Abctract
The phenolic compounds from Oudemansiella radicata were extracted by an ultrasound-assisted extraction method (UAE) using response surface methodology. The extraction with an extraction time 35.5 min, ultrasonic power 88 W and an ethanol concentration of 45.2% was considered to be the optimal UAE conditions with the maximum total phenolic content. The O. radicata extracts (ORE) exhibited distinct antioxidant activities against DPPH radical, ABTS radical and hydroxyl radical, as well as DNA damage. Using UPLC-Triple-TOF–MS, eight phenolic compounds were identified. These data suggest that ORE may be utilized as a natural oxidant and this work provide a basis for the utilization of O. radicata.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) can lead to cell damage and the deterioration of packaged foods [1]. Antioxidants arouse people’s widespread concern in preventing or minimizing the damaging effects of free radicals [2]. Synthetic antioxidants like BHA and BHT have been widely used as food preservatives and additives to enhance food function. However, their side effects have become a matter of serious concern [3]. Therefore, it is important to exploit natural antioxidants to substitute synthetic antioxidants due to their safety and potential therapeutic value [4].
Phenolic compounds are distinguished antioxidants that have preventive activity against ROS [5]. They possess certain characteristic that have attributed them the designation of antioxidant as electron donors (the ability to delocalize and stabilize the unpaired electrons) or metal-chelators and so on [6]. Owing to the beneficial effects on human health, the extraction of phenolic compounds from raw materials has been widely researched [7]. The efficient methods for the extraction of phenolic compounds may help the utilization of natural products [8].
Recently, mushrooms have received much attention due to their abundant biologically active substances [9]. Oudemansiella radicata, a fascinating edible mushroom of the genus Oudemansiella and family Tricholomataceae, has been widely cultivated in China [10]. Accumulated data suggest that O. radicata possesses various pharmacological activities including antioxidant, anti-aging, immunomodulatory, anti-inflammatory, antitumor and antibacterial activities [11]. To date, most previous studies have focused on the polysaccharide compounds and, currently, little information is available about phenolic compounds in O. radicata.
The objective of the present study was to optimize by the ultrasound-assisted extraction (UAE) method to obtain maximum total phenolic content (TP). Box-Behnken design (BBD) was used for optimizing and studying the interaction effects of the three process variables: extraction time (A), ultrasonic power (B) and ethanol concentrations (C). Furthermore, the antioxidant activities of O. radicata extracts (ORE) were investigated and the main compounds were identified using UPLC-Triple-TOF–MS analytical protocols.
Materials and methods
Materials
O. radicata (1000 g) were harvested from the farm of the Zhejiang Academy of Agricultural Science in March 2019. The samples were dried in a hot air oven at 60 °C, ground and passed through a 120-mesh screen. About 120 g powder were obtained and kept in an amber glass at − 20 °C until used. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), butyl hydroxy anisole (BHA), gallic acid (GA) and 2,2-azino-bis (3-ethyl-benzothia-6-sulfonic acid) diammonium salt (ABTS) were obtained from Sigma (St. Louis, MO, USA). All chemicals and solvents were reagent grade or better.
Ultrasound-assisted extraction of phenolic compounds
The phenolic compounds were extracted from O. radicata by ultrasonic-assisted treatment with an ultrasonic generator. One gram of the powdered mushroom was put into a beaker and different volume solvents were added. And then the beaker was immersed in the ultrasonic generator to extract the phenolics compounds. For RSM optimization studies, the experiments were conducted according to the experimental design given in Table 1.
Response surface optimization of UAE
Design Expert software (Version 8.0.6) was employed in this study for optimization and analysis. Three variables, ratio of solid to liquid, sonication power and solvent concentration were chosen for RSM optimization studies. A Box-Behnken design was employed to identify the optimal levels and effects of the process variables on the response. Chosen variable were studied at three levels, i.e., extraction time (20, 30, and 40 min), ethanol concentration (40%, 60%, and 80%) and ultrasonic power (60, 80, and 100 W). Seventeen experiments were carried out according to BBD and the responses (TP) are shown in Tables 1.
After extraction, the supernatant was filtered under vacuum through Whatman paper No. 1. The liquid extract was evaporated in a rotary evaporator at 50 °C to get rid of the ethanol and then dried in a freeze-dryer at − 55 °C. The dried extract was stored at − 20 °C for further analysis.
Determination of TP
The TP was analyzed using the Folin-Ciocalteu method [12]. Briefly, 0.15 mL ORE solution (1 mg/mL) was added 0.225 mL of twofold diluted Folin-Ciocalteu reagent, and this was kept for 5 min at room temperature. Then, 1.125 mL of 2% Na2CO3 solution were added, mixed well and kept for 15 min at room temperature. Finally, the absorbance was measured at 750 nm.
Determination of antioxidant activities of ORE in vitro
The scavenging capability of ORE against DPPH free radical was investigated according to the method reported by Shimadas et al. [13]. Briefly, 1 mL ORE solution at different concentrations (10–160 μg/mL) was added to 1 mL of 0.008% DPPH in 50% ethanol. This mixture was mixed well and then left standing for 30 min at room temperature in the dark. The absorbance of the resulting solution was recorded at 517 nm. BHA was used as control.
The scavenging ability of ORE against ABTS free radical was determined according to the method of Re et al. [14]. Briefly, ABTS radical cation was produced by reacting ABTS stock solution with potassium persulfate. The ABTS radical cation solution was diluted with PBS to an absorbance of 0.7 ± 0.02 at 734 nm. After addition of 1.0 mL of diluted radical cation solutions to 1.0 mL ORE solution at different concentrations for 5 min, the absorbance was recorded at 734 nm. BHA was used as control.
Hydroxyl radical-scavenging activity of ORE used was based on a procedure described by Gay et al. [15]. Briefly, 1 mL of salicylic acid–ethanol solution was mixed with 1 mL FeSO4 solution. Then 2 mL of sample solutions with different concentrations, followed by 1 mL of 0.3% H2O2 solution were added. After the mixture solution was incubated at 37 °C for 30 min, the absorbance was recorded at 510 nm. BHA was used as control.
The inhibition of ORE on supercoiled Pbr322 plasmid DNA strand breakage against hydroxyl radical was conducted using the method described by Jeong et al. [16]. Briefly, the reaction mixture contained of PBS, plasmid DNA, ORE solutions, FeSO4 and H2O2. The mixture was incubated at 37 °C for 30 min. After incubation, 2 μL of a loading buffer was added to stop the reaction and the reaction mixtures was subsequently electrophoresed on 1% agarose gel for 30 min. Ascorbic acid was used as control.
UPLC-Triple-TOF–MS analysis
ACQUITY ultra-high-performance liquid chromatography (Waters Company, USA) equipped with ZORBAX SB C18 (100 × 4.6 mm, Agilent, USA) and a Triple TOF 5600+ time-of-flight mass spectrometer, equipped with an electrospray ionization source (AB SCIEX Co., USA) were used in this study. The eluents and MS conditions were conducted according to the method of Wang et al. [17]. Briefly, the eluents were: A, 0.1% methanolic acid in water; B, 0.1% methanolic acid in acetonitrile. The applied elution conditions were: 0–2 min, 95% A isocratic; 2–25 min, linear gradient from 95 to 50% A isocratic; 25–35 min, linear gradient from 50 to 5% A; 35–37 min, 5% A isocratic; 37–38 min, linear gradient from 5 to 95% A. The MS conditions were as follows: negative ion scanning mode, scanning range: m/z 100–1500; GS1:50 psi; GS2: 50 psi; curtain gas (CUR): 35 psi; temperature of the ion source: 550 °C; voltage of the ion source: − 4500 V; first-order scanning: declustering potential: 100 V; focusing voltage: 10 V; second-order scanning: TOF MS ~ Product Ion ~ IDA mode was used to collect the MS data. CID energy was − 20, − 40 and − 60 V.
Statistical analysis
All the experiments were carried out in triplicate and data are expressed as mean ± standard deviation. Statistical analysis was performed with ANOVA followed by the Student’s t-test. A level of p < 0.05 was taken as statistically significant.
Results and discussion
Response surface optimization of UAE
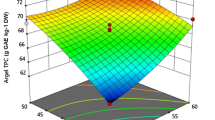
The mean experimental data of the yield of phenolic compounds from the O. radicata under the various experimental UAE conditions are presented in Table 1. The maximum total phenolic content (12.21 mg GA/g) were found in experiment run number 6 with an extraction time of 30 min, power 80 W and ethanol concentration 60% and the minimal value was found in run number 4 (6.06 mg GA/g).
A second-order polynomial equation was set up to fit with the experimental data as follows:
The estimated regression coefficients and ANOVA of the TP yield are manifested in Table 2. The quadratic regression model was significant (p < 0.001), whereas the lack of fit had a high p-value (p > 0.05), which shows that the model was a good fit for the experimental data of the TP yield. The high determination coefficient R2 (99.20%) and adjusted determination coefficient Adj. R2 (98.16%) further support that the model is perfect for prediction of TP yield. The low coefficient of variation (CV = 2.83%) reveals that high precision and good reliability of the experimental values. In summary, the above results indicate that the model can analyze and predict the TP extraction results.
The effects of the variables and their interactions on the yield of phenolic compounds are presented by the 3D response surfaces. Results from Fig. 1 further verified the ANOVA findings. The quadratic term of the ultrasonic power and ethanol concentration indicated that the two variables had a large effect. Using Design-Expert, the optimum values were obtained as follows: extraction time 35.5 min, ultrasonic power 88.0 W and ethanol concentration of 45.2%. The maximum predicted extraction yield of TP was 12.45 mg GA/g, which matched well with the actual yield (12.24 ± 0.36%, n = 3). These data imply that the model used in this study was high-performance.
Antioxidant activities of ORE
DPPH free radical-scavenging activity
DPPH assay has been widely used in antioxidant studies because it is very sensitive and simple [18]. As shown in Fig. 2a, the scavenging activity against DPPH free radical increased with the increasing concentrations of OREand BHA. At 10–160 μg/mL, the DPPH scavenging rates were 8.7–72.2% for ORE and 24.7–94.5% for BHA. The IC50 of ORE and BHA were 31.2 and 56.7 μg/mL, respectively. The DPPH scavenging abilities of antioxidants against DPPH are thought through scavenging free radicals or donating the hydrogen. In the present study, ORE showed outstanding scavenging activity against DPPH, which may be attributable to its strong hydrogen-donating ability.
ABTS free radical scavenging activity
As shown in Fig. 2b, the scavenging abilities of ORE and BHA against ABTS free radical were concentration dependent. The ABTS free radical-scavenging rates of both ORE and BHA were higher than 90% at the concentration of 120 μg/mL, indicating that both ORE and BHA are powerful scavengers of ABTS free radical. At a concentration of 80 μg /mL, the ABTS free radical scavenging ability was 68.2% for ORE and 88.2% for BHA.
Hydroxyl radical scavenging activity
The hydroxyl radical, generated by the Fenton reaction, was scavenged by ORE and BHA in the present study. The scavenging effects of all samples are shown in Fig. 2c. The concentration was discovered to affect the hydroxyl radical scavenging activity. The hydroxyl radical scavenging activity of ORE was comparable to BHA at the tested concentrations. When the concentration was > 120 μg/mL, there was no difference in the hydroxyl radical-scavenging activity between ORE and BHA. Hydroxyl radical is the most highly reactive oxygen radical among the free radicals, which can induce some diseases [19]. Furthermore, it can damage biological molecules through interacting with DNA, abstracting hydrogen atoms or leading to the formation of oxysulphur radical [20].
DNA damage protective activity
As shown in Fig. 3, the capacity of ORE to prevent oxidative damage of DNA induced by hydroxyl radical was evaluated. The normal DNA was mainly of the supercoiled form (Fig. 3, lane 1, control). The supercoiled form of DNA was converted into the relaxed circular or linear forms after the triggering of the Fenton reaction (Fig. 3, lane 2), indicating that the DNA breaks were induced by the hydroxyl radical [21]. ORE significantly reduced the DNA damage in a dose-dependent manner at the concentrations of 50, 100, 150 μg/mL (Fig. 3, Lanes 4–6). Ascorbic acid at 50 μg/mL exhibited the best effect on inhibiting oxidative damage of DNA (Fig. 3, Lane 3). The inhibitory effect of ORE on the oxidative DNA damage may be through suppression of the Fenton reaction or by directly quench of hydroxyl radical [22].
Inhibitory effect of O. radicata extract (ORE) on hydroxyl radical induced DNA damage. Lanes 1 and 2 were normal DNA and treated with 1 mM FeSO4 and 1 mM H2O2, respectively. Lane 3 were treated with ascorbic acid (50 μg/mL), lanes 4–6 were treated with various concentrations of ORE (50, 100 and 150 μg/mL)
The DNA migration assay is sensitive in testing the DNA damage [23]. Free radicals are notorious for damaging DNA strands, which has been doubted to be the main reason of cancer [24]. Hydroxyl radicals are the most predominant radicals, which can give rise to the breaks in DNA stands [25]. In the present study, ORE showed protective activity against DNA damage through its free radical-scavenging activity.
Identification of the compounds
The base peak chromatograms of ORE under the optimal conditions resulting from the UPLC-Triple-TOF–MS method described above are shown in Fig. 4. The peaks were tentatively identified by analyzing their MS and MS/MS spectra and also by considering data from the literature and open-access mass-spectra databases (Scifinder and Reaxy). A total of 10 compounds were tentatively identified. The MS spectra and structures of the identified compounds are shown in Supplementary Fig. 1.
HPLC chromatogram recorded at 320 nm showing the profile of O. radicata extract (ORE). 1, uridine; 2, heliopine lactam; 3, liquiritin 7-β-d-apiofuranoside; 4, luteolin 7-O-glucoside; 5, apigenin 7-O-d-glucoside; 6, glycroside; 7, isoliquiritin 4′-β-d-apiofuranoside; 8, isoliquiritin 7-β-d-apiofuranoside; 9, isoliquiritin; 10, licorice glycoside B
Peak 1 showed an [M−H]− ion at m/z 243. Its MS2 spectrum produced ions at m/z 200 [(M−H)-43]−, which indicates an amide structure. The ions at m/z 200 and m/z 110 implied that this compound was tentatively indentified as uridine.
Peak 2 exhibited an [M−H]− ion at m/z 200. Its MS2 spectrum produced ions at m/z 156 [(M−H)-44]−, which indicates a carboxyl group. The ions at m/z 156 and m/z 110 indicated that it was tentatively identified as heliopine lactam [26].
Peak 3 showed an [M−H]− ion at m/z 549. Its MS2 spectrum produced ions at m/z 429[(M−H)-120]−, m/z 255[(M−H)-294]− and m/z 135[(M−H)-414]−. The characteristic fragment ion at m/z 255 indicates that this compound was liquiritin 7-β-d-apiofuranoside [27].
Peak 4 emerged a molecular ion [M−H]− ion at m/z 449 and was tentatively assigned to luteolin 7-O-glucoside with its MS2 spectrum exhibiting characteristic fragment ions at m/z 287[(M−H)-162]−, m/z241[(M−H)-208]−, and m/z 153[(M−H)-296]−[28].
Peak 5 had an ion at m/z 433[M−H]− and a characteristic fragment ion at m/z 271[(M−H)-162]− tentatively implying that compound 5 was apigenin 7-O-d-glucoside [29].
Peak 6 possessed an ion at m/z 563[M−H]−. Its MS2 spectrum showed a characteristic fragment ions at m/z 419[(M−H)-144]− and m/z 269 [(M−H)-294]− suggesting that compound 6 was glycroside [30].
Peak 7 showed an ion at m/z 549[M−H]−. The negative ion mode of MS2 spectra revealed ions at m/z 417[(M−H)-132]−, m/z 255[(M−H)-294]− and m/z 135[(M−H)-414]−. The peak was tentatively authenticated as isoliquiritin 4′-β-d-apiofuranoside [31].
Peak 8 showed an ion at m/z 549 [M−H]−. Its spectrum produced ions at m/z 429[(M−H)-120]−, m/z 255[(M−H)-294]−, and m/z 135[(M−H)-414]−. The above MS2 spectrum tentatively identified this compound as isoliquiritin 7-β-d-apiofuranoside.
Peak 9 displayed an ion at m/z 419 [M−H]−. Its MS2 spectrum exhibited characteristic fragments ions at m/z 257[(M−H)-162]−, m/z239 [(M−H)-180]−, and m/z 137[(M−H)-296]−. The above MS2 spectrum tentatively deduced that this compound was isoliquiritin [32].
Peak 10 showed an ion at m/z 695 [M−H]−. Its spectrum produced ions at m/z 531[(M−H)-164]−, m/z 255[(M−H)-294]−, and m/z 163[(M−H)-532]−. The above ions indicated that this compound was tentatively inferred as licorice glycoside B [33].
In recent years, studies with mushrooms have become more important due to their beneficial effects on human health, which may be related to their antioxidant potential [34]. The antioxidant properties of mushrooms are attributed mostly to their phenolic compounds [35]. The phenolic compounds have been identified in several species of mushrooms grown in different countries. The profiles of phenolic compounds of 17 different wild species collected in Portugal were reported by Vaz et al. [36]. Palaciose et al. [37] analyzed the profiles of phenolic compounds of eight species of wild mushrooms grown in Spain. The phenolic compounds in 17 types of edible wild mushroom species from Mexico were evaluated by Yahia et al. [38]. In addition, the phenolic compounds profiling of 43 wild mushrooms in Chinawere also identified [39]. These phenolics are biosynthesized by mushrooms adoption to biotic and abiotic stress condition and recognized as excellent antioxidants due to their ability to scavenge free radicals.
In the present study, 10 compounds in O. radicata were identified. As our literature survey revealed, this is the first study on phenolic profiling of this mushroom. Among the ten compounds, eight compounds were phenolic compounds. The identified phenolics were liquiritin 7-β-d- apiofuranoside, luteolin 7-O-glucoside, apigenin 7-O-d-glucoside, glycroside, isoliquiritin 4′-β-d-apiofuranoside, isoliquiritin 7-β-d-apiofuranoside, glycyroside, isoliquiritin and licorice glycoside B. This is the first report of the extraction of a large number of phenolic compounds in a single extraction from O. radicata. Luteolin and its derivatives have been reported to possess strong anti-oxidative activities [40]. Liquiritin and isoliquiritin exhibited the antioxidant activities in B65 neuroblastoma cells [41]. Apigenin and its derivatives can antagonise gamma radiation-induced oxidative damages in human peripheral blood lymphocyte [42]. The antioxidant activities of glycyroside and licorice glycoside B were also verified [43]. The combined effects of the above phenolic compounds have a decisive influence in the antioxidant activities of the O. radicata extract. This finding opens the possibility of development of O. radicata extract as an excellent functional food for preventing relevant disease.
Conclusion
In the present study, RSM was successfully utilized to optimize the extraction process for the effective extraction of phenolic compounds in O. radicata. The optimum extraction conditions were as follows: extraction time 35.5 min, ultrasonic power 88W, and an ethanol concentration of 45.2%. Under these conditions, the maximum extraction yield of TP reached 12.24 mg GA/g. The phenolic extract demonstrated distinct antioxidant activities and eight phenolic compounds were identified. The aforementioned results revealed that UAE is an effective method to extract phenolic compounds from O. radicata and ORE may be used as a natural antioxidant.
References
K. Ghafoor, Y.H. Choi, Optimization of ultrasound-assisted extraction of phenolic compounds and antioxidants from grape peel through response surface methodology. J. Korean Soc. Appl. Biol. 52, 295–300 (2009)
Y. Shukla, M. Singh, Cancer preventive properties of ginger: a brief review. Food Chem. Toxicol. 45, 683–690 (2007)
H. Sies, Strategies of antioxidant defense. Eur. J. Biochem. 215, 213–219 (1993)
C. Lu, C. Li, B. Chen, Y. Shen, Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of Amygdalus pedunculata Pall. Food Chem. 265, 111–119 (2018)
P. Hou, Q. Wang, W. Qi, Y. Zhang, J. Xie, Comprehensive determination of seven polyphenols in Eucommia ulmoides and its anti-oxidative stress activity in C. elegans. J. Food Meas. Charact. 13, 2903–2909 (2019)
Z.H. Rana, M.K. Alam, M. Akhtaruzzaman, Nutritional composition, total phenolic content, antioxidant and α-amylase inhibitory activities of different fractions of selected wild edible plants. Antioxidants 8, 203 (2019)
O. Kadiri, S.O. Gbadamosi, C.T. Akanbi, Extraction kinetics, modeling and optimization of phenolic antioxidants from sweet potato peel vis-a-vis RSM, ANN-GA and application in functional noodles. J. Food Meas. Charact. 13, 3267–3284 (2019)
L. Zhang, Z. Tu, H. Wang, Z. Fu, Q. Wen, H. Chang et al., Comparison of different methods for extracting polyphenols from Ipomoea batatas leaves, and identification of antioxidant constituents by HPLC-QTOF-MS2. Food Res. Int. 70, 101–109 (2015)
S.P. Wasser, Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biot. 60, 258–274 (2002)
S.B. Kim, S.H. Kim, K.R. Lee, J.O. Shim, M.W. Lee, M.J. Shim et al., The optimal culture conditions for the mycelia growth of Oudemansiella radicata. Mycobiology 33, 230–234 (2005)
X. Zou, Optimization of nutritional factors for exopolysaccharide production by submerged cultivation of the medicinal mushroom Oudemansiella radicata. World J. Microbiol. Biotechnol. 21, 1267–1271 (2005)
M.K. Alam, Z.H. Rana, S.N. Islam, Comparison of the proximate composition, total carotenoids and total polyphenol content of nine-orange-fleshed sweet potato varieties grown in Bangladesh. Foods 5, 64 (2016)
K. Shimadas, K. Fujikawas, K. Yaharas, T. Nakamuras, Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 50, 840–845 (1992)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C.A. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 26, 1231–1237 (1999)
C. Gay, J. Colins, J.M. Gebicki, Hydroperoxide assay with the ferric-xylenol orange complex. Anal. Biochem. 273(2), 149–155 (1999)
J.B. Jeong, J.H. Park, H.K. Lee, S.Y. Ju, S.C. Hong, J.R. Lee et al., Protective effect of the extracts from Cnidium offiicinale against oxidative damage induced by hydrogen peroxide via antioxidant effect. Food. Chem. Toxicol. 47, 525–529 (2009)
Y. Wang, G. Luan, W. Zhou, J. Meng, H. Wang, N. Hu et al., Subscritical water extraction, UPLC-Triple-TOF/MS analysis and antioxidant activity of anthocyanins from Lucium ruhtenicum Murr. Food Chem. 249, 119–126 (2018)
J.K. Moom, T. Shibamoto, Antioxidant assays for plant and food components. J. Agric. Food Chem. 57, 1655–1666 (2009)
P. Siddhuraju, K. Becker, The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts. Food Chem. 101, 10–19 (2007)
B. Halliwell, Antioxidant in human health and disease. Annu. Rev. Nutr. 16, 33–50 (1996)
H.J. Hwang, S.W. Kim, J.M. Lim, J.H. Joo, H.O. Kim, H.M. Kim et al., Protective effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotoxin-induced diabetic rats. Life Sci. 76, 3069–3080 (2005)
A. Chandrasekara, F. Shahidi, Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. J. Funct. Foods 3, 159–170 (2011)
Y. Jung, Y. Surh, Oxidative DNA damage and cytotoxicity induced by copper-stimulated redox cycling of salsolinol, a neurotoxic tetrahydroisoquinoline alkaloid. Free Radical Biol. Med. 30, 1407–1417 (2001)
R. Cozzi, R. Ricordy, T. Aglitti, V. Gatta, P. Perticone, R. De Salvia, Ascorbic acid and beta-carotene as modulators of oxidative damage. Carcinogenesis 18, 350–356 (1997)
T. Albishi, J.A. John, A.S. Al-Khalifa, F. Shahidi, Antioxidant: Anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Funct. Foods 5, 930–939 (2013)
W.S. Chilton, A. Petit, M.D. Chilton, Y. Dessaux, Structure and characterization of the crown gall opines heliopine, vitopine and radeopine. Phytochemistry 58, 137–142 (2001)
X.T. Zheng, P.Y. Shi, Y.Y. Cheng, H.B. Qu, Rapid analysis of a Chinese herbal prescription by liquid chromatography-time-of-flight tandem mass spectrometry. J. Chromatogr. A 1206, 140–146 (2008)
R. Yin, F. Han, Z. Tang, R. Liu, X. Zhao, X. Chen et al., UFLC-MS/MS method for simultaneous determination of luteolin-7-O-gentiobioside, luteolin-7-O-β- D-glucoside and luteolin-7- O-β-d-glucurnide in beagle dog plasma and its application to a pharmacokinetic study after administration of traditional Chinese medicinal preparation: Kudiezi injection. J. Pharmaceut. Biomed. 72, 127–133 (2013)
E. Guzelmeric, I. Vovk, E. Yesilada, Development and validation of an HPTLC method for apigenin7-o-glucoside in chamomile flowers and its application for finger print discrimination of chamomile-like materials. J. Pharmaceut. Biomed. 107, 108–118 (2015)
S. Wang, L. Chen, J. Leng, P. Chen, X. Fan, Y. Cheng, Fragment ion diagnostic strategies for the comprehensive identification of chemical profile of Gui-Zhi-Tang by integrating high-resolution MS, multiple-stage MS and UV information. J. Pharmaceut. Biomed. 98, 22–35 (2014)
S. Tao, Y. Huang, Z. Chen, Y. Wang, Rapid identification of anti-inflammatory compounds from Tongmai Yangxin Pills by liquid chromatography with high-resolution mass spectrometry and chemometric analysis. J Sep. Sci. 38, 1881–1893 (2015)
B. Jin, Y. Zhao, P. Yu, B. Yang, C. Zhou, Z. Yu, LC-ESI-MS/MS method for simultaneous determination of eleven bioactive compounds in rat plasma after oral administration of Ling-Gui-Zhu-Gan Decoction and its application to a pharmacokinetics study. Talanta 190, 450–459 (2018)
W. Xu, M. Huang, H. Li, X. Chen, Y. Zhang, J. Liu et al., Chemical profiling and quantification of Gua-Lou-Gui-Zhi decoction by high performance liquid chrimatography/ quadrupole-time-of–flight mass spectrometry and ultra-performance liquid chromatography/ triple quadrupole mass spectrometry. J. Chromatogr. B. 986–987, 69–84 (2015)
Z.F. Zhang, Q.L. Jin, G.Y. Lv, L.J. Fan, H.J. Pan, L.F. Fan, Comparative study on antioxidant activity of four varieties of Flammulina velutipes with different colour. Int. J. Food Sci. Technol. 48, 1057–1064 (2013)
J.L. Mau, H.C. Lin, C.C. Chen, Antioxidant properties of several medicinal mushrooms. J. Agric. Food Chem. 50, 6072–6077 (2002)
J.A. Vaz, L. Barros, A. Martins, J.S. Morais, M.H. Vasconcelos, I.C.F.R. Ferreira, Phenolic profile of seventeen Portuguese wild mushrooms. LWT-Food Sci. Technol. 44, 343–346 (2011)
I. Palacios, M. Lozano, C. Moro, M. Darrigo, M.A. Rostagno, J.A. Martinez et al., Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 128, 674–678 (2011)
E.M. Yahia, F. Gutierrez-Orozco, M.A. Moreno-Perez, Identification of phenolic compounds by liquid chromatography-mass spectrometry in seventeen species of wild mushrooms in Central Mexico and determination of their antioxidant activity and bioactive compounds. Food Chem. 226, 14–22 (2017)
T. Islam, X. Yu, B. Xu, Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT-Food Sci. Technol. 72, 423–431 (2016)
G. Seelinger, I. Merfort, C.M. Schempp, Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 74, 1667–1677 (2008)
Y. Nakatani, A. Kobe, M. Kuriya, Y. Hiroki, T. Yahagi, L. Sakakibara et al., Neuroprotective effect of liquiritin as an antioxidant via an increase in glucose-6-phosphate dehydrogenase expression on B65 neruoblastoma cells. Eur. J. Pharmacol. 815, 381–390 (2017)
N. Begum, P.N. Rajendra, Apigenin, a dietary antioxidant, modulates gamma radiation-induced oxidative damage in human peripheral blood lymphocytes. Biomed. Prev. Nutr. 2, 16–24 (2012)
C.Y. Chen, Y.G. Zu, Y.J. Fu, M. Luo, C.J. Zhao, W. Wang, B.S. Zhao, J. Li, T. Efferth, Preparation and antioxidant activity of Radix Astragali residues extracts rich in calycosin and formononetin. Biochem. Eng. J. 56, 84–93 (2011)
Acknowledgments
We acknowledge the support provide by the New Variety Breeding Project of Science Technology Department of Zhejiang Province, China (2016CO2057), Agriculture, Rural areas and Farmers and Six-party Project of Zhejiang Province(2019SNLF015) and Science and Technology Projects of Qujiang District, Quzhou, Zhejiang Province, China (2018303).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., Lv, G., Song, T. et al. Recovery and analysis of phenolic extracts from Oudemansiella radicata using ultrasonic-assisted extraction. Food Measure 14, 2176–2184 (2020). https://doi.org/10.1007/s11694-020-00464-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00464-7