Abstract
Bioactive compounds, antioxidant activity and extraction yield of Teucrium polium aerial parts by different concentrations of aqueous and alcoholic solvents [water, ethanol and ethanol:water (50:50)] were investigated by ultrasonic and maceration. The total phenolic, tocopherol, tannin, flavonoid content and antioxidant activity (200–1200 ppm) of the extracts were determined and compared with butylated hydroxyanisol (BHA) (as a synthetic antioxidant) by DPPH· assay, β-carotene/linoleic bleaching inhibition and OSI methods. The results showed that the highest extraction yield (40.26%) and total phenolic (65.74 mg/g), tocopherol (112.13 µg/mL), tannin (5.90 mg/g) and flavonoid (5.60 mg/g) content were observed in ethanol:water (50:50) ultrasonic extraction. Moreover, as the highest bioactive components extracted by ethanol:water (50:50) ultrasonic methods, it signified that the highest radical scavenging activity with 91.45% and β-carotene/linoleic bleaching 84.14% inhibition at 1200 ppm of the extract which is higher than BHA (200 ppm) with 88.31% DPPH· radical scavenging activity and 76.83% inhibition in β-carotene/linoleic bleaching assay. Furthermore, addition of water as the safe, available and inexpensive solvent increased efficiency of ethanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The medicinal plants have similar properties of conventional pharmaceutical drugs which were used from ancient times [1]. They are considered as important sources of new bioactive substances with beneficial therapeutic effects. Recently, the consumer interest in natural antioxidants consumption has considerably increased as an alternative of synthetic ones which are being limited owing to their carcinogenic property and the other side effects [2]. These natural herbs and plants not only protect food products from deterioration, but also provide health benefits by reason of preventing from biological damage [3]. As part of our efforts to find an edible herb which was reported its health beneficial properties, we have investigated the bioactive substances of Teucrium polium. The genus Teucrium (family Lamiaceae, subfamily Teucrioideae, tribe Teucriaea) is represented by more than 340 species and about 12 species in Iran [4]. T. polium or Halpeh is a wild-growing flowering plant. It is a perennial shrub, which is distributed in hills and deserts of many countries such as South Western Asia, Europe and North Africa [5].
In Iran, the tea of T. polium was used traditionally for curing many diseases such as abdominal pain, indigestion, type 2 diabetes, common cold and rheumatism [6]. Besides, it was used for various purposes such as anti-inflammatory, anti-mutagenic, anti-cancer, anti-nociceptive, anti-oxidant, anti-bacterial, anti-hypertensive and anti-hyperlipidemic properties [7, 8]. Moreover, it could be helpful for body weight reduction and lowering high blood pressure [9]. Kadifkova Panovska et al. [10] exemplified that extracts of T. polium by using different organic solvents (diethyl ether, ethyl acetate and n-butanol) were effective inhibitors of β-carotene oxidation. Also, the HPLC analysis of T. polium extract revealed the presence of phenolic components such as ferulic acid, caffeic acid and quercetin [11].
Antioxidants from herbs and plants can be extracted by various solvents and extraction methods [12, 13]. Solvent extraction is the most common technique used for isolating bioactive compounds of plants. Solvent properties are important parameters in antioxidative compounds extraction [14]. The types and yield of extracted bioactive compounds are different as affected by the solvent properties such as polarity, vapor pressure and viscosity. Among the different extraction methods, ultrasonic-assisted extraction is one of the successful extraction techniques that which could offer high reproducibility, reduced solvent, energy, temperature and time consumption and also make easier manipulation [15]. Ultrasonic cavitations generate shear forces which disrupt cell walls and also increase mass transfer and extraction yield (EY). Indeed, ultrasonic assisted extraction due to reduction of solvent consumption may provide more economical, environmental, and safety bioactive components isolating method [16].
The main novelties of the present work were (1) to extract bioactive compounds such as total tannin, flavonoid, tocopherol and phenolic compounds from T. polium with ultrasound-assisted extraction and maceration, (2) to compare the yield extract of these methods, (3) to explore the antioxidative activity of these extracts (200–1200 ppm) through the use of several commonly assays.
Materials and methods
Materials
Teucrium polium (200 g) was obtained from Shiraz city, Fars province, Iran in 2015. Voucher specimens of T. polium from are deposited by botanists in Herbarium of Pharmacy School, Mashhad University of Medical Sciences. All chemicals and solvents were provided from Sigma and Merck companies. Folin–Ciocalteu reagent from Merck (Darmstad, Germany), DPPH· (2,2-diphenyl-1-picrylhydrazyl) and β-carotene were purchased from Sigma-Aldrich (St. Louis, MO). The butylated hydroxyanisol (BHA) used as control antioxidant provided from TITRAN. All other chemicals used were of analytical grade.
Preparation of T. polium
Teucrium polium aerial parts were collected, dried in shade and grinded by mixer (Panasonic, MK-G20NR).
Maceration extraction
The powdered plant material (10 g) was mixed with water, ethanol and ethanol:water (50:50), separately. The ratio of solvent to dried plant is 10:1 (v/w). The matrix was extracted in a shaker (LABTRON Ls-100) for 12 h in 160 rpm at room temperature, filtered through Whatman No 1. and the solvent was removed under vacuum oven at 120 mm Hg at 40 °C. The residual extracts by various solvents were weighted and stored at − 18 °C under a nitrogen gas flow [17].
Ultrasound-assisted extraction
The ultrasound-assisted extraction method was used for the extraction of T. polium powder (10 g) with various solvents including water, ethanol and ethanol:water (50:50). The ratio of solvent to the material like as maceration is 10:1 (v/w). The extracts were sonicated for 20 min in an ultrasonic bath (Elma s 30 H model, 280 W and 20 kHz, Heating Power: 200 W and internal dimensions: 198 × 106 × 50 cm). The temperature was being controlled (45 °C) by circulating water. The extracts were filtered and the following steps were done consistent with the procedure mentioned in “Maceration extraction” section [12].
Determination of extraction yield
The EY was calculated from the following equation:
where \({m}_{0}\) is the weight of dried curd extraction (g) and \(\text{m}\) is the weight of T. polium powder (g).
Determination of total tannin content
Total tannin content was established by the Folin–Ciocalteu method [18]. Plant extract was mixed with adsorbent (powder of polyvinyl polypyrrolidone) and then stirred for 1 h; then, stored for 1.5 h at 4 °C to homogenize tannin and polyvinylpyrrolidone complex. Subsequently, the pH was decreased (pH 3) by using polyvinylpyrrolidone. Sample was centrifuged at 4000 rpm for 15 min. No adsorbed phenolics in the supernatant were specified by the Folin–Ciocalteu method. Calculated values were subtracted from total polyphenol contents before and after adding polyvinyl polypyrrolidone and expressed as milligram gallic acid equivalents per gram extract.
Determination of total phenolic content
The total phenolic content (TPC) was determined by using Folin–Ciocalteu’s reagent [19]. The 0.5 mL of T. polium extracts were added, a tenfold diluted Folin–Ciocalteu reagent (2.5 mL) and Na2CO3 (2 mL, 7.5%), in a volumetric flask reaching the volume (50 mL) with water. The samples were stored 12–15 h and analyzed at 765 nm by spectrophotometer. Results were expressed in mg of gallic acid per g of extract.
Determination of total flavonoid content
Flavonoids are polyphenols that found in plants and herbs with a wide variety of biological and functional activities [20]. Total flavonoid content was measured as stated by Dewanto et al. [21]. Each extract (250 µL) was mixed with NaNO2 (75 µL, 5%). After 6 min, AlCl3 (150 µL, 10%) and NaOH (500 µL, 1 M) were added to the mixture. Finally, the mixture was adjusted with distilled water (2.5 mL). The absorbance was read at 510 nm. Total flavonoid content of aerial parts of T. polium was expressed as mg catechin equivalents per g through the calibration curve with catechin.
Determination of total tocopherol content
Tocopherols were evaluated spectrophotometrically [12]. 0.2 g of T. polium extract was solved in toluene (5 mL) and 2.2-bipyridine (3.5 mL, 0.07% in 95% aqueous ethanol) and FeCl3·6H2O (0.5 mL, 0.2% in 95% aqueous ethanol) to make a volume by adding 95% aqueous ethanol. After 1 min, the absorbance was read at 520 nm. Results were expressed as µg of α-tocopherol equivalents per mL of extract.
Determination of radical scavenging activity
The effectiveness of water, ethanol and ethanol:water (50:50) extracts (200–1200 ppm) of T. polium to scavenge DPPH· free radicals was identified by the method explained by Lima et al. [22]. Decrease in methanol solution absorbance at 517 nm containing DPPH· radicals, after 30 min without exposure to light, was the basis of DPPH· assay. DPPH· free radical inhibition (I%) was calculated by following equation:
In this equation, Ablank is control absorbance, Asample is sample extract absorbance, BHA was used as a control antioxidant.
β-Carotene/linoleic bleaching inhibition
Lipid peroxidation inhibition activities of the T. polium extracts (200–1200 ppm) were determined using the β-carotene bleaching method [23]. β-carotene (5 mg) was dissolved in chloroform (10 mL), the solution (600 µL) was mixed with linoleic acid (40 mg) and Tween 40 (400 mg). Chloroform was evaporated by rotary vacuum evaporator. Distilled water (100 mL) was added and shaken intensively, the upper solution (2.5 mL) was conveyed to the test tube and each extract (350 µL, 2 g/L) was added. The absorbance values of samples were read at 470 nm after 2 h at 50 °C. Antioxidant capacity of the extracts was expressed as percentage inhibition:
Determination of oxidative stability index (OSI)
Different concentrations of the T. polium extracts (200–1200 ppm) and BHA (200 ppm) as control antioxidant were exposed to Rancimat (Metrohm model 734, Herisan Switzerland) at 120 °C at an airflow of 15 L/h.
Statistical analysis
Three experimental replicates were done. Data were expressed as the mean and standard deviation and analyzed by one-way ANOVA, followed by Duncan’s multiple range test. Analyses were carried out to a significance level of p < 0.05, using the software program SPSS.
Results and discussion
Extraction yield
The choice of solvent and its polarity is a crucial point for antioxidant activity assessment of extracts. The powder form of T. polium could be boiled in water, which is polar compound, and used as herbal tea. Thus, the polar solvents were preferred for extraction of T. polium materials [24]. Water was an effective solvent to increase the rate of plant particles swelling and as a result, the contact surface of the solvent and plant matrix was developed [25]. Undeniably, efficiency of the diverse solvent in extraction relies on the plant components matrix as well as the type of extractable compounds [26].
The solvents used for the extraction of T. polium aerial parts exhibited statistically significant different yields (Table 1). It can be seen that the EY of ethanol:water (50:50) is higher than pure ethanol or water. This may be caused by the combination of organic solvent and water that facilitates the extraction of all compounds that were soluble in both water and organic solvents. The highest EY was obtained by using ethanol:water (50:50) ultrasonic (p < 0.05). Actually, ultrasonic waves through cavitation phenomena causes cell wall destruction and came out the bioactive content. The results of this study are in agreement with the EYs of some medicinal plants [27,28,29].
Total phenolics content
One of the secondary metabolites of plants is phenolic compounds that are able to obstruct lipid oxidation and chelate redox active metal ions [30]. Different factors, such as the type of solvent used, structure of phenolic compounds and insoluble complexes and also interact ions between the phenolic and other plant matrix effect on the phenolic compound solubility. Thus, there is no general method for the extraction of all plant’s phenolic content. In this study, phenolics were evaluated by Folin–Ciocalteu reagent method and results were expressed as gallic acid equivalents. As shown in Table 1, ethanol:water (50:50) ultrasonic method had the highest (p < 0.05) TPC as well as the ethanol:water (50:50) mixture considering the maceration methods. No difference (p > 0.05) was found between ethanol:water (50:50) maceration and ethanol ultrasonic. Ethanol maceration had intermediate values (p < 0.05) for TPC. Water maceration and water ultrasonic showed the lowest (p < 0.05) values for TPC, which may be attributed to the content of more nonphenol compounds such as terpene in water extracts than in other extracts. It may also be caused by the possible complex formation of some phenolic compounds in the extract that are soluble in ethanol. These phenolic compounds may have more phenol groups or have higher molecular weights than the phenolics in the water extract. Farahmandfar et al. [29] compared antioxidant activity of Tarom Mahali rice bran extracted by ultrasound assisted and traditional solvent [ethanol and ethanol:water (50:50)] extraction methods. The results indicated that the phenolic content may contribute directly to antioxidant activity. In ethanol:water (50:50) extraction, the phenolic content increased as a result of higher polarity (stronger hydrogen bonds) compared to ethanol extraction. Water as a polar solvent improved mass transfer by the viscosity reduction. Ethanol:water (50:50) ultrasonic treatment through cavitation bubbles can make a significant difference in phenolic content compared to other extraction methods.
Moreover, ultrasonic intensity could exceed the surface tension force. So, the cavitation bubbles were produced easily and phenolic compounds extraction was more from plant tissues. Solvent viscosity also could affect the extractability of bioactive components from plant matrix. Low-viscosity solvents have high diffusivity which allows them to rapidly diffuse into the pores of the plant particles to leach out the bioactive constituents [25]. Ethanol:water (50:50) in ultrasonic treatment can help extract more phenolic content of T. polium. Total phenolic and flavonoid content of different extracts with solvents (water, methanol, ethyl acetate, acetone, petroleum ether) from the whole plant and plant parts (leaves, flowers and stems) of T. polium were determined by Stankovic et al. [31] and the results showed that the total phenolic and flavonoid contents ranged between 14.57–157.84 and 6.48–139.87 mg/g, respectively. The methanolic leaves extract was the greatest concentration of phenolic compounds (157.84 mg of GAE/g) and showed strong antioxidant activity in their research.
Total tannin contents
Tannins major features are derived from their phenolic nature. For instance, their antioxidant capacity is linked to the phenolic rings present in their structure, which can act as electron scavenger to trap ions and radicals. Owing to this antioxidant nature, tannins are widely utilized in different areas such as the pharmaceutical, medical or food industry [32]. Total tannin contents were clarified as gallic acid equivalents in milligrams per gram (mg GAE/g). As shown in Table 1, ethanol:water (50:50) maceration and ethanol:water (50:50) ultrasonic T. polium extract had the most tannin content (respectively 5.98 mg/g and 5.90 mg/g), followed by ethanol maceration (4.78 mg/g), water ultrasonic (3.92 mg/g), ethanol ultrasonic (3.77 mg/g) and water maceration (3.31 mg/g). The relationships between tannins contents and extraction solvents can be related to the polymerization degree for the tannins extracted by different solvents [33]. The interactive abilities of solvent and tannins compounds are probably related to chemical compositions and structures. The solubility tannins are correlated to the degree of polymerization due to the increase in the number of hydroxyl groups [34]. This means that this plant contains polymeric and oligomeric tannins. The findings of our research are in accordance with reported by other researchers [35, 36].
Total flavonoids content
Flavonoids are effective lipid-soluble antioxidants that can play as nutritional components and antioxidant agents. These compounds are essential components for the diet of animals because they are only synthesized by oxygenic photosynthetic organisms like plants [37]. Total flavonoids were measured as catechine quivalents in milligrams per gram (mg catechin/g extract). The total flavonoid content of T. polium extract varied from 2.12 to 5.60 mg/g (Table 1) and the highest amount was obtained with ethanol:water (50:50) ultrasonic (5.60 mg/g) while the lowest total flavonoid content was determined with water maceration (2.12 mg/g) (p < 0.05). It was observed that the effect of solvents on total flavonoid content is similar to that on TPC. The result is similar to the extraction of flavonoids from guava, banana [38] and Limnophila aromatic [27]. Sharififar et al. [39] studied the different solvents (petroleum ether, chloroform, methanol and water) for extraction and analyzed the major flavonoids of T. polium. The results showed that fractionation of the methanol extract yielded four major flavonoids including rutin, apigenin, 3,6 dimethoxy apigenin and 4,7 dimethoxy apigenin.
Total tocopherol content
Tocopherols play and important role in minimizing and preventing the oxidation of susceptible lipid molecules. Although, this antioxidant function has been considered to be primary role of tocopherols for several decades, it is becoming increasingly clear that this antioxidant vitamin has a range of other important functions in cell biology [40]. The tocopherols occur in α, β, γ and δ forms, determined by the number and position of methyl groups on the chromanol ring. As shown in Table 1, on the basis of total tocopherol content, ethanol:water (50:50) ultrasonic with 112.13 µg/mL acted better than other extraction procedures and the final level with the lowest total tocopherol content, is ethanol maceration (92.08 µg/mL) (p < 0.05). Hachicha et al. [41] researched the fatty acid, tocopherol and sterol content of three Teucrium species from Tunisia. The results showed that the major tocopherol was the α-isomer in T. polium and this plant is a potential source of tocopherol. As a consequence, T. polium can be a good source of bioactive compounds for the inhibition of lipid peroxidation.
Antioxidant activity
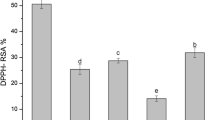
Oxidation is a reaction that occurs in the presence of oxygen and produce free radicals, peroxides and singlet/triplet oxygen which are the main causes of edible oil deterioration [42]. The antioxidative activity of bioactive compounds of plants and herbs extracts due to marvelous redox properties, could easily decompose peroxides and free radicals and also quenching the photooxidation agents [43]. The antioxidative activity of these extracts was considered through the universal assays including DPPH· radical-scavenging activity assay, β-carotene/linoleic bleaching inhibition and OSI, which were be used for evaluating lipid oxidation degree. DPPH· radical-scavenging test is one of the general assays for determination the electron donating potential and antioxidant activity of various biological compounds [44]. DPPH· radical scavenging of T. polium from different extraction methods is presented in Table 2. In this assay, β-carotene deterioration through peroxyl radicals from linoleic acid oxidation is our basis to determine antioxidant activity of the extracts. If an effective antioxidant presents, the loss of yellow color of β-carotene is postponed. So, the β-carotene decomposition is contributed to the extract antioxidant capacity [45]. β-carotene/linoleic bleaching inhibition of T. polium in different concentrations and methods is illustrated in Table 3. The principle of Rancimat test is referred to as the increase of electrical conductivity due to volatile carboxylic acids which are produced during lipid oxidation [46]. Higher OSI values meant higher antioxidant activities. Addition of antioxidant agents with high antioxidant activity is a technique to minimize rancidity, prolong the OSI value and extent the edible oil shelf life. Table 4 showed OSI (h) of different concentrations of T. polium extract at 120 °C and airflow rate of 15 L/h.
Results showed that the extraction method and conditions of extraction had significant effects on antioxidant activity of T. polium extracts (200–1200 ppm). Among various solvent extracts of T. polium, the ethanol:water (50:50) ultrasonic extract possessed the highest antioxidant activity, while the water maceration extract showed the lowest antioxidant activity (p < 0.05). The results show that BHA as a synthetic antioxidant, has better hydrogen donating ability compared to other extracts at the same concertation (200 ppm). Extracts obtained using the combination of ethanol and water accelerates the extraction of polar and nonpolar compounds which were soluble in both water and ethanol solvents. On the other hand, ultrasound increases the efficiency and speed of extraction processes for food components bioactive ingredients, including antioxidants. Hence, there is a positive trend between the antioxidant activity and phenolic content of extracts. Our data clearly confirmed that the extract of T. polium possesses potent antioxidant properties.
Conclusion
The total phenolic, tocopherol, tannin, flavonoid content and antioxidant activity (200–1200 ppm) of T. polium were evaluated by ultrasonic and maceration using aqueous and alcoholic solvents [water, ethanol and ethanol:water (50:50)]. Ethanol:water (50:50) ultrasonic extract of the aerial part of T. polium showed a high antioxidant activity due to high amount of bioactive chemicals, such as flavonoids, tocopherols and phenolics. These components could have a synergistic effect through different antioxidant mechanisms, such as free radical scavenging, interrupting chain reactions, binding metal catalysts and disintegrating peroxides. These T. polium could be a good source of antioxidants that would help to increase the shelf life of food products and protect it against lipid peroxidation.
References
E. Asnaashari, M. Asnaashari, A. Ehtiati, R. Farahmandfar, J. Food Meas. Charact. 9(2), 215–224 (2015)
M. Asnaashari, B. Hashemi, S. Mohammad, H.M. Mehr, S.H. Asadi, Yousefabad, Food Technol. Biotechnol. 53(1), 81–86 (2015)
C. Hu, D. Kitts, Phytomedicine 12(8), 588–597 (2005)
V. Mozafarrian, Dictionary of the Names of Iranian Plants (Farhange Moaser, Tehran, 1998), pp. 43–86
S. Bahramikia, R. Yazdanparast, Phytother. Res. 26(11), 1581–1593 (2012)
S. Bahramikia, A. Ardestani, R. Yazdanparast, Food Chem. 115(1), 37–42 (2009)
M. Abdollahi, H. Karimpour, H.R. Monsef-Esfehani, Pharmacol. Res. 48(1), 31–35 (2003)
H. Rasekh, M. Khoshnood-Mansourkhani, M. Kamalinejad, Fitoterapia 72(8), 937–939 (2001)
M.-S. Suleiman, A.-S. Abdul-Ghani, S. Al-Khalil, R. Amin, J. Ethnopharmacol. 22(1), 111–116 (1988)
T. Kadifkova Panovska, S. Kulevanova, M. Stefova, Acta. Pharm. 55(2), 207–214 (2005)
C. Proestos, I. Boziaris, G.-J. Nychas, M. Komaitis, Food Chem. 95(4), 664–671 (2006)
R. Farahmandfar, M. Asnaashari, R. Sayyad, J. Essent. Oil Bear. Plant 20(1), 196–204 (2017)
S.H. Nile, A.S. Nile, Y.-S. Keum, 3 Biotech 7(1), 1–10 (2017)
M. Asnaashari, R. Tajik, M.H.H. Khodaparast, J. Food Sci. Technol. 52(8), 5180–5187 (2015)
M.K. Khan, M. Abert-Vian, A.-S. Fabiano-Tixier, O. Dangles, F. Chemat, Food Chem. 119(2), 851–858 (2010)
K. Vilkhu, R. Mawson, L. Simons, D. Bates, Innov. Food Sci. Emerg. Technol. 9(2), 161–169 (2008)
C. Chotimarkorn, S. Benjakul, N. Silalai, Food Chem. 111(3), 636–641 (2008)
K. Msaada, M.B. Jemia, N. Salem, O. Bachrouch, J. Sriti, S. Tammar, I. Bettaieb, I. Jabri, S. Kefi, F. Limam, Arab. J. Chem. 10, S3176–S3183 (2017)
F. Pourmorad, S. Hosseinimehr, N. Shahabimajd, Afr. J. Biotechnol. 5(11), 1142–1145 (2006)
V. Farzaneh, I.S. Carvalho, Ind. Crops Prod. 65, 247–258 (2015)
V. Dewanto, X. Wu, K.K. Adom, R.H. Liu, J. Agric. Food Chem. 50(10), 3010–3014 (2002)
C.F. Lima, M. Fernandes-Ferreira, C. Pereira-Wilson, Life Sci. 79(21), 2056–2068 (2006)
D. Zhang, Y. Hamauzu, J. Food Agric. Environ. 1(2), 22–27 (2003)
V. Goulas, V. Exarchou, A.N. Troganis, E. Psomiadou, T. Fotsis, E. Briasoulis, I.P. Gerothanassis, Mol. Nutr. Food Res. 53(5), 600–608 (2009)
M.J.O. Wijekoon, R. Bhat, A.A. Karim, J. Food Compos. Anal. 24(4), 615–619 (2011)
M. Rezaie, R. Farhoosh, M. Iranshahi, A. Sharif, S. Golmohamadzadeh, Food Chem. 173, 577–583 (2015)
Q.D. Do, A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji, Y.-H. Ju, J. Food Drug Anal. 22(3), 296–302 (2014)
M.C. Bowyer, Q. Van Vuong, I.A. Van Altena, C.J. Scarlett, Ind. Crops Prod. 67, 192–200 (2015)
R. Farahmandfar, M. Asnaashari, R. Sayyad, J. Food Sci. Technol. 52(10), 6385–6394 (2015)
Z. Sayyari, R. Farahmandfar, Food Sci. Nutr. 5(2), 266–272 (2017)
M.S. Stankovic, N. Niciforovic, V. Mihailovic, M. Topuzovic, S. Solujic, Acta Soc. Bot. Pol. 81(2), 117–122 (2012)
P.L. de Hoyos-Martínez, J. Merle, J. Labidi, F. Charrier–El Bouhtoury, J. Clean. Prod. 206, 1138–1155 (2019)
R. Naima, M. Oumam, H. Hannache, A. Sesbou, B. Charrier, A. Pizzi, F. Charrier–El Bouhtoury, Ind. Crops Prod. 70, 245–252 (2015)
S. Felhi, A. Daoud, H. Hajlaoui, K. Mnafgui, N. Gharsallah, A. Kadri, Food Sci. Technol. 37(3), 483–492 (2017)
R. Murugan, T. Parimelazhagan, J. King Saud Univ. Sci. 26(4), 267–275 (2014)
W. Pothitirat, M.T. Chomnawang, R. Supabphol, W. Gritsanapan, Pharm. Biol. 48(2), 182–186 (2010)
S. Boussahel, F. Cacciola, S. Dahamna, L. Mondello, A. Saija, F. Cimino, A. Speciale, M. Cristani, Nat. Prod. Res. 32(16), 1911–1919 (2018)
M. Alothman, R. Bhat, A. Karim, Food Chem. 115(3), 785–788 (2009)
F. Sharififar, G. Dehghn-Nudeh, M. Mirtajaldini, Food Chem. 112(4), 885–888 (2009)
F. Galli, A. Azzi, Biofactors 36(1), 33–42 (2010)
S.F. Hachicha, S. Barrek, T. Skanji, H. Zarrouk, Z.G. Ghrabi, Chem. Nat. Compd. 45(3), 304–308 (2009)
M. Asnaashari, R. Farhoosh, R. Farahmandfar, J. Sci. Food Agric. 96(13), 4594–4602 (2016)
P.C.H. Hollman, M.B. Katan, Food Chem. Toxicol. 37(9), 937–942 (1999)
M. Asnaashari, R. Farhoosh, A. Sharif, Food Chem. 159, 439–444 (2014)
P. Ljubuncic, S. Dakwar, I. Portnaya, U. Cogan, H. Azaizeh, A. Bomzon, Evid. Based Complement. Alternat. Med. 3(3), 329–338 (2006)
Y.-E. Sun, W.-D. Wang, H.-W. Chen, C. Li, Crit. Rev. Food Sci. Nutr. 51(5), 453–466 (2011)
Acknowledgements
We are grateful to Sari Agricultural Sciences & Natural Resources University (SANRU) for financial support under Project No. 02-1395-15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farahmandfar, R., Asnaashari, M. & Bakhshandeh, T. Influence of ultrasound-assist and classical extractions on total phenolic, tannin, flavonoids, tocopherol and antioxidant characteristics of Teucrium polium aerial parts. Food Measure 13, 1357–1363 (2019). https://doi.org/10.1007/s11694-019-00051-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00051-5