Abstract
To explore the potential of winged bean seed protein as a food ingredient, an oil-in water emulsion, [25% (w/w), pH 3] stabilized by the protein (5% solution) was prepared by high-pressure homogenizer and subjected to heat (35–75 °C), cold (4–8 °C) and NaCl (1–4%) conditions alone or in combination, and their physicochemical stability to the treatments was assessed by measuring creaming, droplets characteristics and rheology. Result showed a slight increase in mean droplet size at treatment up to 35 °C and 1–2% NaCl. At treatment of ≥ 55 °C and ≥ 3% NaCl, significant increases in mean droplet size were observed, droplet distribution changed from monomodal to bimodal, advanced flocculation and coalescence up to 75 °C, leading to poly-disperse distribution. No flow was observed until a yield value (σ0) was overcome. At 1% NaCl, the σ was 13 Pa s compared to 21 Pa s at 3–4% NaCl, and above 35 °C treatment, emulsions had high apparent viscosity and exhibited Bingham plastic model resulting from increased droplets sizes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several plant proteins have been tested for their dual properties of surface activity as well as stabilizers of food emulsion. Plant proteins such as flax seed [1], soybean proteins [2, 3], pea isolate and concentrates [2, 4], whey protein [5, 6] have been studied, and the influence(s) of pH, ionic strength and thermal processing on the stability of many protein-stabilized emulsions have also been investigated [7,8,9]. The use of protein in stabilizing food emulsions depends on understanding the interfacial behavior of the adsorbed proteins, and the relationship between interfacial characteristics and the bulk physicochemical properties of the emulsion [10]. Isolated plant protein are employed in food products such as salad dressing, mayonnaise, spreads, dressings and other products as ingredients with surface-active properties and as stabilizers. The stability of emulsions with desirable physicochemical, microstructural and rheological characteristics is essential for used as delivery vehicle for photosensitive lipid-soluble micronutrients. According to German, O’Neill [11], a perfect amphiphile should be soluble and flexible enough to rapidly absorb, coat fresh surfaces as they are exposed and then interact sufficiently among adjacent droplets to form a stable film. However, not all proteins possess this capacity, and different proteins of varying sizes, structures and flexibilities differ dramatically in their ability to emulsify and stabilize emulsions [12,13,14]. At pH values below their isoelectric points (pI), proteins produce cationic emulsion droplets that protects unsaturated lipids and micronutrients such as β-carotene from oxidation by inhibiting ferrous and ferric-lipid interactions than do non-ionic surfactants [8, 9, 15,16,17]. The choice of the fat phase is also critical and is usually selected based on nutritional profile, solid fat content (SFC), crystallization behavior, oxidative stability, flavor release and density and viscosity of the intended molecules [16, 18, 19]. Though soybean oil has been employed due in part to its SFC that had been implicated in emulsion destabilization via partial coalescence [20,21,22], the presence of polyunsaturated fatty acids (PUFA) subjects it to rapid oxidation and consequently breakdown. Even though no single fat possesses all the desirable characteristics for a perfect O/W emulsion, the choice of an ideal fat is a matter of compromises in favor of the most critical properties governing emulsion stability. The use of winged bean as emulsifier and stabilizer of o/w emulsion is scanty because until recently, the winged bean remained an underutilized crop in spite of its reported potentials protein and lipids sources [22, 23]. Thus, in the study that followed, soybean oil was used taking advantage of its low SFC [19, 24, 25].
The objective of the present study was to determine the physicochemical and rheological stability of winged bean protein-stabilized o/w emulsion under varying salt, temperature and ionic conditions assessed by droplets size characteristics, flocculation and rheological properties.
Materials and methods
Materials
The winged bean seeds, AKIN-0199 (dark-brown color), was provided by the Malaysia Agricultural Research and Development Institute, MARDI, Malaysia, whereas soybean oil was purchased from a local manufacturer (Daisy, Serdang, Malaysia). Sodium citrate, sodium hydroxide, hydrochloric acid were purchased from Sigma (Sigma Chemical Co. St. Louis, USA) and Merck (Merck Co., Ltd. Darmstadt, Germany). All other chemicals were of analytical grade purchased from their respective suppliers.
Methods
The winged bean seeds were cleaned, manually dehulled and milled using variable speed rotor mill (Pulveristte-14, Fritsch, GmbH, Germany) and passed through a 500 µm test sieve (ASTM Grade) mounted onto an electromagnetic sieve shaker (Analysette 3, Fritsch, Germany), yielding 0.5 mm particle size flour. Flours were fat-extracted with n-hexane and the meal air-dried to remove hexane traces and then stored in sealed aluminium-lined plastic bags at below 4 °C until used. To extract the proteins, 100 g winged bean flour was dispersed in 600 mL distilled water and then adjusted to pH 9 then mixed with a glass rod before extraction using a magnetic stirrer (Cimarec Thermo-Scientific, Massachusetts, USA) at 75% of its speed for 2 h at room temperature. On standing for 30 min, the mixture was centrifuged (Multifuge-3L, Thermo Electric Corp., GmbH, Germany) at 3500×g for 10 min and the pooled supernatants after three extractions were precipitated at isoelectric pH of 4.2 [26]. After 30 min standing, slurry was centrifuged at 3500×g for 10 min and the pellet re-solubilized in distilled water, neutralized, lyophilized and stored at 4 °C until used. The protein content of the winged bean extracted protein was found to be 81.68% [22].
Formulation and preparation of emulsion
The aqueous phase was prepared following the method described by Mundi and Aluko [27] by suspending the protein powder (1–5.0% w/v) in citrate buffer (5 mM, pH 3.0) at room temperature using a magnetic stirrer (Cimarec, Thermo-Scientific, Massachusetts, USA) for 2 h at room temperature to ensure complete dispersion of the powders. Coarse oil-in-water emulsions (n = 6) were prepared by homogenizing weighed amounts of soybean oil (5–30% w/w) with aqueous phase (95–70% w/w) using a homogenizer (Silent Crusher M, Heidolph Instrument, Schwabach, Germany) at 15,000 rpm for 3 min at room temperature. The coarse emulsions were passed three times through a 2-stage high-pressure (valve) homogenizer (Niro Soavi, Parma, Italy) at 35 MPa to reduce the mean droplet diameter (d4,3) to ≤ 1 µm, then kept in capped 250 mL bottles at below 4 °C before further treatments. Based on the different emulsion blends prepared above, a final 25% (w/w) O/W emulsion in 5% protein solution was further prepared in two portions: one portion analyzed after 24 h while the other portion was treated (“Determination of the effect of chilling, salt and/or temperature”) and analyzed 5 days after storage. Each sample (n = 3) was determined in triplicates and results expressed as mean ± standard deviation of three determinations.
Determination of the effect of chilling, salt and/or temperature
Emulsion was divided into three portions: a portion of the emulsion was analyzed after 24 h storage at ambient temperature while the other portions were treated under changing salt and under different temperature conditions (at pH 3) then kept for 5 days. Accordingly, the effects of salt were determined by diluting the final emulsion with 1, 2, 3, and 4% aqueous NaCl in 15 mL test tubes (16-mm internal diameter, 120-mm in height). The effects of high temperature conditions were investigated by heating the different salted emulsions in a water bath (Julabo, SW 22, Labortechnik, Eisenbohnstrabe, Germany) at 35 °C, 55 °C and 75 °C for 30 min and stored at room temperature for 5 days. To determine the effect of chilling on the stability of the emulsions, samples in 15 mL centrifuge tubes were kept at 4, 6 and 8 ± 1 °C then stored for 5 days before assessment for droplets size characteristics such as flocculation and coalescence using polarized light microscopy (PLM) and their flow properties analyzed. The refrigerator condition (for chilling) was earlier obtained by placing a thermometer inside the refrigerator for 10–15 min to equilibrate to the measuring temperatures (4, 6 and 8 °C). Each sample (n = 3), 35 °C, 55 °C and 75 °C treatment, was determined in triplicates for the effects of 1, 2, 3 and 4% NaCl, respectively and results expressed as mean ± standard deviation of three determinations.
Determination of particle-size and distribution
The particle-size distribution of the emulsions was measured using laser light scattering instrument (Malvern-Sizer 2000, Malvern Instruments Ltd, Worcestershire, UK). The refractive index of the oil and of water at 25 °C was taken as 1.47 and 1.33, respectively, yielding an index of 1.09. Emulsions were stirred gently and inverted a few times to ensure sample homogeneity and an aliquot added to 650 mL of distilled water contained in a Hydro 2000S beaker attached to the instrument until the required level of obscuration (≤ 5%) was attained while also avoiding multiple scattering effects. The droplet properties were characterized by their distribution in volume (%) versus droplet diameter (d4.3) index, defined as ∑nidi4/∑nidi3: where ni is the number of emulsion droplets of diameter di as equivalent spherical diameter based on Mie theory [28] and reported as volume-weighted mean (or d4,3) index. Each sample (n = 3) was prepared in triplicates and analyzed three times automatically by the Size Analyzer (Malvern-Sizer 2000).
Determination of creaming index
The stability of emulsions to creaming was ascertained using an accelerated creaming test. Emulsion sample (10 mL) was placed in a 15 mL centrifuge tube and ran in a centrifuge (Z 200 A, Hermle LaborTechnik, GmbH, Germany) at 1000×g for 20 min., rested and the extent of creaming was determined by measuring the height of the interface between the opaque droplet-rich layer at the top and transparent or translucent depleted layer at the bottom. Readings were calculated as accelerated creaming index (ACI) = 100 × (height of interface)/(height of total emulsion).
Polarized light microscopic examination of droplet characteristics
The absence or presence of flocs and/or aggregates was checked using a polarized light microscope (Olympus, Model BH-2, Tokyo, Japan) connected to a colored video camera (Leica Q500 MC, Leica Cambridge Ltd., UK) at a 20 x magnification. A drop of the emulsion sample (n = 3) was placed on a microscope slide, covered with a glass slip and then observed (× 20) under isothermal condition. The observed characteristics of the emulsion droplets after the treatments were recorded (at × 20) and reported as droplets characteristics.
Rheological analysis of the emulsions
The rheological evaluation of the emulsions was carried out using a controlled-stress rheometer (Rheostress RS600, Thermo, GmbH, Germany) equipped with a circulating water bath (DC5, Haake), temperature programmer (HAAKE 6000, Thermo, Karlsruhe, Germany), a Peltier sensor (TC 81, Haake) temperature control unit and a cone and plate geometry with a cone (pp 35/2, Ti-35 mm diameter) with 0.50 mm gap. Determination of the flow properties was conducted at 25 °C and shear rate of 1–100 s−1. Shear stress, shear rate, and steady shear (apparent) viscosity (η) data collection and treatment were performed using an analytical software (RheoWin 3 Ver. 3.12 Thermo, Karlsruhe, Germany). Emulsion sample (n = 3) was placed to cover the lower plate while excess sample that flowed out of the plates was cleared and a thin layer of mineral oil applied to exposed parts of the sample to prevent moisture loss. All determinations (n = 3) were conducted in triplicates (automatically) and results reported as means ± standard deviations.
Statistical analysis
All determinations were carried out in triplicates and data analysis carried out using analysis of variance (ANOVA) with Minitab (V. 16) statistical software. Means of triplicate determinations were compared using 2-sample t test at 5% level of significance and results reported as mean ± standard deviation of at least triplicate determinations.
Results and discussions
Accelerated creaming index
Under the influence of the centrifugal force, the droplets became easily flocculated and gradually, with increasing salt concentration, 1–4%, where the emulsion eventually separated into two phases: the serum and the cream layers. At very low salt concentration where droplets were still held in distance by electrostatic repulsion, there was little effect on their stability, however, the effects became more pronounced at high salt concentration leading to phase separation and later two distinct phases of a sticky cream and a translucent serum became evident (Fig. 1a). The creaming instability were more in the emulsions containing high salt concentration than in those with very low salt concentration attributed to droplet flocculation caused by hydrophobic interaction and low electrostatic repulsion. Generally, the accelerated creaming test showed an opaque or turbid cream layer and a translucent serum layer formed at the interface whose magnitude increased with salt concentration; the effects of which is more with divalent or multivalent ions [29]. Djordjevic et al. [8] reported increased droplets aggregation and consequent formation of two phases: a ‘paste-like’ gel and serum at high salt concentration (250 mM) and syneretic cracks formed by the contraction of protein molecules away from water molecules. This is because the prior addition of salt effectively screened the electrostatic repulsive forces that kept the droplets apart.
Creaming stability as a function of NaCl concentration (left) and superimposed size distribution curves on micrographs (right) of the WBP-stabilized oil-in-water emulsions (at pH 3) in 0% NaCl solution. The salt-treated emulsion (left) was stored (5 days) and subjected to centrifugal force of 1000×g for 20 min. Droplet size (d4,3) distribution (in volume, %) curves of the emulsions were determined by laser light scattering techniques. Emulsion samples (at pH 3) were treated with 0% NaCl solution, and kept (in 15 mL tubes) at ambient temperature (25 ± 1 °C) for 5 days in a vertical position. After storage, samples were analyzed for droplet characteristics using light scattering instrument and reported as droplet size (d4,3) distribution volume (%) by particle size (µm). As shown above, the respective droplets curves were superimposed over the droplets optical micrographs (scale = 50 µm) observed under polarized light microscopy at ambient temperature
Effects of changing salt concentration
The pH of the aqueous phase in many food emulsions may vary substantially from acidic as in most soft drinks to slightly basic in some nutritional beverages but rarely neutral. In low pH soft drinks, i.e., at pH values below their isoelectric points, adsorbed proteins remain in solution and the repulsive forces are at highest level because the negative net charge of the proteins. The mean particle size diameter of the emulsions, measured fresh at ambient temperature showed a mono-modal size distribution on the first day of preparation with more than 50% of the particles < 1 µm in diameter (Table 1). Thus, the mean particle diameter and size distribution did not change appreciably (P < 0.05) from the initial values after the emulsions were aged for 48 h at the ambient temperature (~ 25 °C), indicating relative stability to flocculation under normal environmental conditions (i.e., without the treatment). Under no salt treatment (Fig. 1b), it was difficult to observe the individual droplets clearly (Fig. 1) unless there are flocs of relatively large sizes (d > 1 µm), then, clear picture of the droplets can be obtained [10]. At low pH away from the pI of proteins and/or low ionic strength, with no other environment influence, the droplets were prevented from coming closer, and protein–protein interaction creates strong interfacial membrane that provide a protective barrier against droplets coalescence [10, 30]. Thus it can be seen that the emulsions were sparingly stable to droplet aggregation at low (1–2%) salt concentration as indicated by the fact that there were small though significant (P ≤ 0.05) changes in droplets sizes (d4,3 = 0.33 to 0.55 µm) and specific surface area of 40 m2/g with no visible sign of aggregation, Table 1. This may be attributed to the strong electrostatic repulsion between the droplets [31]. Oil-in-water emulsions with small droplet size < 1 µm could have a great potential technological application in emulsion design because of relative stability to destabilization [32]. The particle sizes however increased with salt concentration (2–4%), where, between 2 and 3% salt concentration, the droplet diameters (d4,3) increased significantly (P ≤ 0.05) from 0.55 to 0.77 µm leading to slight flocculation at 3%, and at 4%, the specific surface area reduced from 39.3 to 32.5 m2/g, interaction became advanced and consequently, flocculation occurred. Surface denaturation resulting from adsorption also increased the amounts of exposed hydrophobic residues and resulted in increased zeta potential of the emulsion droplets with consequent increase in stability against flocculation [33, 34]. At temperature above their Td and high salt concentration, globular proteins unfold extensively with consequent increase in flocculation because the electrostatic repulsion between the droplets is not sufficiently strong to overcome the various attractive interactions, e.g., van der Waals or hydrophobic interaction at the interface [19]. At the low salt concentration and temperatures below their Td, ˂ 110 for winged bean (WBP) any observed flocculation could be due to surface electrostatic attractions between the protein-coated lipid droplets [10]. Also, at the low salt (1–2%) (Fig. 2a, b), there was probably a fairly high positive charge of the aqueous phase because the adsorbed protein layer was far away from its pI and the resulting large electrostatic repulsion between protein-coated droplets prevents them slightly from coming closer to one another [19].
The emergent of bimodal curves resulted from poly-disperse nature of the emulsion at the high salt concentration as droplets come together due to reduced electrostatic repulsion and increase in size [31]. Presumably, the predominant positive charges on the surface were not strong enough to prevent droplets aggregation, and consequently poly-disperse distribution resulted with larger mean droplet size than emulsions at the high salt concentration. The influence of salt on the interactions between protein-coated emulsion droplets leading to flocculation and consequent increase in droplet sizes have been reported [19, 31]. Thus, it can be seen that at high salt (4%) and high temperature (75 °C), droplets flocculation were more pronounced than it was at low salt (1%) and high temperature (Fig. 4), an indication of synergistic effect of the heat and salt [8]. As a consequence, the particle size distribution changed from mono-modal to bimodal, well distinct at the high temperatures (55–75 °C) and high salt (3–4%) treatments (Fig. 4c, d). Previous studies by McClements [10], Kim et al. [31] showed that heating emulsion after salt treatment promoted droplets flocculation than heating before salt treatment because the electrostatic repulsion between droplets is already weakened by the salt treatment and cannot overcome interactions due to van der Waals, hydrophobic and/or steric attractions [19, 35]. Thus the electrostatic repulsion reduced greatly and the droplets come much closer to each other as a result of ‘salting-out’ phenomenon of the proteins, and consequently, there was aggregation and coalescence, marked by a bimodal, skewed size distribution of the emulsion droplet.
Effect of changing temperature
In the freshly prepared WBP-stabilized emulsion at pH 3, a monomodal droplets size distribution was obtained that remained for up to 5 days, but when subjected to alternating salt-temperature treatments, small quantities of large flocs and/or coalesced droplets formed as evidenced by the appearance of a second peak in the droplets size distribution. When no salt was added to the emulsion at ambient temperature, no appreciable increase in mean droplets sizes (d4,3 = 0.23 µm) was observed, as shown in Table 1. However, when increasing amount of salt was added, there were progressively but significant (P < 0.05) increases in droplets diameter probably due to low disulphide bond formation at the low pH [31]. The influence of heat treatment (35–75 °C) in the presence of salt (1–4%) on the droplets characteristics (Fig. 1 (0% NaCl), 2, 3, 4) of the proteins stabilized O/W emulsions showed no significant changes in mean particle diameter (d4,3) at 35 °C, but slight increases at 55–75 °C; a phenomenon attributed to strong electrostatic repulsion between the protein stabilized emulsion droplets that cancels out attractive forces due to Van der Waals and hydrophobic interactions at the higher salt concentrations [36, 37]. In the presence of salt (1–4%) followed with heat treatment (35–75 °C), the protein-stabilized emulsion droplets sizes increased significant (P < 0.05) followed by appreciable droplet flocculation at the salt-heat treatments of 3–4% at 55 °C and 3–4% at 75 °C (Table 1).
Thus the mono-modal size distribution (Fig. 3c, d) superimposed on the size distribution of the same emulsion, became broadened with bimodal distribution of droplets sizes ranging between 0.98 and 1.48 µm, and a large span (83.23), Table 1. It can thus be seen that extensive flocculation occurred at 75 °C (and 3, 4% NaCl) than at 75 °C (1–2% NaCl), indicating that the effects of salt condition (NaCl) was no more pronounced under high temperature than under low temperature, attributed to greater protein–protein intermolecular interactions between layers adsorbed onto different droplets [10]. Structurally, it is suggested that in emulsion stabilized by proteins, α-helix proteins have greater impact on the surface activity of proteins, followed by the aperiodic structure and then β-sheets because the latter is more rigid [34]. However, it is reported that adsorbed proteins can exist in conformations much different from their native states [34]. Because of the propensity of their hydrophobic sides to adsorb to the hydrophobic interface with resulting from distortion of protein structure [38].
Similarly, at the 55 °C treatments and with increasing salt concentration, droplets sizes increased steadily from 0.25 µm at 1% to 1.48 µm at 4%. Of note also is that the presence of salt during heating may have altered the thermal denaturation temperature of the adsorbed proteins compared with zero salt treatment, thereby altering their stability to heat-induced flocculation [39]. The creaming instability was high in emulsions containing salt than in those containing no added salt at all temperatures, an indication that salt promoted droplet flocculation. At high temperature above 55 °C up to 75 °C, droplets became unstable and flocculation advanced leading to coalescence. The droplets sizes changed significantly (P ≤ 0.05) from 1.65 µm at 1% NaCl to 2 µm at 2% NaCl until there is flocs build-up and aggregation when the droplets sizes reached 9 µm (Table 1). At this point, the droplets distribution assumed multi-modal feature and distinct droplets can visibly be distinguished as individual entities (Fig. 4) characterized by formation of advanced coalescence and separation (Table 1).
Effect of chilling temperature
Under chilling conditions, the mean particle diameter (d4,3) of the emulsions remained relatively constant from 0.30 to 0.65 µm at 4 °C, to 0.74 to 1.95 µm at 8 °C (Table 1). In all the samples assessed, no appreciable increase in sizes was observed at the lower chilling temperature, however, slight but significant (P < 0.05) increase in droplet sizes occurred at chilling temperature between 6 and 8 °C. It is of note here to state that at 1% NaCl of each chilling temperature, 4, 6 and 8 °C, the mean droplets diameter (d4,3), 0.30, 0.44 and 0.74 µm, respectively, did not increase appreciably, but at 3 and 4% NaCl, droplet size increased noticeably from 0.48 to 0/65; 0.67 to 0.89 and from 1.12 to 1.95 µm, respectively (Table 1). Thus, the very slight changes in droplets diameter, when compared to the characteristics of the salt-heat treated emulsion droplets, it could be said that in the latter case, severe deterioration of droplets sizes was aggravated by decreased hydrophobic attraction and disulfide bond formation between droplets [10].
Thus the emulsions chilled at temperature from 4 to 8 °C and aged for 5 days were remarkably stable and no meaningful flocculation could be noticed from the droplets morphology, indicating mild effects of low salt concentration under quiescent conditions of 0% NaCl treatment with chilling at 6 °C for 5 days. The mild effect of chilling on the emulsion stability could be due to the low solid fat content of the oil phase (soybean oil) whose major fatty acids (about 53% linoleic) has a very low (− 5 °C) melting point.
This low melting point could be responsible for preventing instability phenomenon such as partial coalescence in chilled emulsion [19]. High SFC oil promote partial coalescence as crystal from a droplet can penetrate into neighboring droplets then act as nucleation sites for further crystal growth, the effect of which is more deleterious at SFC of 10–50% [10, 40]. Similar distribution curves and d4,3 values were obtained for the other chill-stored emulsions after storage at the different (1–4%) salt treatments, indicating that salt-chill treatment did not have deleterious effects as salt-heat treatment on the stability of protein-stabilized emulsion system, but may likely have in high SFC oil. This phenomenon is important in applications where protein-stabilized emulsions, partly or wholly, are a starting or intermediate material in the manufacture of other food products [19].
Rheological characteristics of the emulsions
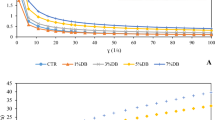
Figure 5 shows the flow curves for the emulsions treated at 1, 2, 2.5, 3.5 and 4% NaCl solutions, pH 3 and aged for 48 h. As can be seen, the flow was not linear with shear rate. At the very low shear rate, no observable flow was noted until a certain yield value (σ0) was overcome before flow became linear and thus, the flow index (n) for the emulsions is not equal to 1, until shear rate (ý) was about 11 s−1, depicting the Bingham plastic model where n ≠ 1. This showed that there were appreciably large emulsions droplets sizes, increasing with salt concentrations. In the emulsions treated with 1% NaCl, the σ0 was smaller; around 13 Pa s compared to the 4% NaCl treatment with about 21 Pa s. The transient behavior at the low shear is representative for all the samples, indicating that emulsions prepared at 1 and 2% NaCl have a significantly low apparent viscosity (Fig. 5a) that further suggest a shearing time dependent behavior. This is due to the fact that the 4% NaCl treated emulsion had larger droplets than the low NaCl-treated (1, 2%) emulsion where electrostatic screening was very low, and the droplets remained sparingly apart. As shearing progressed, all the samples assumed a Newtonian fluids model with a linear, straight line passing through the origin when all droplets might have broken down, indicating a shear-thinning behavior. Thus the viscosity of the emulsion increases with increasing salt concentration of the aqueous that caused an increase in the droplet sizes as a result of the ‘salting-out’ phenomenon. All the emulsions exhibited shear-thinning behaviors within the shear rates range of 0–11 s−1 in the protein-stabilized emulsion, with a linear step afterward, indicating that the thixotropic property was completely eliminated after the initial shearing effect. The large droplets sizes and probably their low spatial distribution due to particle–particle interaction enhanced their deformability since smaller drop sizes are less deformable than larger ones [41, 42], lest the viscosity might be larger than observed. Thus, as the viscosity decreases with the increase shear, a disruption of the average droplet sizes caused a decrease in internal viscosity of the emulsion systems at any given shear rate thereafter. Tang and Liu [42] observed that the linear viscoelastic properties of emulsions treated at salt solution up to 500 mM at pH 3 and 5 in oscillatory shear flow mode depends on the droplet size, and the storage modulus increased with the decrease in droplet size due to an increase in interfacial stress. A quite, distinct behavior was however reported for emulsions with sodium-caseinate and laccase enzyme at pH 7, where anti-thixotropic behavior was observed with slight increase in apparent viscosity with shear time (r) attributed to droplets aggregation under shear [19]. Shear-thinning behaviors of a gradual and linearly decreasing η with increasing shear rate in the range 0.4–100 s−1 has been reported for emulsions treated with increasing ionic strength from 10 to 500 mM [42].
Rheological properties of the WBP-stabilized emulsion at shear rate of 0–100 s−1 and 25 °C (above). The yield stress (σ0) is the shear stress (σ) at shear rate (γ) zero and the viscosity (η) is the slope of the curve at stresses above the yield stress. [For brevity of the diagrams the shear stress-shear rate curves (passing through the intercept) was removed]. Below: Changes in the viscosity of the WBP-stabilized emulsion as a function of salt concentration (1–4%) and aged for 5 days
The viscosity of the emulsion plotted with increasing salt (NaCl) concentration is shown in Fig. 5. Though, initial response to salty environment was slow probably due to partial flexibility conferred on the winged bean protein by its β-sheets content and it’s tendency to unfold, undergo conformational rearrangement and form a cohesive film at the interface [26, 34]. When the initial barrier was overwhelmed, however, emulsions showed upsurge in viscosity due to advanced droplet–droplet interaction leading to increases in droplet sizes and flocculation. Chilling did not caused emulsion destabilization significantly even at 3–4% salt concentration. Thus it is recommended that application of winged bean protein stabilized emulsion as carrier for lipid-soluble micronutrients or where the emulsion is a contemplated intermediate raw material, should preferably be employed at low temperature.
Conclusions
The winged bean exhibited considerable properties as an emulsifier protein that could be employed in oil in water emulsion for protection and delivery of fat-soluble micronutrients that may be susceptible to oxidation and photo-sensitive. Even under stress, high temperature and accelerated storage conditions, the emulsion droplets showed relative resistance to destabilization, making it a potential ingredient for use in oil-in-water food emulsions.
References
B. Wang et al., Ability of flaxseed and soybean protein concentrates to stabilize oil-in-water emulsions. J. Food Eng. 100(3), 417–426 (2010)
O. Benjamin et al., Emulsifying properties of legume proteins compared to β-lactoglobulin and Tween 20 and the volatile release from oil-in-water emulsions. J. Food Sci. 79(10), E2014–E2022 (2014)
J. Ji et al., Preparation and stabilization of emulsions stabilized by mixed sodium caseinate and soy protein isolate. Food Hydrocoll. 51(0), 156–165 (2015)
A. Gharsallaoui et al., Pea (Pisum sativum, L.) protein isolate stabilized emulsions: a novel system for microencapsulation of lipophilic ingredients by spray drying. Food Bioprocess Technol. 5(6), 2211–2221 (2012)
M. Ray, D. Rousseau, Stabilization of oil-in-water emulsions using mixtures of denatured soy whey proteins and soluble soybean polysaccharides. Food Res. Int. 52(1), 298–307 (2013)
K. Demetriades, J.N. Coupland, D.J. McClements, Physicochemical Properties of whey protein-stabilized emulsions as affected by heating and ionic strength. J. Food Sci. 62(3), 462–467 (1997)
C. Bengoechea et al., Temperature and pH as factors influencing droplet size distribution and linear viscoelasticity of O/W emulsions stabilised by soy and gluten proteins. Food Hydrocoll. 24(8), 783–791 (2010)
D. Djordjevic et al., Physical stability of whey protein-stabilized oil-in-water emulsions at pH 3: potential ω-3 fatty acid delivery systems (part A). J. Food Sci. 69(5), C351–C355 (2004)
C. Qian et al., Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 132(3), 1221–1229 (2012)
D.J. McClements, Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 9(5), 305–313 (2004)
J. German, T. O’Neill, J. Kinsella, Film forming and foaming behavior of food proteins. J. Am. Oil Chem. Soc. 62(9), 1358–1366 (1985)
D. Graham, M. Phillips, Proteins at liquid interfaces. V. Shear properties. J. Colloid Interface Sci. 76(1), 240–250 (1980)
P. Hailing, CRC critical review. Food Sci. Nutr. 155, 13 (1981)
J.E. Kinsella, Functional properties of proteins: possible relationships between structure and function in foams. Food Chem. 7(4), 273–288 (1981)
M. Hu, D.J. McClements, E.A. Decker, Impact of whey protein emulsifiers on the oxidative stability of salmon oil-in-water emulsions. J. Agric. Food Chem. 51(5), 1435–1439 (2003)
M. Hu, D.J. McClements, E.A. Decker, Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 51(6), 1696–1700 (2003)
D. Djordjevic, D.J. McClements, E.A. Decker, Oxidative Stability of whey protein-stabilized oil-in-water emulsions at pH 3: potential ω-3 fatty acid delivery systems (part B). J. Food Sci. 69(5), C356–C362 (2004)
S. González-Pérez et al., Emulsion properties of sunflower (Helianthus annuus) proteins. J. Agric. Food Chem. 53(6), 2261–2267 (2005)
D.J. McClements, Food emulsion: principles, practices and techniques, in Contemporary Food Science, 2nd edn. (CRC Group, Boca Raton, 2005), p. 609
F. Liu, C.-H. Tang, Emulsifying properties of soy protein nanoparticles: influence of the protein concentration and/or emulsification process. J. Agric. Food Chem. 62(12), 2644–2654 (2014)
Y. Shao, C.-H. Tang, Characteristics and oxidative stability of soy protein-stabilized oil-in-water emulsions: influence of ionic strength and heat pretreatment. Food Hydrocoll. 37, 149–158 (2014)
M.U. Makeri et al., Comparative analysis of the physico-chemical, thermal, and oxidative properties of winged bean and soybean oils. Int. J. Food Prop. 19(12), 2769–2787 (2016)
N.Q. Ng, Conserving Tropical Leguminous Food Crops, in Conservation of Tropical Plant Species, (Springer, New york, 2013), pp. 213–247
K. Boode, C. Bisperink, P. Walstra, Destabilization of O/W emulsions containing fat crystals by temperature cycling. Colloids Surf. 61, 55–74 (1991)
D. Rousseau, Fat crystals and emulsion stability—a review. Food Res. Int. 33(1), 3–14 (2000)
M.U. Makeri et al., Comparative physico-chemical, functional and structural characteristics of winged bean [Psophocarpus tetragonolobus DC] and Soybean [Glycine max.] Protein isolates. J. Food Meas. Charact. 11(2), 835–846 (2017)
S. Mundi, R.E. Aluko, Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res. Int. 48(1), 299–306 (2012)
T. Allen, Particle size measuremnet, 4th edn. (Chapman and hall, London, 1992)
E. Keowmaneechai, D. McClements, Effect of CaCl2 and KCl on physiochemical properties of model nutritional beverages based on whey protein stabilized oil-in-water emulsions. J. Food Sci. 67(2), 665–671 (2002)
E. Dickinson, Emulsion gels: the structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 28(1), 224–241 (2012)
H.J. Kim, E.A. Decker, D.J. McClements, Comparison of droplet flocculation in hexadecane oil-in-water emulsions stabilized by β-lactoglobulin at pH 3 and 7. Langmuir 20(14), 5753–5758 (2004)
P. Bellalta et al., Rheological and microstructural characterization of WPI-stabilized O/W emulsions exhibiting time-dependent flow behavior. LWT—Food Sci. Technol. 46(2), 375–381 (2012)
R.J.B.M. Delahaije et al., Protein concentration and protein-exposed hydrophobicity as dominant parameters determining the flocculation of protein-stabilized oil-in-water emulsions. Langmuir 29(37), 11567–11574 (2013)
S. Damodaran, L. Razumovsky, Molecular bases of surface activity of proteins, in Functional Properties of Proteins and Lipids, ed. by F.S.J.R. Whitaker, A.L. Munguia, R.Y. Yada, G. Fuller (American Chemical Society, Washington, DC, 1998), pp. 2–18
A. Kulmyrzaev, H. Schubert, Influence of KCl on the physicochemical properties of whey protein stabilized emulsions. Food Hydrocoll. 18(1), 13–19 (2004)
H. Kim, E. Decker, D. McClements, Impact of protein surface denaturation on droplet flocculation in hexadecane oil-in-water emulsions stabilized by β-lactoglobulin. J. Agric. Food Chem. 50(24), 7131–7137 (2002)
H.-J. Kim, E. Decker, D. McClements, Role of postadsorption conformation changes of β-lactoglobulin on its ability to stabilize oil droplets against flocculation during heating at neutral pH. Langmuir 18(20), 7577–7583 (2002)
D.G. Dalgleish, Food emulsions—their structures and structure-forming properties. Food Hydrocoll. 20(4), 415–422 (2006)
M. Corredig, D.G. Dalgleish, A differential microcalorimetric study of whey proteins and their behaviour in oil-in-water emulsions. Colloids Surf. B 4(6), 411–422 (1995)
E. Davies, E. Dickinson, R. Bee, Shear stability of sodium caseinate emulsions containing monoglyceride and triglyceride crystals. Food Hydrocoll. 14(2), 145–153 (2000)
H.A. Barnes, Rheology of emulsions—a review. Colloids Surf. A 91, 89–95 (1994)
C.-H. Tang, F. Liu, Cold, gel-like soy protein emulsions by microfluidization: emulsion characteristics, rheological and microstructural properties, and gelling mechanism. Food Hydrocoll. 30(1), 61–72 (2013)
Acknowledgements
The first author, Makeri, U. M. thank the Malaysian Government for awards of a Ph.D. Scholarship through the Malaysian International Scholarship (MIS) and the study fellowship by the Ahmadu Bello University Zaria. The research was funded by the Universiti Putra Malaysia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared they have no conflict of interest in any form.
Rights and permissions
About this article
Cite this article
Makeri, M., Muhammad, K., Ghazali, H. et al. Influence of temperature and ionic conditions on the rheology and droplets characteristics of winged bean protein stabilized oil-in-water emulsion. Food Measure 13, 97–106 (2019). https://doi.org/10.1007/s11694-018-9922-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9922-1