Abstract
The study was aimed to determine the phytochemical content, antioxidant and cytotoxic activities along with exploring antineoplastic potency of Momordica subangulata subsp. renigera seeds. Methanolic extract (5 g) of seeds of Momordica subangulata (MESMS) was prepared by maceration of 250 g powdered sample with 500 mL methanol at room temperature. Different in vitro assays were used to estimate total phenolic and flavonoid contents as well as determine free radical scavenging activities of MESMS. Total phenolic and flavonoid contents of MESMS were found to be 113.25 and 48.6 mg (g of extract)−1 in terms of gallic acid and catechin equivalent, respectively. The MESMS showed very good scavenging activity on DPPH (IC50: 7.78 μg mL−1) and ABTS (IC50: 9.10 μg mL−1) radicals in respect to nitric oxide (IC50: 52.45 μg mL−1) and superoxide (IC50: 68.16 μg mL−1) radicals. In vitro MESMS showed 21.76–89.37% inhibitory effect against Ehrlich ascites carcinoma (EAC) cells at the concentration range of 3.125–100 μg mL−1 and the inhibitory effect was increased with increasing of concentration. In addition, MESMS (at 10 and 20 mg kg−1) significantly (P < 0.05) decreased the viable EAC cell count and body weight gain of mice due to tumor burden as well as increased 38.35% and 55.35% life span and rescued the deteriorated haematological parameters of cancer cell bearing mice. Moreover, apoptotic characteristics and induction of p53 gene expression in MESMS-treated EAC cells were noted during fluorescence microscopic observation and RT-PCR analysis. Finally it can be summarized that MESMS with rich phenolic content and antioxidant activity, inhibited EAC through p53-dependent apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In human body, reactive oxygen species (ROS) which comprises both free radical and no free radical like oxygen containing molecule, are continually formed as part of normal aerobic life. If free radicals overcome the body’s ability to regulate them, a condition known as oxidative stress arises [1]. Oxidative stress can disturb DNA, proteins and lipids, and can stimulate chronic illnesses such as cancer and cardiovascular diseases. Antioxidants inhibit or restrain the oxidation of molecules in the cells and are considered to confer positive effects against chronic diseases caused by oxidative stress. So a balance between free radicals and antioxidants is necessary for proper physiological function [1, 2]. Plant-derived polyphenolic compounds are well-known antioxidants and possess a wide range of pharmacological properties, the mechanisms of which have been the subject of considerable interest [3]. In recent years, several reports have documented that polyphenolic compounds like curcumin, resveratrol, and gallotannins induce apoptosis in various cancer cell lines [4,5,6,7]. Moreover, various polyphenols such as epigallocatechin-3-gallate, gallic acid, and resveratrol induce apoptotic cell death in various cancer cell lines but not in normal cells [5,6,7]. Polyphenolic compounds of plant origin have been strongly correlated with cancer cell inhibition through their antioxidant potentials [6, 7]. For this reason, medicinal herbs and their constituents have the merit to be a source of safe and effective antineoplastic agents.
On the basis of above observations, we had selected an important medicinal plant namely Momordica subangulata subsp. renigera, popularly known as Kakrol (Bengali). It belongs to the family Cucurbitaceae and has a wide spread occurrence in Bangladesh, India, China, Indonesia and Malaysia. In our country, Momordica subangulata subsp. renigera are mainly cultivated that can be differentiated from Momordica dioica & Momordica cochinchinensis by the comparative morphology and ecological studies (tuber’s morphology, germination pattern, fruits and seed’s size, flowers etc.) [8, 9].
Different parts of Momordica subangulata subsp. renigera were used in traditional folk medication system of Bangladesh without having knowledge about its side effects and toxicity. In experiment, it has been found that lycopene and beta-carotene contents of the seeds are much greater than pulp [10]. Saponin, terpenoid, triterpenoid, alkaloids, carbohydrates are recorded in fruits [11]. However, to the best of our knowledge there have so far been no reports available on the antioxidant status as well as anticancer efficacy of seeds of Momordica subangulata subsp. renigera. Therefore, the present study was undertaken in order to evaluate antioxidant, cytotoxic and antineoplastic potentials of the seeds of Momordica subangulata subsp. renigera. (MESMS) using Ehrlich ascites carcinoma (EAC) in Swiss albino mice. The study also focused on the chemical composition of MESMS.
Materials and methods
Chemicals and reagents
2,2′-Diphenyl-1-picrylhydrazyl (DPPH·), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS·+), catechin (CAT), sulphanilamide, hydroxylamine hydrochloride, Hoechst 33342, RPMI 1640-medium, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA) whereas N-(1-naphthyl)ethylenediamine dihydrochloride, aluminum chloride (AlCl3·6H2O), gallic acid (GAE), NBT (p-nitro blue tetrazolium), sodium carbonate (Na2CO3), sodium nitrite (NaNO2), trypan blue, isopropanol and RNA extraction kit (TRIzol) were from Carl Roth GmbH (Karlsruhe, Germany). Penicillin–streptomycin and fetal calf serum were obtained from country distributer of Invitrogen (USA) and Folin Ciocalteu (FC) reagent was supplied from Sisco Research Laboratory, Mumbai, India. Methanol and other solvents were purchased from E-Mark (Germany) and all the chemicals used here were of analytical and HPLC grade with 99.9% purity.
Collection of plant materials and authentication
The genus Momordica belongs to the family Cucurbitaceae and comprises of about 40 species distributed chiefly in Africa in South-East Asia [12]. Momordica subangulata subsp. renigera is one of the species available in Bangladesh and like other Momordica species, germination of Momordica subangulata seeds is very difficult or impossible because of the hard seed coat. So seeds of this plant are formally not available in the market. First we collected Momordica subangulata plants with mature fruits from Rajshahi district (Geographically located at 24°22′26″N88°36′04″E, 23 m i.e., 75 ft above sea level) using random sampling techniques. The collection period of this plant was February, 2018. This plant was authenticated by Professor Dr. A. H. M. Mahbubur Rahman (a taxonomist), Department of Botany, University of Rajshahi and a sample specimen was preserved (No. 17) in the herbarium of this department for further reference. The seeds were then removed from the mature fruits and were washed thoroughly with tap water, dried in shade for 1 week and stored in airtight glass container till further use.
Extraction of plant materials
After shade drying, collected seeds of Momordica subangulata were immediately pulverized into a coarse powder with the help of grinder (FFC-15, China). Then according to the previously described method for extraction [13], 250 g of the powdered sample was kept immersed in 500 mL methanol contained in an aspirator bottle for 7 days at room temperature. This methanolic extraction was carried out in triplicate. After filtration, the solvent was completely removed by rotary vacuum evaporator (Model: RE-2000E, Kori instrument, China) to have 5 g the crude methanol extract of seed of Momordica subangulata subsp. renigera (designated as MESMS) and stored in a vacuum desiccator for further uses.
Estimation of total phenolic content
Total phenolic contents of MESMS were estimated according to the Folin-ciocalteu reagent (FCR) method [14, 15]. Briefly, 1 mL methanolic solution of gallic acid of different concentration (0.00005–0.005 mg mL−1) and MESMS (0.0025 mg mL−1) was added to 5 mL of FCR. The each mixture was shaken and left to stand for 5 min before the addition 1 mL of 5% (w v−1) sodium carbonate (Na2CO3) solution. Then 3 mL distilled water was added to make the total solution 10 mL. The mixture was allowed to stand for 60 min at room temperature. The absorbance was taken at 760 nm using a UV–visible spectrophotometer (HALORB-10, Dynamica Scientific, Australia) with noise (500 nm) of ≤ 0.0005 and the experiment was repeated three times. The total phenolic content (TPC) of MESMS was calculated using the equation of gallic acid based calibration curve (y − 0.076x = 0.013, R2 = 0.992) and expressed as in mg of gallic acid (GAE) equivalent (g of the extract)−1.
Estimation of total flavonoid content
Flavonoid content was measured by the aluminum chloride colorimetric assay [16]. 1 mL of extract (0.04 mg mL−1) and catechin of different concentration (0.001–0.003 mg mL−1) were taken in different test tubes and mixed with an equal volume of 2% (wv−1) AlCl3·6H2O solution in methanol. The mixture was vigorously shaken and after 10 min of incubation, the absorbance was taken at 430 nm. Calibration curve (y − 0.1557x = 0.0321, R2 = 0.979) for catechin was drawn and the total flavonoid content (TFC) of MESMS was calculated by the same way as described in the estimation of total phenolic content and expressed as in mg of catechin (CAT) equivalent (g of the extract)−1.
DPPH radical scavenging assay
DPPH radical scavenging activity of MESMS was measured according to the method described previously [17]. A solution of 0.1 mM DPPH was prepared in methanol and 3 mL of this solution was mixed with 1 mL of extract/catechin (standard) in methanol at different concentrations (4.0–9.0 μg mL−1 for extract; 0.25–8.0 μg mL−1 for catechin). The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517 nm. The ability to scavenge the DPPH radical was calculated using the following equation:
where Ao is the absorbance of the control (blank, without extract); A1 is the absorbance in the presence of the extract/standard; A2 is the absorbance without DPPH. All samples were analyzed in triplicate. Finally percentage DPPH radical scavenging activity was plotted against concentration and IC50 was calculated from the graph.
ABTS radical scavenging assay
Free radical scavenging activity of MESMS was determined by ABTS radical cation decolorization assay [18]. ABTS·+ cation radical was produced by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate (1:1), stored in the dark at room temperature for 12–16 h before use. The ABTS·+ solution was then diluted with water to obtain an absorbance of 0.70 ± 0.02 at 734 nm. ABTS·+ solution (3 mL) was added to 0.1 mL of MESMS with various concentrations (2.0–32.0 μg mL−1) and mixed vigorously. The absorbance was measured at 734 nm after standing for 6 min. Catechin (0.24–2.0 μg mL−1) was used as standard substance. The percentage (%) scavenging effect for ABTS·+ and IC50 were calculated by the similar way as described in DPPH· assay.
Nitric oxide radical scavenging assay
We had used previously described method where sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide which interacts with oxygen to produce nitric ions that can be estimated by using Griess reagent [19]. Scavengers of nitric oxide compete with oxygen leading to reduce production of nitric oxide. The sodium nitroprusside (5 mM) in phosphate buffer saline (PBS) was mixed with 3.0 mL of different concentrations (6.25–100 μg mL−1) of MESMS and incubated at 25 °C for 150 min. The samples were added to Griess reagent containing 1% (wv−1) sulphanilamide, 2% (v/v) H3PO4 and 0.1% (wv−1) napthylethylenediamine dihydrochloride. The absorbance of the chromophore formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with napthylethylenediamme, was measured at 546 nm. The percentage of scavenging was calculated by the following formula:
where Ao was the absorbance of the control (blank, without extract) and A1 was the absorbance in the presence of the extract. Catechin was used here as standard. All the tests were performed in triplicate and the graph was plotted with the mean values for determination of IC50.
Superoxide radical scavenging assay
Superoxide radical scavenging activity was measured using NBT (nitro blue tetrazolium reagent) method [20]. In this assay, 1 mL of 5 mM sodium carbonate, 0.4 mL of 0.24 mM NBT and 0.2 mL of 0.1 mM EDTA solutions were added to the test tubes containing MESMS with different concentrations (3.12–100 μg mL−1) and reading was taken immediately at 560 nm. Then 0.4 mL of 1 mM hydroxylamine hydrochloride was added to initiate the reaction and reduction of NBT was measured at 560 nm after incubation at 25 °C for 15 min. Decrease in the absorbance of reaction mixture indicates increased superoxide anion scavenging activity. Absorbance was recorded and the percentage of scavenging effect was calculated according to the following equation:
where Ao is the absorbance of the initial reading of sample/standard and A1 is the absorbance of final reading. Catechin was used as the reference compound.
Brine shrimp lethality assay
Cytotoxicity of the MESMS was screened against brine shrimp nauplii according to published protocol [21]. Artemia salina Leach (brine shrimp eggs) was allowed to hatch and mature as nauplii (Larvae) in seawater for 48 h at 25 °C. Six doses (10, 20, 40, 80, 100, and 160 μg mL−1) of MESMS were applied from their corresponding stock solutions to the seawater (5 mL), containing 10 nauplii and the percentage of mortality was determined after 24 h. The LD50 values were calculated using probit analysis.
Tumor cells
EAC cells required for this study were collected from the Indian Institute for Chemical Biology (IICB), Kolkata, India and maintained by weekly i.p. inoculation of 106 cells mouse−1 under laboratory conditions.
In vitro cell viability test by MTT colorimetric assay
In vitro EAC cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum, and 1% (vv−1) penicillin–streptomycin, in a humidified atmosphere of 5% CO2 at 37 °C. To find out the possible link between in vivo and in vitro anticancer effects of MESMS, MTT assays were applied to examine cell viability as demonstrated by Kabir et al. [22] with little modification. In brief, EAC cells (2.5 × 105 in 200 µL RPMI-1640 media) were plated in the 96-well culture plate in the presence of different concentrations (3.125–100 µg mL−1) of MESMS. These cells were then incubated for 24 h. Subsequently, 20 µL of MTT (0.5 mg mL−1 final concentration) was added to each well and again incubated for 8 h at 37 °C. After removal of the supernatant again, 200 µL of acidic isopropanol was added into each well to dissolve the crystals. The absorbance values were recorded at 570 nm by an automatic ELISA plate reader (DR-200Bs Microplate Reader, Diatek Instruments, China). Cell proliferation inhibition ratio was calculated by the following equation:
where Ao is the OD570 nm of the cellular homogenate without MESMS (control) and A1 is the OD570 nm of the cellular homogenate with MESMS.
Animals and ethical clearance
Adult (5–6 weeks old) Swiss albino male mice, weighing 25 ± 5 g were obtained from International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B). The mice were housed in polypropylene cages (maximum six mice per cage). Sterile paddy husk was used as bedding material and the mice were fed with standard mouse food-pellets (collected from ICDDR’B) and water ad libitum. Permission and approval (289/345-IAMEBBC/IBSc) for animal studies were achieved from Animal Ethics board of Institute of Biological Sciences, University of Rajshahi.
Acute toxicity study
The acute toxicity study was performed in order to determine the LD50 value of MESMS in mice according to the procedure of Lorke [23]. Briefly, a single intraperitoneal injection was done on each group of mice (n = 6) with various doses [100, 200, 400, 800, 1600, 3200 mg (kg b wt)−1]. After 24 h of treatment, the LD50 values were determined by recording mortality.
Study on MESMS-induced cell growth inhibition (in vivo)
Cell growth inhibition activity of MESMS was examined by using four groups of Swiss albino mice (n = 6) weighing 26 ± 4 g. For this purpose, 1.5 × 106 EAC cells were inoculated into the mice of each group on day 0 and after 24 h of inoculation, treatment was commenced and continued for 5 days. Groups 1 and 2 mice received MESMS at the doses of 10 and 20 mg kg−1 day−1, respectively, via intraperitoneal injection. Group 3 was treated with bleomycin, a standard anticancer drug (0.3 mg kg−1 day−1) whereas group 4 was used as untreated control. After 5 days of treatment, EAC cells were harvested in normal saline (0.9% NaCl) and then viable cells were counted with a haemocytometer under inverted microscope (XDS-1R, Optika, and Bergamo, Italy) using trypan blue [24]. EAC cells derived from MESMS-treated (20 mg kg−1 day−1) and untreated (control) were also used for studying the morphological characteristics and p53 gene expression.
Studies on morphological appearance of MESMS treated EAC cells
Morphological changes in the nuclear chromatin of EAC cells obtained from MESMS-treated (20 mg kg−1 day−1) and control mice were detected by staining with Hoechst 33342 fluorochrome [25]. Briefly, the collected EAC cells were washed with phosphate buffered saline (PBS) and fixed with Hoechst 33342 (final concentration 10 µg mL−1 in PBS) at room temperature for 10 min in the dark. Nuclear morphology was examined using a fluorescence microscope (Olympus iX71, Korea). Intact blue nuclei and condensed or fragmented nuclei were considered viable and apoptotic cells, respectively.
Study on p53 gene expression using RT-PCR
PCR specificity was determined using both a melting curve analysis and gel electrophoresis. In RT-PCR (Real time-PCR), TRIzol was applied to extract total RNA from EAC cells of MESMS-treated and untreated mice groups. Then 3 μg RNA was reversed transcribed into cDNA. Expression of p53 gene was studied using this cDNA as template for PCR. GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) was used as the housekeeping gene. A BioRad (USA) gradient thermal cycler was used for amplification. The cycling condition was initial PCR activation step of 5 min at 95 °C, followed by 35 cycles of 95 °C min−1, 55 °C min−1, 72 °C min−1, and final 72 °C (10 min)−1 (elongation). All the PCR reactions were analyzed in 1.0% agarose gel and Gene Rular 1 kb DNA ladder (Fermentus, USA) was used as marker. Moreover, we also measured relative quantities (RQ) of p53 tumor suppressor mRNA of control and treated EAC cells by quantitative real time PCR using a melting curve analysis [26]. Primer sequences used for the RT-PCR assay of p53 are given below: 5′-GCGTCTTAGAGACAGTTGACT-3′ and 5′-GGATAGGTCGGCGGTTCATGC-3′.
Studies on survival time and average weight gain of EAC cell bearing mice
The effect of MESMS on survival time and weight gain (due to tumor burden) of EAC cell bearing mice were also examined [27]. Briefly, mice were divided into four groups (n = 6). On the initial day, each mouse of each group was inoculated with 1.5 × 106 EAC cells and treatment was started following 24 h of tumor inoculation and continued for ten days. Groups 1 and 2 received MESMS at the doses of 10 and 20 mg kg−1 day−1 whereas group 3 was treated with bleomycin, as a standard anticancer drug. Animals of group 4 were used as untreated EAC (control). Daily weight change and survival time of each mouse were recorded to monitor the weight gain and mean survival time (MST), respectively. MST and percent increase of life span, (% ILS) were calculated using the formula as described previously [27].
Studies on hematological parameters
The effect of MESMS on hematological parameters was studied using the method of Islam et al. [28]. Briefly, 24 mice in four groups (n = 6) were injected with EAC cells (1.5 × 106 cells mouse−1) intraperitoneally except the normal group (group 1) at the day zero. Group 2 was used as EAC control. MESMS at 10 and 20 mg kg−1 day−1 doses were administered in groups 3 and 4, respectively for 10 days. On the 12th day after EAC cell inoculation, blood was collected from the tail vein of each mouse of each group to determine the hematological parameters (Hemoglobin, RBC and WBC).
GC–MS analysis of MESMS
Separation and identification of the components of MESMS were performed by gas chromatography/mass spectrometry (GC/MS) detector analysis using GCMS-QP2010S (Shimadzu Kyoto, Japan) spectrometer equipped with a Rxi-5MS fused silica capillary column (5% diphenyl/95% dimethyl polysiloxane) and AOC-20i+s (autosampler) of 0.25 mm diameter, 30 m length, and 0.25 μm film thickness [29]. The sample size of 2 μL was injected through the injector. The inert gas helium was used as carrier gas. The MS was taken at 70 eV of ionization energy. Column flow was 1.21 mL min−1 and total flow was 16.3 mL min−1. Flow control with linear velocity was 39.9 cm s−1. The initial oven temperature was set at 60 °C for 1 min and ramped at 10 °C min−1 to 180 °C for 1 min and then ramped at 20 °C min−1 to 280 °C for 15 min. Scan range 20–550 mz−1 and a scan interval of 0.50 s, 260 °C with a split ratio of 10:0 and signal to noise ratio (S/N) ≥ 200:1. Total running time of GC–MS was 65 min. The relative % amount of each component was expressed as a percentage with peak area.
Statistical analysis
Analysis of data were performed by one way ANOVA (analysis of variance) followed by Dunnett’s ‘t’ test using SPSS software of 16 version. All results were represented as mean ± standard deviation (SD). Differences at P < 0.05 level were considered to be statistically significant.
Results and discussion
Phytochemical content and antioxidant activity of MESMS
Plants containing high phenolic compounds can be a good source of antioxidants. This information has led to the determination of the total phenolic and flavonoid contents of MESMS. The Folin–Ciocalteu method is simple and this method has been applied to measure the total phenolic content of many plants [30, 31]. Moreover, flavonoids are one kind of phenolic compounds that is considered to have antioxidant and antineoplastic activities [32]. The data for total phenolic and flavonoid contents of MESMS are shown in Table 1. MESMS contained 113.25 ± 3.78 mg of GAE equivalent (g of dry extract)−1 where as its flavonoid content was found to be 48.6 ± 1.17 mg of CAT equivalent (g of dry extract)−1.
In this study DPPH, ABTS, nitric oxide and superoxide scavenging assays were used here to accurately assess the antioxidant activity of MESMS. As a free radical, DPPH accepts an electron or hydrogen radical donated from the antioxidant to become a stable diamagnetic molecule, which is widely used in the evaluation of free radical scavenging activity [33]. On the other hand, ABTS is more reactive than DPPH· and unlike the reactions with DPPH, which involves H atom transfer; the reactions with ABTS involve an electron transfer process [34]. The scavenging activity of MESMS on DPPH and ABTS assays was shown in Table 1. A dose–response relationship was found in DPPH scavenging activity of MESMS and an increase in concentration was synonymous with an increase in scavenging capacity. Catechin (used as the positive standard) showed high scavenging activity whereas the IC50 values of MESMS were 7.78 ± 1.06 and 9.10 ± 1.72 µg mL−1 in case of DPPH and ABTS activities, respectively. This radical-scavenging activity of MESMS could be related to the phenolic compounds, thus contributing to their electron transfer/hydrogen donating ability.
Nitric oxide is an essential bioregulatory molecule required for several physiological processes like neural signal transmission, immune response, control vasodilatation and control of blood pressure [35]. Nitric oxide plays an important role in various types of inflammatory processes in the animal body. In this study, nitric oxide radical generated from sodium nitroprusside at physiological pH, was found to be inhibited by MESMS in a dose dependent manner (IC50: 52.45 ± 2.26 µg mL−1).
Superoxide radical is known to be very harmful to cellular components as a precursor of the more reactive oxygen species, contributing to tissue damage and various diseases like cancer [36]. Different concentrations of MESMS scavenged superoxide radical and its IC50 value on superoxide radical scavenging activity was found to be 68.16 ± 2.94 µg mL−1, whereas the IC50 value of catechin was found to be 17.45 ± 0.94 µg mL−1 (Table 1).
Plant kingdom with rich amount of phenolic and flavonoid compounds has produced remarkable antioxidant effects thereby preventing numerous oxidative stress associated diseases as well as cancer [37,38,39,40]. In this investigation, we found MESMS as a rich source of phenolic and flavonoid compounds and it acts as a strong free radical scavenger (Table 1). These data are in accordance with others, which have shown that a high total phenolic content of seeds and other plant parts increases antioxidant activity and there is a linear correlation between phenolic content and antioxidant activity [24, 41, 42].
Cytotoxic effect of MESMS against brine shrimp nauplii and EAC cells (in vitro)
The brine shrimp lethality bioassay in which the simple zoological organism, brine shrimp nauplii (Artemia salina, Leach) is used, is efficient, rapid and inexpensive tests [43] that correlates reasonably well with cytotoxic and other biological properties [44]. In this study, the results of brine shrimp lethality bioassay demonstrated that MESMS showed a moderate activity against the nauplii and the LD50 value was found to be 106.33 ± 2.17 µg mL−1 for MESMS whereas ampicillin trihydrate (standard) had a LD50 of 7.21 ± 0.47 µg mL−1. As brine shrimp lethality bioassay is a primary assay to detect cytotoxic property of plant extract, it is necessary to establish the cytotoxicity of MESMS against human cancer cell lines. As a continuation along this direction, in vitro MESMS was tested against EAC cell line using MTT cytotoxicity assay. A decrease of viable cells was observed at a concentration of MESMS as low as 3.125 µg mL−1 and loss of cell viability was found to be increased with the increasing its concentrations (Fig. 1a). The IC50 value of the MESMS was determined as 13.12 µg mL−1 against EAC cell (Fig. 1b).
Previously many scientists have got findings of similar pattern when they used seed extract against cancer cell lines [45]. In MTT assay study, exposure of EAC cells to various concentrations of MESMS for 24 h triggered significant cell death in a dose dependent fashion (Fig. 1a) which indicated the in vitro capacity of an extract to inhibit the growth of cancer cell [46]. Moreover, previous reports also showed a strong correlation between polyphenolic content of plants and their cytotoxic efficacy [47]. So the high polyphenolic content of MESMS is a strong justification of its cytotoxic effects.
LD50 of MESMS from acute toxicity study
Acute toxicity studies mainly aims at establishing the safe therapeutic dose and intraperitoneal administration of various doses of MESMS to Swiss albino mice showed a LD50 of 2658.70 mg (kg b wt)−1. So MESMS was very safe at 10 and 20 mg (kg b wt)−1 therapeutic doses.
The effect of MESMS on cell proliferation, survival time and weight gain of EAC bearing mice
EAC cells are also expedient for anticancer drug test due to their suitability to study in mice model. Ehrlich ascites carcinoma (EAC) is a swiftly growing experimental tumor with very aggressive behavior and resembles human tumors [48]. Therefore, antineoplastic activity of MESMS against EAC cell bearing mice was assessed by the parameters such as viable cell count, mean survival time and increase in body weight due to tumor burden. Treatment with MESMS at 10 and 20 mg (kg b wt)−1 doses, significantly (P < 0.05) reduced the viable tumor cell count in respect to untreated EAC control group. MESMS at 10 mg (kg b wt)−1 and 20 mg (kg b wt)−1 doses induced 54.72 and 72.13% inhibition of EAC cell growth, respectively whereas it was 90.29% for the standard bleomycin [0.3 mg (kg b wt)−1] (Table 2). The EAC cells implantation induces per se a local inflammatory reaction with increasing vascular permeability, which results in an intense edema formation, cellular migration and a progressive ascitic fluid formation [49]. The ascitic fluid is essential for tumor growth, since it constitutes a direct nutritional source for tumor cells [50]. The inhibition of EAC cells in this study could indicate either a direct cytotoxic effect of MESMS on EAC cells or an indirect local effect, which may involve macrophage activation, vascular permeability inhibition and nutritional fluid deficiency.
On the other hand, The MST was increased to 27.67 ± 1.6 (% ILS = 38.35) and, 31.25 ± 1.5 (% ILS = 55.35) days upon administration of MESMS at doses of 10 and 20 mg (kg b wt)−1, respectively, while the EAC control and the reference drug bleomycin showed survival times of 20.25 ± 1.29 and 39.25 ± 0.79 (% ILS = 93.82) days, respectively (Table 2). The average weight gain of untreated control group was 16 ± 0.75 g whereas it was significantly (P < 0.05) reduced by treatment of MESMS and bleomycin (Table 2). The prolongation of the animal life span was being considered as a reliable criterion for the depiction of efficacy of an anticancer agent [51]. This study showed that MESMS can significantly inhibit the EAC cells growth, decrease average weight gain and enhance survival time of the EAC-cell bearing mice that are deliberated for the verdict of effectiveness of a convinced compound as anticancer agent and trustworthy condition for evaluating the efficiency of any anticancer drug [52].
The effect of MESMS on hematological parameters of EAC cell bearing mice
Hematological parameters of EAC bearing mice were found to be significantly altered relative to the normal group (Table 3). The total WBC count was found to be increased (P < 0.05) with a reduction of Hgb content and RBC count in EAC control animals when compared with the normal saline group. On the other hand, administration of MESMS at 10 and 20 mg (kg b wt)−1 doses significantly (P < 0.05) restored all hematological parameters to normal levels in EAC cell bearing mice (Table 3).
In cancer chemotherapy myelosuppression and anemia are the foremost troubles due to the reduction of RBC or hemoglobin percentage [53]. Recovery of the hemoglobin content, RBC and WBC cell count in the experimental mice indicates the protective action of MESMS on the hemopoietic system. In justifying the potency of a compound in cancer chemotherapy, all these factors are considered as important and promising subjects [28].
Effect of MESMS on morphological appearance and p53 gene expression in EAC cell
Apoptosis is a central cell suicidal mechanism that can be synchronized by frequents cellular signaling pathways and portrayed morphologically by cell shrinkage, chromatin condensation, nuclear fragmentation and formation of apoptotic body [48]. EAC cells harvested from the mice treated with and without methanol extract were stained with Hoechst 33342 to examine the cell morphological changes and fluorescence microscopic observation revealed that untreated EAC cells exhibited a round and homogeneously stained nucleus. But EAC cells treated with MESMS triggered cell membrane blebbing, nuclear condensation and fragmentation of EAC cells (Fig. 2) that are noteworthy demonstration of apoptotic cell death [52]. The above findings confirmed the MESMS-induced apoptosis in EAC cells.
In case of apoptosis, the multifunctional transcriptional factor p53 has been reported to play a key role in many apoptotic cell death models. p53 is one of the most momentous proapoptotic peacekeepers and it is inactivated in more than 50% of human cancers [54]. Preneoplastic and malignant cells correspond to a protective mechanism against induction of p53 [55]. We sought to investigate whether MESMS treatment could upregulate the p53 gene expression or not. The quantitative analysis of p53 mRNA level by RT-PCR showed a marked increase mRNA level of p53 gene in EAC cells obtained from mice challenged with MESMS [20 mg (kg b wt)−1] compared to untreated control (Fig. 3a). The relative mRNA expression in EAC cells from untreated control was found to be 0.241 ± 0.09 whereas it was 1.157 ± 0.19 in case of EAC cells from MESMS treated group. The products of RT-PCR for MESMS treated EAC cells had also given clear band on agarose gel stained with ethidium bromide (Fig. 3b). So this finding indicates that apoptosis induced in MESMS treated EAC cell was probably mediated by p53-dependent pathway.
Effect of MESMS on p53 mRNA expression in EAC cells. a Real-time PCR relative quantification of p53 mRNA levels in untreated (EAC control) and MESMS-treated EAC cells; b PCR reaction products separated on agarose gel stained with ethidium bromide. L DNA ladder, C RNA from EAC cells of untreated control, T RNA from EAC cells of MESMS-treated mice
In addition, polyphenols can directly influence different points of the apoptotic process, and/or the expression of regulatory proteins. A growing body of evidence provides new insight in the comprehension of the cellular and molecular mechanisms responsible for the induction of apoptosis by polyphenols [56]. Moreover, plant extracts induced apoptosis in isolated rat hepatic mitochondria by increasing the mitochondrial membrane permeability pore opening [57]. These evidences indicate a correlation between induction of apoptosis by MESMS and its high polyphenolic content with antioxidant activity.
Chemical profile of MESMS analyzed by GC–MS
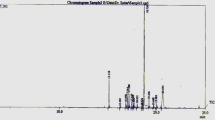
The chemical profile of MESMS identified by GC–MS spectrum (Fig. 4), are summarized in Table 4. The GC–MS analysis revealed that m-pentadecylphenol (62.321%), palmitic acid (5.947%), oleic acid (4.355%), arachidic acid (2.533%) linoleic acid (0.550%) and phthalic acid, 6-ethyloct-3-yl 2-ethylhexyl ester (24.294%) are the major compounds present in MESMS. The previous studies has reported on the antitumor activity of palmitic acid, oleic acid and linoleic acid [58,59,60]. So the chemical profile of MESMS confirmed by GC–MS act as a strong support for its antineoplastic effect.
Conclusion
In conclusion, the overall novel outcomes from this study indicate that seeds of Momordica subangulata have rich phenolic content as well as promising antioxidant, cytotoxic and apoptotic properties as revealed by the in vitro and vivo studies. Nevertheless, it is indispensable to accomplish inclusive research on further parts of this plant via miscellaneous solvent extracts to represent the most dexterous extract adjacent to cancer as well as to appreciate the exact mechanisms of action of these activities throughout applying diverse cancer cell lines and our future research plan is oriented in this direction.
References
P.A. Riley, Int. J. Radiat. Biol. 65, 27–33 (1994)
B. Halliwell, J.M. Gutteridge, C.E. Cross, J. Lab. Clin. Med. 119, 598–620 (1992)
W.V. Berghe, Pharmacol. Res. 65, 565–576 (2012)
E. Jaruga, S. Salvioli, J. Dobrucki, S. Chrul, J. Bandorowiz-Pikula, E. Sikora, C. Franceschi, A. Cossarizza, G. Bartosz, FEBS Lett. 433, 287–293 (1998)
M.V. Clement, J.L. Hirpara, S.H. Chawdhury, S. Pervaiz, Blood 92, 996–1002 (1998)
M. Inoue, R. Suzuki, T. Koide, N. Sakaguchi, Y. Ogihara, Y. Yabu, Biochem. Biophys. Res. Commun. 204, 898–904 (1994)
N. Ahmad, D.K. Feyes, A.L. Nieminen, R. Agarwal, H. Mukhtar, J. Natl. Cancer Inst. 89, 1881–1886 (1997)
J.K. Joseph, V.T. Antony, Y.C. Roy, Resour. Crop. Evol. 54, 1327–1332 (2006)
K.J. John, V.T. Antony, J. Marydas, R. Karuppaiyan, Genet. Res. Crop. Evol. 56, 861–868 (2006)
S. Singh, S. Swain, M. Nisha, V.S. Banu, D.R. Singh, S.D. Roy, Ind. Crops Prod. 73, 154–163 (2015)
A.N. Madane, S.K. Kamble, B.J. Patil, V.T. Aparadh, Asian J. Pharm. Res. 3, 53–55 (2013)
M. Ali, H. Okubo, T. Fujii, K. Fujieda, Sci. Hortic. 47, 335–343 (1991)
J. Azmir, I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, A.K.M. Omar, J. Food Eng. 117, 426–436 (2013)
F. Alhakmani, S. Kumar, S.A. Khan, Asian Pac. J. Trop. Biomed. 3, 623–627 (2013)
R.K. Sahu, M. Kar, R. Routray, J. Med. Plants Stud. 1, 21–27 (2013)
L. Silva, B.R. Pezzini, L. Soares, Pharmacogn. Mag. 11, 96–101 (2015)
K. Pavithra, S. Vadivukkarasi, Food Sci. Hum. Well. 4, 42–46 (2015)
Y. Cai, Q. Luo, M. Sun, H. Corke, Life Sci. 74, 2157–2184 (2004)
S. Banerjee, A. Chanda, A. Ghoshal, R. Debnath, S. Chakraborty, R. Saha, A. Das, Asian J. Exp. Biol. Sci. 2, 595–599 (2011)
M. Fontana, L. Mosca, M.A. Rosei, Biochem. Pharmacol. 61, 1253–1257 (2001)
R. Perveen, F. Islam, J. Khanum, T. Yeasmin, Asian Pac. J. Trop. Med. 5, 121–125 (2012)
S.R. Kabir, A. Hossen, A. Zubair, F. Islam, A. Hossain, Y. Kimura, Protein Pept. Lett. 18, 1140–1149 (2011)
D. Lorke, Arch. Toxicol. 54, 275–287 (1983)
S. Islam, S. Nasrin, M.A. Khan, S.M. Hossain, F. Islam, P. Khandokhar, M.N. Mollah, M. Rashid, G. Sadik, M.A. Rahman, A.H. Alam, BMC Complement. Altern. Med. 13, 142 (2013)
S.R. Kabir, M.M. Nabi, A. Haque, R. Zaman, Z.H. Mahmud, M.A. Reza, Phytomedicine 20, 1288–1296 (2013)
K.M. Ahsanul Kabir, R. Amin, I. Hasan, A.K.M. Asaduzzaman, H. Khatun, S.R. Kabir, Int. J. Biol. Macromol. 125, 92–98 (2019)
M.R. Habib, M.A. Aziz, M.R. Karim, J. Appl. Biomed. 8, 47–54 (2010)
F. Islam, H. Khatun, M. Khatun, S.M. Ali, J.A. Khanam, Pharm. Biol. 52, 281–290 (2014)
F. Pragst, V. Auwaerter, F. Sporkert, K. Spiegel, Forensic Sci. Int. 121, 76–88 (2001)
S.P. Mazur, A. Nes, A.B. Wold, S.F. Remberg, K. Aaby, Food Chem. 160, 233–240 (2014)
S. Sellappan, C.C. Akoh, G. Krewer, J. Agric. Food Chem. 50, 2432–2438 (2002)
H. Kim, J.Y. Moon, A. Mosaddik, S.K. Cho, Food Chem. Toxicol. 48, 2435–2442 (2010)
J.R. Soares, T.C.P. Dins, A.P. Cunha, L.M. Ameida, Free Radic. Res. 26, 469–478 (1997)
S. Kaviarasan, G. Naik, R. Gangabhagirathi, C. Anuradha, K. Priyadarsini, Food Chem. 103, 31–37 (2007)
G.C. Jagetia, M.S. Baliga, K.J. Malagi, M.S. Kamath, Phytomedicine 9, 99–108 (2002)
A. Phaniendra, D.B. Jestadi, Ind. J. Clin. Biochem. 30, 11–26 (2015)
N.G. Shehab, A. Mahdy, S.A. Khan, S.M. Noureddin, Res. J. Med. Plant 5, 531–546 (2011)
T. Fotsis, M.S. Pepper, E. Aktas, S. Breit, S. Rasku, H. Adlercreutz, K. Wahala, R. Montesano, L. Schweigerer, Cancer Res. 57, 2916–2921 (1997)
D.K. Singh, S.M. Lippman, Oncology (Williston Park) 12, 1643–1653 (1998)
P.J. Ferguson, E. Kurowska, D.J. Freeman, A.F. Chambers, D.J. Koropatnick, J. Nutr. 134, 1529–1535 (2004)
I.A. Adebayo, H. Arsad, M.R. Samian, Pharmacogn. Mag. 14, 191–194 (2018)
M. Holasova, V. Fiedlerova, H. Smrcinova, M. Orsak, J. Lachman, S. Vavreinova, Food Res. Int. 35, 207–211 (2002)
S. Ramachandran, M. Vamsikrishna, K.V. Gowthami, B. Heera, M.D. Dhanaraju, Asian J. Sci. Res. 4, 90–94 (2011)
Q.S. Sarah, F.C. Anny, M. Misbahuddin, Bangladesh J Pharmacol. 12, 186–189 (2017)
P. Graidist, M. Martla, Y. Sukpondma, Nutr. J. 7, 2707–2718 (2015)
S.R. Kabir, M.F. Islam, M.J. Alom, M.A. Zubair, N. Absar, Protein Peptide Lett. 19, 360–368 (2012)
T. Ismail, P. Sestili, S. Akhtar, J. Ethnopharmacol. 143, 397–405 (2012)
J.A. Segura, L.G. Barbero, J. Marquez, Immunol. Lett. 74, 111–115 (2000)
M. Shimizu, C. Azuma, T. Taniguchi, T. Murayama, J. Pharmacol. Sci. 96, 324–332 (2004)
P.K. Samudrala, B.B. Augustine, E.R. Kasala, L.N. Bodduluru, C. Barua, M. Lahkar, Pharmacognosy Res. 7, 66–73 (2015)
P.K. Haldar, S. Bhattacharya, B. Kar, A. Bala, U.K. Mazumder, Toxicol. Environ. Chem. 92, 1749–1763 (2010)
J. Hirsch, JAMA 296, 1518–1520 (2006)
H.C. Hoagland, Semin. Oncol. 9, 95–102 (1982)
D. Hanahan, R.A. Weinberg, Cell. 100, 57–70 (2000)
H. Mukhtar, Cancer Lett. 326, 123–127 (2012)
M. D’Archivio, C. Santangelo, B. Scazzocchio, R. Varì, C. Filesi, R. Masella, C. Giovannini, Int. J. Mol. Sci. 9, 213–228 (2008)
J.A.O. Olugbuyiro, D. Odugbesan, S.O. Rotimi, S.L. Uchechukwu, Int. J. Pharm. Sci. Res. 8(7), 3122–3127 (2017)
H. Harada, U. Yamashita, H. Kurihara, E. Fukushi, J. Kawabata, Y. Kamei, Anticancer Res. 22, 2587–2590 (2002)
C. Carrillo, M. Cavia, S.R. Alonso-Torre, Nutr. Hosp. 27, 1860–1865 (2012)
Y. Zhou, T. Wang, S. Zhai, W. Li, Q. Meng, Public Health Nutr. 19, 1457–1463 (2016)
Acknowledgements
The authors are thankful to IICB (Indian Institute of Chemical Biology), Kolkata, India, authority for providing the EAC cells and also to ICDDRB, for supplying the Swiss albino mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karmakar, P.C., Yesmin, R., Ali, H. et al. Antioxidant, cytotoxic and apoptotic potentials of seeds of Momordica subangulata subsp. renigera inhibit the growth of Ehrlich ascites carcinoma in mice. Food Measure 13, 3049–3059 (2019). https://doi.org/10.1007/s11694-019-00227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00227-z