products of these reactions result in darkened, less green and more red-brown coloured aqueous green tea extracts.
Optimisation
For simultaneous optimisation of multiple responses, desirability is considered a useful approach [34]. The same importance degree (+++++) was assigned to all responses to obtain optimum process conditions. TPC, TFC, and CTC yields of the extracts were maximized, while IC50 values and colour changes were minimised. In other words, we aimed to obtain bright and intense green coloured aqueous green tea extracts with high TPC, TFC, CTC and antioxidant activity by means of microwave application. Based on these criteria, eight different microwave power-irradiation time combinations were suggested by the tool as summarized in Table 4. These optimum conditions indicate that moderate microwave power application for long time is more suitable to improve water extraction of green tea phenolics, flavonoids, condensed tannins, as well as to protect colour of infusions. Optimum process conditions were as follows: 350.65 W microwave power and 5 min irradiation time, with the highest desirability value of 0.671. At this point, the investigated responses were predicted as 111.579 mg GAE/g of TPC, 49.334 mg CE/g of TFC, 9.888 mg CE/g of CT, 294.464 µg/mL of IC50 value, 13.727 L* value, 2.05 a* value and 7.09 b* value for optimised aqueous green tea extracts.
Conclusions
In this study, the optimisation of microwave-assisted extraction in green tea was confirmed with improved antioxidant and total phenolic capacities and targeted colour quality. Response surface model analysis revealed that all models were well-fitted and adequate for representing relationship between variables and responses. Extraction efficiency of bioactive compounds and colour attributes of extract was highly dependent on irradiation time and microwave power.
By using microwave extraction technique, increased antioxidative capacity, TPC, TFC, CTC and desired colour values were achieved at moderate microwave powers for long application time. This combination (moderate power-long time) is a convenient way for protection of heat sensitive components and prevention of undesirable oxidation reactions. Therefore, based on the tested functional and quality responses, it was concluded that microwave-assisted extraction can be considered a rapid and healthy sustainable alternative to conventional extraction techniques for food and medicinal industries.
Further studies should be carried out to find out effects of extraction medium temperature on functional and colour attributes of microwave-assisted aqueous green tea extracts. Moreover, temperature should be considered a response as well as other sensory characteristics such as taste, aroma, and general acceptability for further microwave-assisted aqueous green tea extraction optimisation studies.
This article does not contain any studies with human participants or animals performed by any of the authors.
References
H.N. Graham, Prev. Med. (Baltim). 21, 334 (1992)
C. Cabrera, R. Artacho, R. Giménez, J. Am. Colloid Nutr. 25, 79 (2006)
R. Assis Jacques, L. dos Santos Freitas, V. Flores Petes, C. Dariva, J.V. de Oliveira, E. Bastos, Caramão, J. Sep. Sci. 29, 2780 (2006)
N. Li, L.S. Taylor, M.G. Ferruzzi, L.J. Mauer, Food Res. Int. 53, 909 (2013)
J.M. Lorenzo, P.E.S. Munekata, Asian Pac. J. Trop. Biomed. 6, 709 (2016)
T. Sultana, G. Stecher, R. Mayer, L. Trojer, M.N. Qureshi, G. Abel, M. Popp, G.K. Bonn, J. Agric. Food Chem. 56, 3444 (2008)
D.C. Li, J.G. Jiang, Int. J. Food Sci. Nutr. 61, 837 (2010)
G. Tea, D.H.M. Bastos, L.A. Saldanha, R.R. Catharino, C. Alexandra, H.F. Sawaya, I.B.S. Cunha, P.O. Carvalho, M.N. Eberlin, Molecules 12, 423 (2007)
L.S. Lee, N. Lee, Y.H. Kim, C.H. Lee, S.P. Hong, Y.W. Jeon, Y.E. Kim, Molecules 18, 13530 (2013)
C.J. Chang, K.L. Chiu, Y.L. Chen, C.Y. Chang, Food Chem. 68, 109 (2000)
J. Xi, D. Shen, S. Zhao, B. Lu, Y. Li, R. Zhang, Int. J. Pharm. 382, 139 (2009)
Y. Huang, J. Sheng, F. Yang, Q. Hu, J. Food Eng. 78, 687 (2007)
X. Pan, G. Niu, H. Liu, Chem. Eng. Process. 42, 129 (2003)
Q.V. Vuong, S.P. Tan, C.E. Stathopoulos, P.D. Roach, J. Food Compos. Anal. 27, 95 (2012)
J.H. Park, J.M. Lee, Y.J. Cho, C.T. Kim, C.J. Kim, K.C. Nam, S.C. Lee, J. Food Biochem. 33, 149 (2009)
D. Pasrija, C. Anandharamakrishnan, Food Bioprocess. Technol. 8, 935 (2015)
E. Nkhili, V. Tomao, H. El Hajji, E.S. El Boustani, F. Chemat, O. Dangles, Phytochem. Anal. 20, 408 (2009)
B. Bekdeşer, N. Durusoy, M. Özyürek, K. Güçlü, R. Apak, J. Agric. Food Chem. 62, 11109 (2014)
X. Jun, S. Deji, L. Ye, Z. Rui, Int. J. Pharm. 408, 97 (2011)
G. Serdar, E. Demir, S. Bayrak, M. Sökmen, Int. J. Second. Metab. 3, 3 (2016)
L. Wang, P. Qin, Y. Hu, Front. Chem. Eng. China 4, 307 (2010)
V. Ghasemzadeh-mohammadi, B. Zamani, M. Afsharpour, A. Mohammadi, Food Sci. Biotechnol. 26, 1281 (2017)
Q.V. Vuong, C.E. Stathopoulos, J.B. Golding, M.H. Nguyen, P.D. Roach, J. Sep. Sci. 34, 2468 (2011)
V.L. Singleton, J.A. Rossi, Am. J. Enol. Vitic. 16, 144 (1965)
Y. Li, C. Guo, J. Yang, J. Wei, J. Xu, S. Cheng, Food Chem. 96, 254 (2006)
S. Castiglioni, E. Damiani, P. Astolfi, P. Carloni, Int. J. Food Sci. Nutr. 66, 491 (2015)
R.B. Broadhurst, W.T. Jones, J. Sci. Food Agric. 29, 788 (1978)
R.P. Singh, K.N. Chidambara, Murthy, G.K. Jayaprakasha, J. Agric. Food Chem. 50, 81 (2002)
W. Brand-Williams, M.E. Cuvelier, C. Berset, LWT – Food Sci. Technol. 28, 25 (1995)
S. Roshanak, M. Rahimmalek, S.A.H. Goli, J. Food Sci. Technol. 53, 721 (2016)
J. Cornell, Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data, 3rd edn. (Wiley, New York, 2002)
Stat-Ease, Stat-Ease, Inc (2018)
ŽR Lazić, Design of Experiments in Chemical Engineering: A Practical Guide (Wiley, Weinheim, 2004)
D.C. Montgomery, Design and Analysis of Experiments, 8th edn. (Wiley, Hoboken, 2013)
W. Vermerris, R. Nicholson, Phenolic Compound Biochemistry (Springer, Dordrecht, 2006)
C. Proestos, M. Komaitis, LWT - Food Sci. Technol. 41, 652 (2008)
S. Medouni-Adrar, L. Boulekbache-Makhlouf, Y. Cadot, L. Medouni-Haroune, F. Dahmoune, A. Makhoukhe, K. Madani, Ind. Crops Prod. 77, 123 (2015)
W. Xiao, L. Han, B. Shi, Sep. Purif. Technol. 62, 614 (2008)
F.Y. Fan, M. Shi, Y. Nie, Y. Zhao, J.H. Ye, Y.R. Liang, Food Chem. 196, 347 (2016)
Z.Y. Chen, Q.Y. Zhu, D. Tsang, Y. Huang, J. Agric. Food Chem. 49, 477 (2001)
H. Liang, Y. Liang, J. Dong, J. Lu, J. Sci. Food Agric. 87, 1748 (2007)
I. Asofiei, I. Calinescu, A. Trifan, I.G. David, A.I. Gavrila, Chem. Eng. Commun. 203, 1547 (2016)
S. Jokić, M. Cvjetko, D. Božić, S. Fabek, N. Toth, J. Vorkapić-Furač, I.R. Redovniković, Int. J. Food Sci. Technol. 47, 2613 (2012)
Y. Hara, S.J. Luo, R.L. Wickremasinghe, T. Yamanishi, Food Rev. Int. 11, 371 (1995)
L.-F. Wang, S.-C. Park, J.-O. Chung, J.-H. Baik, S.-K. Park, J. Food Sci. 69, S301 (2004)
S.C. Ho, S.P. Wu, S.M. Lin, Y.L. Tang, Food Chem. 122, 768 (2010)
H. Wang, K. Helliwell, Food Chem. 70, 337 (2000)
R. Wang, W. Zhou, X. Jiang, J. Agric. Food Chem. 56, 2694 (2008)
Y. Jin, J. Zhao, E.M. Kim, K.H. Kim, S. Kang, H. Lee, J. Lee, Molecules 24, 1735 (2019)
C. Kılıç, Z. Can, A. Yılmaz, S. Yıldız, H. Turna, Int. J. Second. Metab. 4, 148 (2017)
P. Shao, J. He, P. Sun, P. Zhao, J. Food Sci. Technol. 49, 66 (2012)
S. Liao, Y.H. Kao, R.A. Hiipakka, Vitam. Horm. 62, 1 (2001)
R.A. Frazier, E.R. Deaville, R.J. Green, E. Stringano, I. Willoughby, J. Plant, I. Mueller-Harvey, J. Pharm. Biomed. Anal. 51, 490 (2010)
A. Rauf, M. Imran, T. Abu-Izneid, S. Iahtisham-Ul-Haq, X. Patel, S. Pan, A. Naz, F. Sanches Silva, F. Saeed, H.A. Rasul Suleria, Biomed. Pharmacother. 116, 108999 (2019)
V. Koleckar, K. Kubikova, Z. Rehakova, K. Kuca, D. Jun, L. Jahodar, L. Opletal, Mini-Rev.. Med. Chem. 8, 436 (2008)
L.S. Ramírez Aristizabal, A. Ortíz, M.F. Restrepo Aristizabal, J.F. Salinas Villada, Rev. Vitae 24, 133 (2017)
P.C. Veggi, J. Martinez, M.A.A. Meireles, Microwave assisted extraction for bioactive compounds, in Theory Practice. ed. by F. Chemat, G. Cravotto (Springer, New York, 2012), pp. 15–52
S.B. Bagade, M. Patil, Crit. Rev. Anal. Chem. 15, 1–12 (2019)
N. Rhazi, H. Hannache, M. Oumam, A. Sesbou, B. Charrier, A. Pizzi, F. Charrier-El, Bouhtoury, Arab. J. Chem. 12, 2668 (2019)
D. Huang, O.U. Boxin, R.L. Prior, J. Agric. Food Chem. 53, 1841 (2005)
C. Zhao, C. Li, S. Liu, L. Yang, Sci. World J. 2014, 283024 (2014)
A. Azimi Mahalleh, P. Sharayei, E. Azarpazhooh, J. Food Meas. Charact. (2019). https://doi.org/10.1007/s11694-019-00314-1
S. Chopra, S.K. Motwani, Z. Iqbal, S. Talegaonkar, F.J. Ahmad, R.K. Khar, Eur. J. Pharm. Biopharm. 67, 120 (2007)
L.S. Badwaik, P.K. Borah, S.C. Deka, Sep. Sci. Technol. 50, 1814 (2015)
F. Dahmoune, B. Nayak, K. Moussi, H. Remini, K. Madani, Food Chem. 166, 585 (2015)
Q. Guo, B. Zhao, S. Shen, J. Hou, J. Hu, W. Xin, Biochim. Biophys. Acta Gen. Subj. 1427, 13 (1999)
J. Ze Xu, S.Y. Venus Yeung, Q. Chang, Y. Huang, Z.-Y. Chen, Br. J. Nutr. 91, 873 (2004)
K. Hayat, X. Zhang, H. Chen, S. Xia, C. Jia, F. Zhong, Sep. Purif. Technol. 73, 371 (2010)
K. Krishnaswamy, V. Orsat, Y. Gariépy, K. Thangavel, Food Bioprocess Technol. 6, 441 (2013)
J.L. Lu, S.S. Pan, X.Q. Zheng, J.J. Dong, D. Borthakur, Y.R. Liang, Int. J. Food Sci. Technol. 44, 2505 (2009)
S. Saklar, E. Ertas, I.S. Ozdemir, B. Karadeniz, J. Food Sci. Technol. 52, 6639 (2015)
M. Wang, R. Yang, W. Zhao, Int. J. Food Eng. 4, 1–12 (2008)
E.S. Kim, Y.R. Liang, J. Jin, Q.F. Sun, J.L. Lu, Y.Y. Du, C. Lin, Food Chem. 103, 1263 (2007)
C. Lantano, M. Rinaldi, A. Cavazza, D. Barbanti, C. Corradini, J. Food Sci. Technol. 52, 8276 (2015)
L.F. Wang, D.M. Kim, C.Y. Lee, Effect of cooking on in vitro iron bioavailability of various vegetables, in Bioactive Compounds in Foods. ed. by C.-T.H. Tung-Ching Lee (American Chemical Society, Washington, 2002), pp. 58–72
S. Sang, I. Yang, B. Buckley, C.T. Ho, C.S. Yang, Free Radic. Biol. Med. 43, 362 (2007)
N. Donlao, Y. Ogawa, LWT 116, 108567 (2019)
Q. Dai, Y. He, C.T. Ho, J. Wang, S. Wang, Y. Yang, L. Gao, T. Xia, J. Food Sci. Technol. 54, 2919 (2017)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taşkın, B., Aksoylu Özbek, Z. Optimisation of microwave effect on bioactives contents and colour attributes of aqueous green tea extracts by central composite design. Food Measure 14, 2240–2252 (2020). https://doi.org/10.1007/s11694-020-00471-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00471-8