Abstract
The present scientific work envisaged the effect of fermentation, starter culture, solvent type, concentration of solvent and extraction conditions on pearl millet phenolics. Fermentation of pearl millet grains was performed using two filamentous fungal strains (Aspergillus oryzae and Rhizopus azygosporus) for a specific period (336 h). Pearl millet koji was extracted under optimized conditions and studied for the presence of specific bioactive compounds using high performance liquid chromatography (HPLC). Results confirmed the presence of ascorbic acid (1.55 mg g−1), p-coumaric acid (1.04 mg g−1) and cinnamic acid (0.71 mg g−1 as major compounds in unfermented pearl millet and ascorbic acid (10.23 mg g−1), gallic acid (8.95 mg g−1), resorcinol (2.90 mg g−1, catechol (7.60 mg g−1), vanillin (4.58 mg g−1), p-coumaric acid (3.96 mg g−1), quercetin (2.74 mg g−1), benzoic acid (5.10 mg g−1) and cinnamic acid (5.14 mg g−1) in A. oryzae fermented millet (AoFM). Specific phenolics in R. azygosporus fermented millet (RaFM) were ascorbic acid (8.80 mg g−1), gallic acid (4.70 mg g−1) and p-coumaric acid (1.27 mg g−1) along with other minor phenolics. DNA damage protection activity was observed maximum till 288 h of fermentation for AoFM and 240 h of fermentation for RaFM.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pearl millet (Pennisetum glaucum) is widely grown cereal grain which belongs to kingdom: Plantae; family Poaceae; order: Poales and genus: Pennisetum. Presence of certain bioactive compounds (gallic acid, p-coumaric acid, syringic acid, ferulic acid, ascorbic acid) and minerals in pearl millet makes it an important cereal among other cereal grains [1,2,3]. Pearl millet is an easily available, low cost cereal grain with natural bioactive constituents, minerals and other phytochemicals. Increased interest of researchers in bioactive constituents attributes to radical scavenging properties of phytochemicals especially phenolic derivatives, flavonoids and tannins [4,5,6]. Bioactive phytochemicals have wide ranging effects on humans including improved immunity, anti-ageing properties, healing effects and DNA damage protection [7,8,9,10].
Fermentation especially solid state fermentation (SSF) is widely adopted method for the production of secondary metabolites, energy production, drugs, food supplements and fortified functional food products [11,12,13]. Fermentation results in enzymatic changes, structural modification and microbial transformation of phenolic compounds. Phenolic compounds include flavonoids, phenolic derivatives, saponins, condensed tannins, catechol, steroids and many important mineral constituents [8, 14,15,16,17]. Among microbial consortia, fungal strains are widely used for the improvement of phenolic compounds in natural substrates [1]. Microbial consortia used for the improvement of phenolic compounds of steam sterilized moist substrates includes: Monascus anka [18]; A. niger [19]; A. awamori [20]; A. oryzae [20, 21]; A. sojae [1, 22]; R. azygosporus [22]; R. oryzae [23,24,25] and R. oligosporus [23, 26].
Substrates (natural form) as well as processed food products have mixture of solvent specific phenolic compounds in their cellular matrix. To extract out maximum phenolic compounds, it is essential to choose an appropriate experimental design that exactly determines the effects of the major factors involved in extraction (extraction phase, temperature and time duration) [27, 28]. Response surface methodology (RSM) has been proved to be an effective statistical tool for optimizing the best conditions for extraction of bioactive compounds. It allows determining the effect of experimental parameters on output response [29]. RSM has been widely used to find a combination of factors that produce an optimum response. Therefore, RSM has become most popular optimization technique which has been successfully used to model and optimize biochemical and biotechnological processes. It has been used for optimizing extraction of phenolic compounds from a number of natural resources [30,31,32,33,34,35,36]. RSM provide optimized conditions which are economically feasible and scientifically important [37, 38]. Experimental runs during RSM analysis provide a large amount of information which could check the interaction between independent experimental variables in the form of output/response [27, 39,40,41].
Researchers use aqueous as well as various organic phases to extract out phenolic compounds from various experimental samples. Some studies also report the effect of combination of solvents on overall recovery of phenolic compounds from experimental samples [28, 37, 38]. However, before selecting particular solvent for extraction, it should be standardized for various conditions. Thus, keeping in view the importance of specific solvent during extraction of phenolic compounds, the present study was designed to optimize the extraction conditions (extraction phase type, concentration, temperature and time) for phenolic compounds present in pearl millet. Further the effect of fermentation on phenolic compounds (PC’s) and antioxidant properties (AP’s) was evaluated and bioactive compounds were identified using HPLC method.
Experimental sample and starter culture
Substrate (Pearl millet cultivar, HHB-197) was procured from Chaudhary Charan Singh Haryana Agricultural University, Hisar (29.1503° N, 75.7056° E) India. Before performing fermentation, substrate (pearl millet grains) was washed with water, dried in an oven (NSW, India) at 40 ± 2 °C for 72 h and stored in airtight containers till further experimental work. Fungal strains A. oryzae (MTCC-3107) and R. azygosporus (MTCC-10195) were procured from Institute of Microbial Technology, Chandigarh, India. They were maintained on potato dextrose agar (PDA) for a period of 120 h at 28 ± 2 °C in an incubator (NSW, India). After specific growth period (120 h) spore suspensions were prepared using sterilized distilled water (Millipore, Merck, Delhi, India).
Chemicals
Solvents (hexane, ethanol, chloroform, methanol and acetone) and chemicals used in the present study were of analytical grade and purchased from HiMedia Pvt. Ltd (India), Fisher Scientific (India) and Sigma-Aldrich (USA).
Preparation of substrate for SSF
Pearl millet grains (50 g) were soaked in potato dextrose broth (PDB, 1:1 w/v) for overnight at room temperature. Next day excess of PDB was discarded and the grains were autoclaved (YSI-402, Yorco, India) (121 °C, 15 min) and subsequently cooled before microbial suspension was sprayed. Both fungal strains (A. oryzae and R. azygosporus) were sprayed (5 ml, 1 × 105 spores/ml) separately on steam sterilized grains, mixed properly and incubated for 336 h at 28 ± 2 °C in an incubator (NSW-152, India).
Preparation of extracts
Pearl millet koji was taken out from Erlenmeyer flasks after a specific fermentation period (48 h), dried in an oven (NSW, India) at 45 °C for 48–72 h. Grains were then milled to flour using mixer grinder (Sujata-2632, India) and stored in airtight containers till further processing. Flour (fermented and unfermented) was defatted using Hexane (1:5 w/v 10 min, thrice) at room temperature. Defatted flour was then dried in an oven at 40 °C and stored at − 20 °C till further analysis. Dried defatted flour (fermented and unfermented) was then extracted with water, ethanol, methanol, acetone and chloroform in waterbath (MSW-273, Macro Scientific Works Pvt. Ltd. India) at experimental conditions as described by Salar et al. [37]. Flour slurry in extraction phase was filtered with Whatman No.1 filter paper. The extracts were prepared and stored at 4 °C. These were further analyzed for PC’s and antioxidant properties (AP’s). In order to avoid repetition, the codes have been used for unfermented millet (UM), A. oryzae fermented millet (AoFM) and R. azygosporus fermented millet (RaFM).
Estimation of total polyphenols content (TPC)
TPC were estimated in extracts (UM, AoFM and RaFM) using Folin-Ciocalteu reagent (FCR) method as reported by Yu et al. [42]. Gallic acid (HiMedia, mg/ml) was used as standard and the equation derived from the curve was used to calculate the amount (mg GAE/g dwb) of TPC in extracts. All determinations were carried out in triplicates.
Sequential strategy for optimized extraction conditions
Three independent variables (concentration of extraction phase, extraction temperature and extraction time) were selected for optimization of PC’s from UM, AoFM and RaFM using mixture design. Maximal amount of PC’s was taken as output response in relation with selected variables. The experiments during sequential strategy were carried out in two phases: phase-1 optimization of concentration of extraction phase concentration; phase-2 optimization of extraction temperature (°C) and time duration (min). Mixture design consisting of experimental runs followed by center composite design (CCD) was employed. The variables during optimization process were the extraction phase composition (%, v/v, water/ethanol/methanol), extraction temperature (°C) and duration of extraction time (min) while output response in relation to selected variables were PC’s (mg GAE g−1 dwb).
Verification experimental
During the single factor experimental analysis, the best extraction phase concentration, extraction temperature and extraction time was used for the verification of experimental runs. Under the optimized conditions maximum amount of bioactive constituents with antioxidant properties from pearl millet flour (unfermented and fermented) was obtained. Experimental verifications were determined from the contour plots generated using response surface methodology.
DPPH-radical scavenging capacity (DPPH-RSC)
DPPH-RSC in extracts (UM, AoFM and RaFM) was measured using the method as reported by Yen and Chen [43]. Aliquot of each extracts (0.1 mL) were added in to test tube followed by addition of purple colored DPPH reagent (3 mL). The reaction mixture (RM) was incubated at room temperature for a period of 30 min. The changes in absorbance at 517 nm were recorded after incubation time (30 min). All determinations were carried out in triplicates. Percent (%) DPPH-RSC was calculated using formula:
Hydroxyl free radical scavenging capacity (HFRSC)
HFRSC in extracts was evaluated using the method as described by Smirnoff and Cumbes [44]. Extracts (0.1 mL) was added to Smirnoff reagent (3 mL) and the mixture was incubated in waterbath at 40 °C for 20 min. All determinations were carried out in triplicates. Absorbance of colored RM was observed at 562 nm.
AC is absorbance of control and AE is absorbance of extracts.
Cupric reducing antioxidant capacity (CUPRAC)
CUPRAC value of extracts (UM, AoFM and RaFM) was calculated by following the method as described by Apak et al. [45]. Aliquot of each extracts (0.1 mL) were added to different test tubes followed by addition of CUPRAC reagent (3 mL). The reaction mixture was incubated at room temperature for 30 min. CUPRAC reagent was prepared using ammonium acetate buffer (1 M), cupric chloride (10 mM) and neocuproine (7.5 mM) in ratio (1:1:1). After incubation period, the absorbance was recorded at 450 nm. Ascorbic acid (AA, HiMedia) was used as standard to prepare the curve and equation derived from curve was used for calculation of CUPRAC value. The results were expressed as mg AAE g−1 dry weight basis. All determinations were carried out in triplicates.
Reducing power capacity (RPC)
RPC of extracts (UM, AoFM and RaFM) was calculated by following the method as reported by Oyaizu [46]. Each extract (100 μl) was mixed separately with potassium ferricyanide (100 μl, 1%) followed by heating at 50 °C for 30 min. After heating trichloroacetic acid (100 μl, 1%) and ferric chloride (100 μl, 0.1%) was added to reaction mixture followed by incubation at ambient temperature for 20 min. Reaction mixture was diluted with double distilled water to prepare final volume (10 mL). Absorbance of reaction mixture was recorded at 700 nm. Quercetin (mg/ml HiMedia) was used as standard for the analysis of RPC in extracts and the results were expressed as mg QE g−1 dry weight basis. All determinations were carried out in triplicates.
Ferric reducing antioxidant power (FRAP)
Wheat-straw colored FRAP reagent was used to assess the FRAP value of extracts (UM, AoFM and RaFM) as reported by Benzie and Strain [47]. Extracts (0.1 ml) was allowed to react with FRAP reagent (3 ml) at room temperature. Absorbance of blue colored RM was recorded at 595 nm (GENESYS 10S UV–VIS, Thermo Scientific, India). For the preparation of standard curve and calculation of FRAP value, FeSO4.7H2O (HiMedia) was used as standard. All determinations were carried out in triplicates.
FRAP value of extracts = (AE/AC) × FRAP value of Standard Solution.
AE is the absorbance of extracts and AC is the absorbance of control, respectively.
DNA damage protection activity (DDPA)
DDPA of extracts (UM, AoFM and RaFM) was measured by agarose gel electrophoresis method using pBR322 plasmid DNA (GeNei™, India, Cat No. 1,600,670,501,730) against Fenton’s reagent (FR). FR was prepared by following the method described by Kumar et al. [48]. pBR322 isolated from Escherichia coli by alkaline lysis method and further purified using density gradient method. pBR322 DNA used during agarose gel electrophoresis was 4361 base pairs in length. DDPA of extracts in the form of specific DNA bands were observed under trans-illumination of UV light using a gel documentation system (Gel Doc™ XR+ Imaging system, BIO-RAD, Japan).
High performance liquid chromatography
Effect of SSF on specific bioactive components of pearl millet was confirmed using HPLC. Qualitative and quantitative analysis to check the presence of specific bioactive compounds in extracts were carried out in a Shimadzu 10 AVP HPLC system comprising a SCL10 AVP system controller, two LC-10 AVP pumps CTO-10, AVP column oven with Rheodyne 7120 injection value (20 μL sample loop) and SPD-M10 AVP photodiode-array detector (all from Shimadzu, Tokyo, Japan). Experiments were carried out Gemini-NX C18 analytical HPLC column (250 × 4.6 mm, 3 μm) with a guard column (40 × 3 mm, 3 μm) both from Phenomenex (Torrance, CA, USA). Specific analysis was performed at a rate of 0.5 mL/min using 2% v/v acetic acid (solvent A) and methanol:acetonitrile (40:50 v/v) mixture (solvent B) under the following gradient program: 0–8 min acetic acid (70%), 8–19 min acetic acid (60%) and 19–30 min acetic acid (30%). Injection volume during the analysis was 10 μL. The analytes were detected at 280 nm. Quantification was carried out using four different mobile phases. Mobile phase A comprises methanol followed by phase B (0.1% glacial acetic acid); phase C (0.1% ortho-phosphoric acid) and phase D (HPLC grade 100% acetonitrile). Optimized gradient phase was prepared using above discussed phases in ratio 25:30:30:15. Flow rate of the optimized phase was set at 1.2 mL/min at temperature range 30 °C.
Statistical analysis
Process parameters were optimized using Design expert software (Version 11). The data reported in the tables were carried out in triplicate and they were subjected to one-way analysis of variance (ANOVA) using Minitab Statistical Software version 14 (Minitab, Inc, USA). Tukey’s test was used to test significant differences among them (Minitab version 14, Inc, USA). Differences among means at 5% level (P < 0.05) were considered statistically significant.
Results and discussion
Effect of starter culture, fermentation duration and extraction phase on PC’s
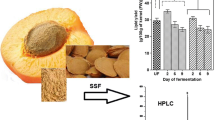
Duration of fermentation (344 h) significantly (P < 0.05) affected the amount of PC’s present in UM, AoFM and RaFM extracts (Table 1). The PC’s for UM was found to be 3.31 mg GAE g−1. Siroha et al. [49] studied different cultivars of pearl millet (HC-10, HHB-67, HHB-223, HHB-226, W-445 and GHB-732) in terms of phenolic compounds. They observed significant (P < 0.05) difference among pearl millet cultivars for TPC with values ranging from 2.39 to 3.13 mg GAE g−1. Chandrasekara et al. [3] reported 8.63 µmol ferulic acid equilvalent.g−1 phenolic compounds in pearl millet. The difference in cultivars for phenolic compounds may be due to difference in soil profile, growing season, availability of moisture, temperature, pH and rainfall. For AoFM and RaFM, the maximum increase in PC’s was till 288 h and 240 h respectively (Table 1). PC’s in natural resources are present in two forms (i) free form (ii) bound form. Free phenolics are easily extractable however, bound form of PC’s is difficult to extract as they may be present in complex forms/as conjugate with fatty acid and sugars. During the process of fermentation fungal strains starts producing some specific kind of enzymes (α-amylase, β-glucosidase and xylanase) and the activity of these enzymes convert bound form of PC’s in to free forms which are easily detectable by spectro-photometric methods. SSF with A. oryzae and R. azygosporus results in an increase in PC’s of HHB-197 by 10.54 folds (954%) and 3.76 folds (276%) respectively. Further increase in duration (h) of fermentation resulted in decrease in pearl millet phenolics for both starter cultures (A. oryzae and R. azygosporus).
Efficacy of extraction phase (water, ethanol, methanol, aqueous methanol 50%, acetone and chloroform) on UM, AoFM and RaFM was evaluated on the basis of amount of PC’s present in the extracts (Table 1). PC’s in extracts (UM) prepared from different extraction solvents were: water (3.31 mg GAE g−1), ethanol (0.96 mg GAE g−1), methanol (3.18 mg GAE g−1), methanol 50% (1.71 mg GAE g−1), acetone (0.11 mg GAE g−1) and chloroform (0.10 mg GAE g−1). After 288 h of fermentation PC’s in AoFM extracts were: water (34.90 mg GAE g−1), ethanol (4.60 mg GAE g−1), methanol (24.44 mg GAE g−1), methanol 50% (24.67 mg GAE g−1), acetone (2.63 mg GAE g−1) and chloroform (2.74 mg GAE.g−1). However, after 240 h of fermentation PC’s in RaFM extracts were water (10.79 mg GAE g−1), ethanol (1.59 mg GAE g−1), methanol (8.67 mg GAE g−1), methanol 50% (12.45 mg GAE g−1), acetone (1.34 mg GAE g−1) and chloroform (1.45 mg GAE g−1). Results showed that PC’s in UM were soluble in water whereas those of AoFM and RaFM were soluble in both aqueous as well as organic solvents. Keeping in view the importance of solubility factors, a mixture design was selected to extract out maximal PC’s from UM, AoFM and RaFM. In mixture design strategy, three different phases (water, ethanol and methanol, v/v) were used in specific ratios (Table 2). Aqueous phase ratio was in range between 65–80%, followed by ethanol (10–20%) and methanol (5–15%). Significant effect on PC’s of UM, AoFM and RaFM was observed with varying concentration of extraction phases. The results of extraction phase ratio revealed that PC’s start increasing with increase in concentration of water and ethanol. However, ethanol and methanol concentrations were inversely proportional to each other. Maximal portion in optimized extraction phase was water (73%) followed by ethanol (16%) and methanol (11%). Under these specific ratios PC’s remain higher in extracts (UM, AoFM and RaFM). Maximal amount of PC’s present in UM was 4.99 mg GAE g−1 whereas in AoFM and RaFM it was 56.95 and 16.99 mg GAE g−1, respectively. Minimal amount of PC’s in UM (3 mg GAE g−1), AoFM (52.17 mg GAE g−1) was found in extraction phase [(water: ethanol: methanol) (80:15:5 v/v)]. However, in RaFM (14.08 mg GAE g−1 dwb) minimal amount was observed with extraction phase ratio [water: ethanol: methanol (80:10:10 v/v)].
Effect of extraction temperature (°C) and time duration (min) on PC’s
The effect of temperature on PC’s depends on type and anatomy of experimental samples selected for extractions. Some of the natural resources possess PC’s in their inner matrix and they require temperature in the range of 50–70 °C for their extraction. However, in some botanical resources PC’s are present in lesser complex form and they require moderate range of temperature (40–60 °C) to leach out from their inner matrix to surrounding extraction medium. Earlier published scientific reports suggest variable range of temperature for extraction of PC’s from experimental samples: pearl millet (44.5 °C) [37]; wheat (61 °C) [38]; Centella asiatica (65 °C) [50]; roasted barley (250 °C) [51].
Optimization of extraction temperature was part of Phase-2 of this study and the temperature range selected was in range 38–62 °C (Tables 3 and 4). For the purpose to fit the response (data generated from experimental work) the linearity and quadratic effect of the independent variables, interactions among them and regression coefficients on the response variables were evaluated using analysis of variance (ANOVA) (Table 5). The model was highly significant due to a very low probability value (p < 0.0001). To determine the relationship between the tested independent variables and the response, multiple regression analysis was applied (Eq. 1):
Final equation in terms of coded factors was:-
Y = Phenolic compounds; A = Extraction temperature; B = Extraction time.
The equation in terms of coded factors can be used to make predictions about the response for given levels of each factor. By default, the high levels of the factors were coded as + 1 whereas for low levels the code was −1. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.
Final equation in terms of actual factors was:-
Y = Phenolic compounds; A = Extraction temperature; B = Extraction time.
Model equation for predicting the response values was verified by experimental runs under the set of optimized conditions. Optimization method was adopted to find a point that maximizes the response.
Increase in temperature range up to a specific degree (45 °C and 50 °C) led to maximal recovery of PC’s from millet flour (UM, AoFM and RaFM). The optimal range of temperature for UM was 45 ± 1 °C as the amount of PC’s recovered was maximum (5.08 mg GAE g−1). Enzymes produced by starter fungal strains during fermentation time remain active only up to a certain temperature range and thereafter their activity decreased. The optimal temperature for extraction of maximum PC’s from AoFM was 45 ± 1 °C however, for R. azygosporus fermented sample was 50 ± 1 °C. The results clearly demonstrate that even within the same substrate, PC’s may require different temperature conditions depending on starter fungal strains used. Duration of time (min) during the extraction process also affected the recovery of PC’s from experimental samples. Lesser time duration for extracting PC’s is one of the desired conditions in food processing industries [28]. Solubility of compounds in specific extraction phases is one of the key factors which determine the overall recovered amount of bioactive phyto-constituents from natural resources. Extraction phase may vary for experimental samples as it depends on the substrate used, types of processing applied and duration of processing. Experiments were performed under moderate range of temperature and time. The data was normally distributed as presented in Fig. 1. The regression equations in coded and actual forms are quoted as Eqs. 1 and 2. The coded equation indicates that extraction temperature was more significant than extraction time. But exceeding temperature beyond a specific range (45 ± 1 °C for UM, AoFM and 50 ± 1 °C for RaFM) had cascading effect on PC’s [Figs. 2 (a, b) and 3 (a, b)]. The optimized conditions for maximal extraction of bioactive compounds from UM, AoFM and RaFM were extraction phase [water: ethanol: methanol 73:16:11 v/v], extraction temperature (45 ± 1 °C for UM, AoFM and 50 ± 1 °C for RaFM) followed by extraction time duration (42 min for UM, AoFM and 40 min for RaFM). Under above said optimized conditions, flour from UM, AoFM and RaFM were extracted and amount of PC’s was determined in the corresponding extracts. Amount of PC’s in UM, AoFM and RaFM varied from 5.2–56.64 (mg GAE g−1 dwb) (Table 6).
Antioxidant properties (AP’s)
Microbial transformation during SSF induces various biochemical changes in millet koji and these specific changes resulted in increment in AP’s of the extracts prepared from AoFM and RaFM. Results from the antioxidant tests revealed that SSF significantly (P < 0.05) enhanced the AP’s (Table 6). Inhibition (%) during DPPH assay was maximum for AoFM on 288 h (98.41%) and RaFM on 240 h (96.17%) as compared to UM (78.32%). Microbial transformation during fermentation resulted in increased CUPRAC value for both AoFM (288 h; 24.50 mg AAE g−1 dwb) and RaFM (288 h; 20.24 mg AAE g−1 dwb). Increase in RPC was observed till 336 h for AoFM and 288 h for RaFM thereafter a decrease was observed. FRAP value of the extracts studied (UM, AoFM and RaFM) varied from 4.64–10.54 (mM Fe2+ g−1) (Table 6). HFRSA value for extracts (UM, AoFM and RaFM) varied from 23.4–37.46%.
Specific PC’s in extracts
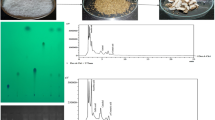
HPLC analysis (qualitative and quantitative) of extracts UM, AoFM and RaFM were carried out. Ten standards (ascorbic acid, gallic acid, catechin, resorcinol, catechol, vanillin, p-coumaric acid, quercetin, benzoic acid and cinnamic acid) were used during the analysis. UM extracts showed the presence of six PC’s [ascorbic acid (1.55 mg g−1), gallic acid (0.69 mg g−1), catechol (0.48 mg g−1), p-coumaric acid (1.04 mg g−1), benzoic acid (0.16 mg g−1) and cinnamic acid (0.71 mg g−1)]. Fermentation modulates the phenolic profile of millet koji which was confirmed from the amount and types of PC’s present in their extracts [Fig. 4 (a–c) and Table 7]. AoFM showed the presence of different PC’s [ascorbic acid (10.23 mg g−1), gallic acid (8.95 mg g−1), catechin (2.15 mg g−1), resorcinol (2.90 mg g−1), catechol (7.60 mg g−1), vanillin (4.58 mg g−1), p-coumaric acid (3.96 mg g−1), quercetin (2.74 mg g−1), benzoic acid (5.10 mg g−1) and cinnamic acid (5.14 mg g−1)]. SSF of pearl millet with A. oryzae proved to be beneficial as it enriched the millet flour with 4 important PC’s (catechin, vanillin, quercetin and resorcinol) in addition to PC’s present naturally in the millet cultivar studied. PC’s present in RaFM extract were ascorbic acid (8.80 mg g−1), gallic acid (4.70 mg g−1), catechin (0.99 mg g−1), resorcinol (0.30 mg g−1), catechol (1.10 mg g−1), vanillin (0.11 mg g−1), p-coumaric acid (1.27 mg g−1) and cinnamic acid (0.86 mg g−1). Thus, fungal fermentation could be a used method for the production of millet based products with health benefiting properties. As during the fermentation process, microbial consortia (starter culture) incorporate their own bioactive compounds into substrate being fermented by them [52].
DNA damage protection activity (DDPA)
Extracts (UM, AoFM and RaFM) were evaluated for the presence of DDPA. Quercetin solution (mg/ml in ethanol) was used as standard during agarose gel electrophoresis. Fenton’s reagent (10 µl) used during the electrophoresis assay showed its affects by degradation of pBR-322 DNA band. All the extracts studied (UM, AoFM and RaFM) possessed DDPA which was characterized by the presence of specific DNA bands during analysis. Presence of sharpness in bands indicates the maximal activity of potent antioxidants whereas mild band indicate minimal activity. AoFM extracts showed sharp bands up to 288 h of fermentation (Fig. 5a). However, DDPA in RaFM extracts remains significant in millet koji only up to 240 h of fermentation (Fig. 5b). DDPA in extracts prepared from fruits, vegetable, cereal grains and microbial extracts as well as processed food products against fenton’s reagent has also been reported in earlier published scientific reports [1, 53, 54].
a DNA damage protecting activity of HHB-197 extracts (UM and AoFM) against hydroxyl radicals induced DNA damage of pBR322. Lane 1: native pBR322 plasmid DNA; Lane 2: DNA + Quercetin (mg/ml positive control) + Fenton’s reagent; Lane 3: DNA + Fenton’s reagent; Lane 4: DNA + UM extract + Fenton’s reagent; Lane 5: DNA + AoFM extract (48 h) + Fenton’s reagent; Lane 6: DNA + AoFM extract (96 h) + Fenton’s reagent; Lane 7: DNA + AoFM extract (144 h) + Fenton’s reagent; Lane 8: DNA + AoFM extract (192 h) + Fenton’s reagent; Lane 9: DNA + AoFM extract (240 h) + Fenton’s reagent; Lane 10: DNA + AoFM extract (288 h) + Fenton’s reagent; Lane 11: DNA + AoFM extract (336 h) + Fenton’s reagent. b DNA damage protecting activity of HHB-197 extracts (UM and RaFM) against hydroxyl radicals induced DNA damage of pBR322. Lane 1: native pBR322 plasmid DNA; Lane 2: DNA + Quercetin (mg/ml positive control) + Fenton’s reagent; Lane 3: DNA + Fenton’s reagent; Lane 4: DNA + UM extract + Fenton’s reagent; Lane 5: DNA + RaFM extract (48 h) + Fenton’s reagent; Lane 6: DNA + RaFM extract (96 h) + Fenton’s reagent; Lane 7: DNA + RaFM extract (144 h) + Fenton’s reagent; Lane 8: DNA + RaFM extract (192 h) + Fenton’s reagent; Lane 9: DNA + RaFM extract (240 h) + Fenton’s reagent; Lane 10: DNA + RaFM extract (288 h) + Fenton’s reagent; Lane 11: DNA + RaFM extract (336 h) + Fenton’s reagent
Conclusions
The present research work explored the effect of SSF and extraction conditions on PC’s and antioxidants of pearl millet. Individual PC’s identified by HPLC revealed the diversity of properties present in pearl millet flour (unfermented and fermented). DDPA of extracts prepared from UM, AoFM and RaFM were greatly affected by presence of maximal PC’s. Organic extraction phases has their own effects on PC’s however aqueous phase was the most important factor to achieve maximal amount of PC’s from unprocessed and processed millet flour. Output from design expert revealed that higher ratio of water was required for good PC’s extraction in millet (unfermented and fermented). In addition, combination of temperature and time duration during optimization analysis was considered to be an effective way to elucidate their effects on phenolic profile of pearl millet. To assess the benefits of increased phenolics, moderate range of temperature and time is necessary to maintain the phenolic profile. The obtained results have shown that fermented pearl millet will be an antioxidant rich and healthy food supplement compared to unfermented pearl millet. After analysis of the other nutritional properties under optimized conditions, the fermented pearl millet can be utilized for preparation of different food products.
Abbreviations
- HPLC:
-
High performance liquid chromatography
- AoFM:
-
Aspergillus oryzae Fermented millet
- RaFM:
-
Rhizopus azygosporus Fermented millet
- SSF:
-
Solid state fermentation
- RSM:
-
Response surface methodology
- PC’s:
-
Phenolic compounds
- AP’s:
-
Antioxidant properties
- MTCC:
-
Microbial type culture collection
- PDA:
-
Potato dextrose agar
- PDB:
-
Potato dextrose broth
- UM:
-
Unfermented millet
- TPC:
-
Total phenolic compounds
- FCR:
-
Folin-Ciocalteu reagent
- GAE:
-
Gallic acid equivalent
- CCD:
-
Center composite design
- DPPH:
-
2, 2-Diphenyl–1′ picrylhydrazyl
- RSC:
-
Radical scavenging capacity
- HFRSC:
-
Hydroxyl free radical scavenging capacity
- RM:
-
Reaction mixture
- CUPRAC:
-
Cupric reducing antioxidant capacity
- AA:
-
Ascorbic acid
- AAE:
-
Ascorbic acid equivalent
- RPC:
-
Reducing power capacity
- QE:
-
Quercetin equivalent
- FRAP:
-
Ferric reducing antioxidant power
- DDPA:
-
DNA damage protection activity
- FR:
-
Fenton’s reagent
- dwb:
-
Dry weight basis
References
R.K. Salar, S.S. Purewal, K.S. Sandhu, Food Res. Int. 100, 204 (2017)
F. Shahidi, A. Chandrasekara, J. Funct. Food. 5, 570 (2013)
F. Chandrasekara, F. Naczk, F. Shahidi, Food Chem. 133, 1 (2012)
S.S. Purewal, K.S. Sandhu, R.K. Salar, P. Kaur, Food Measure. 13, 1479 (2019)
P. Kaur, S.S. Purewal, K.S. Sandhu, M. Kaur, R.K. Salar, Food Measure. 12, 793 (2019)
F. Shahidi, M. Nackz, Food Phenolics: Sources, Chemistry, Effects and Applications (Technomic Publishing Company Inc., Lancaster, PA, 1995), p. 281
P. Kaur, S.S. Purewal, K.S. Sandhu, M. Kaur, Bioresour. Bioprocess. 6, 1 (2019)
S. Singh, M. Kaur, D.S. Sogi, S.S. Purewal, Food Measure. 13, 347 (2019)
P. Kaur, S.B. Dhull, K.S. Sandhu, R.K. Salar, S.S. Purewal, Food Measure. 12, 1530 (2018)
R.K. Salar, S.S. Purewal, Food Measure. 11, 126 (2017)
P.D. Postemsky, M.A. Bidegain, R. Gonzalez-Matute, N.D. Figlas, M.A. Cubitto, Bioresour. Technol. 231, 85 (2017)
P.D. Postemsky, N.R. Curvetto, Int. Biodeterior. Biodegrad.100, 52 (2015)
H. Smith, S. Doyle, R. Murphy, Food Chem. 185, 389 (2015)
R.K. Salar, S.S. Purewal, K.S. Sandhu, Biocatal. Agric. Biotechnol. 11, 201 (2017)
S.B. Dhull, P. Kaur, S.S. Purewal, Resour. Eff. Technol. 2, 168 (2016)
R.K. Salar, P. Sharma, S.S. Purewal, Tang 5, 1 (2015)
P. Sharma, H.S. Gujral, Food Chem. 120, 673 (2010)
Q. Bei, G. Chen, F. Liu, S. Wu, Z. Wu, Food Chem. 245, 297 (2018)
F.V. Dulf, D.C. Vodnar, E.H. Dulf, Z. Diaconeasa, C. Socaciu, LWT Food Sci. Technol. 87, 241 (2018)
H.Y. Shin, S.M. Kim, J.H. Lee, S.T. Lim, Food Chem. 272, 235 (2019)
C.L. Handa, F.S. de-Lima, M.F.G. Guelfi, M.S. Fernandes, S.R. Georgetti, E.I. Ida, Food Chem. 271, 274–283 (2019).
Y. Lee, C. Hung, C Chou, Int. J. Food Microbiol. 121, 150 (2008)
S. Huang, Y. Ma, D. Sun, J. Fan, S. Cai, Int. J Food Sci. Technol. 52, 1 (2017)
K.C. Massarolo, T.D. de-Souza, C.C. Collazzo, E.B. Furlong, L.A. Soares, Food Chem. 228, 43–49 (2017).
C.G. Schmidt, L.M. Goncalves, L. Prietto, H.S. Hackbart, E.B. Furlong, Food Chem. 146, 371 (2014)
D.L. Abd-Razak, N.Y. Abd Rashid, A. Jamaluddin, S.A. Sharifudin, K. Long, Biocatal. Agric. Biotechnol. 4, 33 (2015).
S. Zemouri-Alioui, M. Bachir-bey, B.Z. Kurt, F. Sonmez, H. Louaileche, Food Measure. 13, 321 (2018)
C.Y. Cheok, N.L. Chin, A.Y. Yusof, R.A. Talib, C.L. Law, Ind. Crops. Prod. 40, 247 (2012)
K.N. Chen, M.J. Chen, in Optimization in Food Engineering, ed. By F. Erdogdu (CRC Taylor & Francis Group, Boca Raton, 2008), pp. 115–140
N. Teslic, N. Bojanic, D. Rakic, A. Takaci, Z. Zekovic, A. Fistes, M. Bodroza-Solarov, B. Pavlic, Chem. Eng. Process 143, 107634 (2019)
A. Pandey,T. Belwal, K. Chandrasekar, ID. Bhatt, RS Rawal, Indus. Crops. Prod. 119, 218 (2018)
G. Sharmila, V.S. Nikitha, S. Ilaiyarasi, K. Dhivya, V. Rajasekar, N.M. Kumar, K. Muthukumaran, C. Muthukumaran, Indus. Crops. Prod. 84, 13 (2016)
M. Chen, Y. Zhao, S. Yu, Food Chem. 172, 543 (2015)
J. Saha, A. Biswas, A. Chhetri, P.K. Sarkar, Food Chem. 129, 507 (2011)
C.Y. Gan, A.A. Latiff, Carbohydr. Polym. 83, 600 (2011)
K.W. Kong, A.R. Ismail, S.T. Tan, K.N. Prasad, A. Ismail, Int. J. Food Sci. Technol. 45, 1739 (2010)
R.K. Salar, S.S. Purewal, M.S. Bhatti, Resour. Eff. Technol. 2, 148 (2016)
C. Liyana-Pathirana, F. Shahidi, Food Chem. 93, 47 (2005)
H.I. Odabas, I. Koca, Indus. Crops. Prod. 91, 114 (2016)
M. Majeed, A.I. Hussain, S.A.S. Chatha, M.K.K. Khosa, G.M. Kamal, M.A. Kamal, X. Zhang, M. Liu, Saudi J Biol. Sci. 23, 389 (2016)
M.A. Bezerra, R.E. Santelli, E.P. Oliveira, L.S. Villar, L.A. Escaleira, Talanta 76, 965 (2008)
L. Yu, S. Haley, J. Perret, M. Harris, Food Chem. 86, 11 (2004)
G.C. Yen, H.Y. Chen, J. Agric, Food Chem. 43, 27 (1995)
N. Smirnoff, Q.J. Cumbes, Phytochem. 28, 1057 (1989)
R. Apak, K. Guclu, M. Ozyurek, S.E. Karademir, J. Agric, Food Chem. 52, 7970 (2004)
M. Oyaizu, Jpn. J. Nutr. Diet. 44, 307 (1986)
I.F.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70 (1996)
V. Kumar, M. Lemos, M. Sharma, V. Shriram, Free Radic. Antiox. 3, 55 (2013)
A.K. Siroha, K.S. Sandhu, M. Kaur, Food Measure. 10, 311 (2016)
K.K. Chew, M.Z. Khoo, S.Y. Ng, Y.Y. Thoo, W.M. Wan Aida, C.W. Ho, Int. Food Res. J. 18, 571 (2011).
M. Omwamba, Q. Hu, Eur. Food Res. Technol. 229, 907 (2009)
R.K. Salar, S.S. Purewal, K.S. Sandhu, 3 Biotech 7, 164 (2017)
R. Kaur, M. Kaur, S.S. Purewal, J Food Process. Preserv. 42, e13585 (2018)
K. Sevgi, B. Tepe, C. Sarikurkcu, Food Chem. Toxicol. 77, 12 (2015)
Acknowledgements
Authors want to thank Dr. Dev Vart Yadav, Bajra Section, Chaudhary Charan Singh Haryana Agricultural University for providing experimental sample for the present research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
This article does not contain any studies with human and animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Purewal, S., Salar, R., Bhatti, M. et al. Solid-state fermentation of pearl millet with Aspergillus oryzae and Rhizopus azygosporus: effects on bioactive profile and DNA damage protection activity. Food Measure 14, 150–162 (2020). https://doi.org/10.1007/s11694-019-00277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00277-3