Abstract
The effect of toasting (mild heat treatment) and cooking (severe heat treatment) on color and antioxidant properties of flours from pearl millet cultivars grown in India were investigated. The antioxidant properties studied were total phenolic content (TPC), total flavonoids content (TFC), antioxidant activity (AOA), metal chelating activity (MCA), and ABTS+ scavenging activity. Toasting resulted in increase in antioxidant properties (TPC, TFC, AOA, MCA, and ABTS+) as well as hunter a* and b* values. The hunter L* value was, however, decreased. Except for increase in hunter L* value and MCA all other antioxidant properties of flours studied showed a decrease after cooking as compared to flours from their native counterparts. Comparison of both the processing methods revealed that except for MCA, all other antioxidant properties of flours after toasting had significantly (p < 0.05) higher values than after cooking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pearl millet (Pennisetum glaucum L.) is a drought tolerant cereal crop grown primarily as a food grain in India and Africa. India is the largest producer of the pearl millet in the world. Cereals and millets are the most commonly consumed food items in India. They contain a wide range of phenolic compounds which are good sources of natural antioxidants [1]. Pearl millet is a principal source of energy, protein, vitamins and minerals for millions of the poorest people in the regions where it is cultivated [2]. Due to good nutritional and phytochemical properties it has gained considerable attention as a botanical dietary supplement in many functional foods. Nutritional knowledge of functional foods also helps to improve the health of consumers [3].
Thermal treatments may reduce or increase the phenolic content and the antioxidant activities of cereals, depending on heat treatment, provided time period and the type of cereal tested [4, 6]. Therefore, it is necessary to find an effective processing method to enhance these compounds. As consumers are becoming more health conscious, they demand for mild processing treatments which have fewer effects on the nutritional compounds. In India, both toasting and cooking treatments are widely used to process variety of cereals and millets including pearl millet. The study of these treatments will provide useful information to consumers, food industry as well as breeders. The literature searched also pointed towards scarce information on the effect of different processing conditions on the antioxidant properties of pearl millets. Therefore, the present investigation was undertaken to study the antioxidant properties of commercially important pearl millets cultivars grown in India after thermal treatments (toasting and cooking).
Materials and methods
Materials
Pearl millet cultivars (HC-10, HHB-67, HHB-223, HHB-226 & W-445) were procured from Chaudhary Charan Singh Haryana Agriculture University, Hissar, India whereas GHB-732 cultivar was procured from Pearl Millet Research Station, Jamnagar, Gujarat, India.
Toasting
Grains were toasted according to method described by Fares and Menga [7]. Different pearl millet cultivars (200 g each) were conditioned to a moisture content of 10% to maintain uniformity during toasting process and toasted at 115 ± 5 °C in an oven (NSW-143, New Delhi, India). Grains were then milled in a flour mill and the flour so obtained was passed through the 70 mesh sieve. It was then packed in the polythene bags till further use.
Cooking
Pearl millet (25 g) grains were cooked in 150 ml boiling distilled water on a hot plate (MAC, New Delhi, India). Few grains were collected after 10 min interval and there after 1 min interval during the cooking operation to determine the extent of cooking by pressing between the two glass slides. Cooking time was recorded as the time taken until the disappearance of white core in more than 95% of collected grains. Grains were then milled in a flour mill and the flour so obtained was passed through the 70 mesh sieve. It was then packed in the polythene bags till further use.
Hunter color characteristics
Color measurements of pearl millet flour samples were carried out using a Hunter colorimeter Model D 25 optical Sensor (Hunter Associates Laboratory Inc., Reston, VA, USA) on the basis of L*, a* and b* values [8]. A glass cell containing flour was placed above the light source, covered with a white plate and L*, a* and b* color values were recorded. The instrument (45°/0° geometry, 10° observer) was calibrated against a standard red-colored reference tile (Ls = 25.54, as = 28.89, bs = 12.03).
Where the L* value indicates the lightness, 0–100 representing dark to light, a* value gives the degree of the red–green color, with a higher positive a* value indicating more red. The b* value indicates the degree of the yellow–blue color, with a higher positive b* value indicating more yellow.
Antioxidant activity using DPPH
Antioxidant activity (AOA) was measured following a modified version of the method described by Brand-Williams et al. [9]. Ground pearl millet samples (100 mg) were extracted with 1 ml of methanol for 2 h and centrifuged at 3000×g for 10 min. The supernatant (100 µl) was reacted with 3.9 ml of a 6 × 10−5 mol/l of DPPH solution. Absorbance (A) at 515 nm was read at 0 and 30 min taking a methanol as a blank. AOA was calculated as % discoloration.
Total phenolic content
The total phenolic content (TPC) was determined by following the Folin–Ciocalteu specterophotometric method described by Gao et al. [10]. Pearl millet flour samples (200 mg) were extracted with 4 ml acidified methanol (HCl/methanol/water, 1:80:10, v/v/v) at room temperature (25 °C) for 2 h using wrist action shaker. The mixture was centrifuged at 3000×g for 10 min on a centrifuge. The supernatant was used for determination of TPC. Aliquot of extract (200 µl) was added to 1.5 ml freshly diluted (20-fold) Folin–Ciocalteu reagent. The mixture was allowed to equilibrate for 5 min and then mixed with 1.5 ml of sodium carbonate solution (60 g/l). After incubation at room temperature (25 °C) for 90 min, the absorbance of the mixture was read at 725 nm. Acidified methanol was used as a blank and the results were expressed as a µg of gallic acid equivalents (GAE)/g of flour.
Total flavonoids content
The total flavonoids content (TFC) was determined by following the method as described by Jia et al. [11]. Pearl millet flour samples (200 mg) were extracted with 4 ml acidified methanol (HCl/methanol/water, 1:80:10, v/v/v) at room temperature (25 °C) for 2 h using wrist action shaker. The mixture was centrifuged at 3000×g for 10 min on a centrifuge. The supernatant was used for determination of TFC. Extract (250 μl) was diluted with 1.25 ml distilled water. Sodium nitrite (75 μl of 5% solution) was added and the mixture was allowed to stand for 6 min. Further, 150 μl of a 10% aluminium chloride was added and the mixture was allowed to stand for 5 min. After that, 0.5 ml of 1 M sodium hydroxide was added and solution was mixed well. The absorbance was measured immediately at 510 nm using a spectrophotometer (Systronics-106, Ahmadabad, India). Catechin was used as standard and the results were reported as µg of catechin equivalents (CE)/g of flour.
Metal chelating activity
The metal chelating activity (MCA) of pearl millet extract was measured by following the method described by Dinis et al. [12]. The extract (0.5 ml) was mixed with 50 μl of ferrous chloride (2 mM/l) and 1.6 ml of 80% methanol was added. After 5 min, the reaction was initiated by the addition of 5 mM/l ferrozine (100 μl) and the mixture was shaken on vortex. The mixture was incubated at room temperature (25 °C) for 10 min. Absorbance of solution was measured at 562 nm on a spectrophotometer. The chelating activity of the extract for Fe2+ was calculated as follows:
ABTS+ scavenging capacity
ABTS+ scavenging capacity was measured by following the method described by Re et al. [13]. This involved the use of free radical ABTS, 2,2-azinobis(3-ethylbenzothiazoline-6 sulfonic acid) diammonium salt. ABTS+ was generated by oxidation of ABTS with potassium persulfate. 3 ml of ABTS+ solution was mixed with 30 µl extract in disposable microcuvette and the decrease of absorption was measured after 1 min of incubation.
Statistical analysis
The data shown in all the tables are an average of triplicate observations and were subjected to one way analysis of variance (ANOVA) using Minitab statistical software version 14 (Minitab Inc, USA).
Results and discussion
Hunter color values of flours
Color parameters of flours from toasted and cooked grains were evaluated using hunter color lab (Table 1). After toasting, L* value was significantly (p < 0.05) reduced in comparison to control or unprocessed counterpart sample, the highest for flours from cv.W-445 whereas the lowest was observed for cv.HHB-67 flour. Toasting resulted in higher a* values indicating more redness with values ranging from 0.36 to 1.76, flour from cv.W-445 showed the maximum redness. b* value was also increased upon toasting with flours from cv.W-445 showing the maximum yellowness. Sandhu et al. [14] observed the similar trend of decrease in L* value and increase in the both a* and b* values of flours from oat cultivars after toasting. L* value of flours, however, increased in comparison to control counterparts after the cooking and the values ranged from 75.5 to 83.5; the highest and the lowest being observed for flours from cv.W-445 and cv.HC-10 respectively. Both a* and b* values were decreased after cooking. Yadav et al. [15] reported increase in L* value and decreased in a* and b* values after hydrothermal treatment till 20 min. Comparison of L* values of flours after toasting and cooking methods revealed higher L* values after cooking. On the contrary a* and b* values for flours after toasting were significantly (p < 0.05) higher than those after cooking.
Total phenolic content
The total phenolic content (TPC) of flours from control cultivars varied significantly (p < 0.05) and ranged from 2394 to 3137 μg GAE/g, with cv.GHB-732 and cv.HHB-223 having the highest and the lowest values (Data already published, Siroha et al. [16]). Toasting led to a significant increase in the TPC by 4.3 to 22.1% (Table 2). Sandhu et al. [14] also reported the increase in TPC of oat cultivars after toasting. The increase in TPC after toasting which is less severe heat treatment may be due to the release of bound phenolic compounds. Boateng et al. [17] also concluded that increase of phenolic compounds could be associated with release of more bound phenolics from the breakdown of cellular constituents during thermal treatment. Wanyo et al. [18] also reported the increase in phenolic component in rice sample after the far-infrared (FIR) radiation heat treatment.
On contrary to toasting, cooking led to decrease in the TPC by 16.4 to 39.1% (Table 2), with cv.HHB-223 showing the maximum effect. Chandrasekara et al. [19] reported that after processing cooking in pearl millet the TPC was decreased. They concluded that the reduced TPC after boiling in millet grains could be due to the degradation of phenolics upon heat treatment or leaching into the endosperm to form complexes with proteins and other macromolecules, thus making phenolics less extractable. Siah et al. [20] also reported that boiling decreases the TFC of faba bean cultivars. Cardoso et al. [21] also reported that cooking in water reduced the content and retention of phenolic compounds in sorghum flour. These reductions may be due to the loss of the compounds in the cooking water [22]. When both toasting and cooking methods were compared, toasting showed higher TPC values as compared to cooking.
Total flavonoids content
Flavonoids have generated interest because of their broad human health promoting effects, most of which are related to their antioxidant properties and to synergistic effects with other antioxidants [23]. Total flavonoids component (TFC) of flours from different pearl millet from control cultivars ranged from 1721 to 2484 µg catechin equivalent (CE)/g, the highest for cv.HC-10 and the lowest for cv.HHB-223 was observed [Data already published, 16]. Upon toasting, TFC of flours increased by 3.4–62.6% (Table 3), the largest increase being observed for cv.HHB-223. Pradeep and Guha [24] reported that heat treatment increased the TFC in millets. On the contrary when pearl millet was cooked their flours showed decrease in TFC by 6.9 to 81.3% (Table 3), with cv.W-445 having the maximum effect. Sharma et al. [23] reported that a significant decrease in the TFC was observed upon extrusion cooking. Sharma and Gujral [25] reported the decrease in flavonoids after heat treatment. The flavonoids are heat susceptible phenolic compounds therefore heat exposure during heat treatment decrease these components [26, 27]. Comparison of both processing methods for TFC values revealed toasting as a better processing alternative.
Metal chelating activity
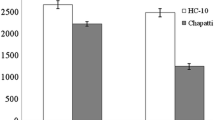
The transition metals have been reported to be catalysts for the initial formation of free radicals. The metal chelators may stabilize metal ions in the living system and inhibit the radical producer hence reduce the free radical damage [28]. Metal chelating activity (MCA) of different control pearl millet cultivars ranged between 6.7 and 20.3% [Data already published, 16]. MCA after toasting and roasting is shown in Fig. 1. Toasting led to a significant increase in the MCA by 29.5–67.9%, the largest increase was observed for cv. HHB-223 whereas cv.GHB-732 had the smallest increase. Sandhu et al. [14] observed that after toasting, the MCA increased significantly (p < 0.05) for oat cultivars. The increase in metal chelation after heat treatment may be due to alteration of phenolic structure and/or degradation of phenolic compounds to different maillard reaction products like melanoids which could also act as antioxidants [29, 30]. Upon cooking, MCA of pearl millet flours were increased and this increased by 39.6 to 94.7%, with cv.HHB-223 showing the maximum effect. Sharma and Gujral [25] reported that after the cooking processing the MCA among barley cultivars was increased. When both methods were compared cooking showed higher MCA values.
Antioxidant activity
DPPH is a stable free-radical compound widely used to test the free-radical scavenging ability of various samples [31]. Antioxidant activity (AOA) of different control pearl millet cultivars varied significantly (p < 0.05) and ranged between 31.8 and 46.7% [Data already published, 16]. Antioxidant activity (%) after toasting and roasting is shown in Fig. 2. Upon toasting, AOA of pearl millet flours increased and this increased by 6.5 to 23.5%, the highest increase was for cv.HHB-223 whereas lowest was observed for cv.W-445. Dewanto et al. [32] found an increase in AOA after thermal treatment for sweet corn. Pradeep and Guha [24] reported that the radical scavenging activity of processed millet extracts increased when compared to their native counterpart sample. An increased AOA activity can be attributed to the formation of non-enzymatic browning products especially melanoids at high temperatures which contribute to AOA [25]. Cooking led to a significant decrease in AOA by 9.6 to 37.4%, the highest and the lowest decrease was observed for flours from cv.W-445 and cv.GHB-732, respectively. Chandrasekara et al. [19] reported a decrease in AOA in the millets after cooking. Xu and Chang [33] also found that after processing different grains, the DPPH radical scavenging was significantly reduced when compared to their counterpart sample. A decrease in AOA after cooking may be due to the activity leached into the boiling water [34]. AOA after toasting had higher values than the values after cooking.
ABTS+ scavenging activity
The antioxidative capacity of test compounds is assessed by measuring their ability to reduce the ABTS radical to its non radical form. ABTS+ activity of different control pearl millet cultivars varied from 18.3 to 25.0%, with cv.W-445 showing the maximum activity. Salar and Purewal [35] observed ABTS+ activity from 27.0 to 52.01% for different pearl millet cultivars. ABTS+ (%) scavenging activity of flours after toasting and cooking is shown in Fig. 3. After toasting ABTS+ activity of flours increased by 0.3 to 25.5% with cv.GHB-732 showing the maximum effect. Chandrasekara and Shahidi [19] also reported an increase in AOA values of thermally treated cashew kernels compared to that of its unroasted counterparts. It has been also reported that during thermal treatment Maillard products such as HMF (5-hydroxymethyl-2-furaldehyde) are formed and contributed to AOA [36]. Cooking, however, had the reverse effect as compared to toasting and ABTS+ scavenging activity decreased by 22.8 to 54.1%. Most of the phenolic compounds are found to be heat sensitive, which could be the reason for their significant decrease in scavenging activity upon cooking [37]. Comparison of both processing methods revealed that toasting resulted in higher ABTS+ scavenging activity than cooking.
Conclusions
Antioxidant as well as hunter color properties of flours from pearl millet cultivars were significantly (p < 0.05) affected after toasting and cooking. Comparison of both the methods of processing revealed that toasting significantly (p < 0.05) increased TPC, TFC, AOA, and ABTS+ scavenging activity as compared to cooking. MCA, however, was higher for flours after cooking. Toasting thus resulted in increase of all the antioxidant properties whereas except for MCA, cooking decreased all the antioxidant properties. Cv.HHB-223 was affected the most for TPC, TFC, MCA and AOA. Further research is required to study the enhancement or degradation of antioxidant compounds after toasting and cooking processing of pearl millets.
References
F.S. Pushparaja, A. Urooj, Antioxidant activity in two pearl millet (Pennisetum typhoideum) cultivars as influenced by processing. Antioxidants 3, 55–66 (2014)
R. K. Salar, S. S. Purewal, M. S. Bhatti, Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resour-Efficient Technol. 2, 148–157 (2016)
D. Peressini, A. Sensidoni, Effect of soluble dietary fibre on rheological and bread making properties of wheat dough’s. J. Cereal Sci. 49, 190–201 (2009)
P.S. Hegde, T.S. Chandra, ESR spectroscopic study reveals higher free radical quenching potential in kodo millet (Paspalum scrobiculatum) compared to other millets. Food Chem. 92, 177–182 (2005)
E.E. Towo, U. Svanberg, G.D. Ndossi, Effect of grain pre-treatment on different extractable phenolic groups in cereals and legumes commonly consumed in Tanzania. J. Sci. Food. Agric. 83, 980–986 (2003)
H. Zielinski, A. Michalska, M.K. Piskula, H. Kozlowska, Antioxidants in thermally treated buckwheat groats. Mol. Nutr. Food Res. 50, 824–832 (2006)
C. Fares, V. Menga, Effects of toasting on the carbohydrate profile and antioxidant properties of chickpea (Cicer arietinum L.) flour added to durum wheat pasta. Food Chem. 131, 1140–1148 (2012)
M. Kaur, N. Singh, Relationships between various functional, thermal and pasting properties of flours from different Indian black gram (Phaseolus mungo L.) cultivars. J. Sci. Food. Agric. 87, 974–984 (2007)
W. Brand-Williams, M.E. Cuvelier, C. Berset, Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25–30 (1995)
L. Gao, S. Wang, B.D. Oomah, G. Mazza, Wheat quality: antioxidant activity of wheat millstreams, in wheat quality elucidation, ed. by P. Ng, C.W. Wrigley (AACC International, St Paul, 2002), pp. 219–233
Z. Jia, M. Tang, J. Wu, The determination of flavonoids content in mulberry and their scavenging effects on superoxides radicals. Food Chem. 64, 555–559 (1999)
T.C. Dinis, V.M. Madeira, L.M. Almeidam, Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and peroxyl radicals scavengers. Arch. Biochem. Biophy. 315, 161–169 (1994)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 26, 1231–1237 (1999)
K.S. Sandhu, P. Godara, M. Kaur, S. Punia, Effect of toasting on physical, functional and antioxidant properties of flour from oat (Avena sativa L.) cultivars. J. Saudi Soc. Agric. Sci. (2015). doi:10.1016/j.jssas.2015.06.004
D.N. Yadav, J. Kaur, T. Anand, A.K. Singh, Storage stability and pasting properties of hydrothermally treated pearl millet flour. Int. J. Food Sci. Technol. 47, 2532–2537 (2012)
A.K. Siroha, K.S. Sandhu, M. Kaur, Physicochemical, functional and antioxidant properties of flour from pearl millet varieties grown in India. Food Meas. 10, 311–318 (2016)
J. Boateng, M. Verghese, L.T. Walker, S. Ogutu, Effect of processing on antioxidant contents in selected dry beans (Phaseolus vulgaris L.). LWT-Food Sci. Technol. 41, 1541–1547 (2008)
P. Wanyo, N. Meeso, S. Siriamornpun, Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 157, 457–463 (2014)
A. Chandrasekara, M. Naczk, F. Shahidi, Effect of processing on the antioxidant activity of millet grains. Food Chem. 133, 1–9 (2012)
S. Siah, J.A. Wood, S. Agboola, I. Konczak, C.L. Blanchard, Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chem. 142, 461–468 (2014)
L.M. Cardoso, T.A. Montini, S.S. Pinheiro, V.A. Queiroz, H.M. Pinheiro-Sant Ana, H.S. Martino, A.V. Moreira, Effect of processing with dry heat and wet heat on the antioxidant profile of sorghum. Food Chem. 152, 210–217 (2014)
A.-M. Afify, H.S. El-Beltagi, S.M. El-Salam, A. A. Omran, Biochemical changes in phenols, flavonoids, tannins, vitamin E, β-carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac. J. Trop. Biomed. 2, 203–209 (2012)
P. Sharma, H.S. Gujral, B. Singh, Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 131, 1406–1413 (2012)
S.R. Pradeep, M. Guha, Effect of processing methods on the nutraceutical and antioxidant properties of little millet (Panicum sumatrense) extracts. Food Chem. 126, 1643–1647 (2011)
P. Sharma, H.S. Gujral, Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res. Int. 44, 235–240 (2011)
M. Zhang, H. Chen, J. Li, Y. Pei, Y. Liang, Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing. LWT-Food Sci. Technol. 43, 181–185 (2010)
F. Zhu, Y. Cai, J. Bao, H. Corke, Effect of γ-irradiation on phenolic compounds in rice grain. Food Chem. 120, 74–77 (2010)
W.W. Nawar, Lipids, in Food Chemistry, ed. by O.R. Fennema, 3rd edn. (Marcel Dekker, New York, 1996), pp. 225–319
H.M. Woffenden, J.M. Ames, S. Chandra, M. Anese, C. Nicoli, Effect of kilning on the antioxidant and pro-oxidant activities on pale malt. J. Agr. Food Chem. 50, 4925–4933 (2002)
R. Randhir, Y. Kwon, K. Shetty, Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedling. Innov. Food Sci. Emerg. Technol. 9, 355–364 (2008)
S. Sakanaka, Y. Tachibana, Y. Okada, Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 89, 569–575 (2005)
V. Dewanto, X. Wu, R.H. Liu, Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 50, 4959–4964 (2002)
B. Xu, S.K. Chang, Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agr. Food Chem. 56, 7165–7175 (2008)
A.M. Chuah, Y. Lee, T. Yamaguchi, H. Takamura, l. Yin, T. Matoba, Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 111, 20–28 (2008)
R. K. Salar, S. S. Purewal, Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of north Indian region. Food Meas. Charact. (2016). doi:10.1007/s11694-016-9379-z
P. Sharma, H. S. Gujral, Antioxidant potential of wheat flour chapattis as affected by incorporating barley flour. LWT-Food Sci. Technol. 56, 118–123 (2014)
H.S. Gujral, M. Angurala, P. Sharma, J. Singh, Phenolic content and antioxidant activity of germinated and cooked pulses. Int. J. Food Prop. 14, 1366–1374 (2012)
Acknowledgements
The financial support provided by Chaudhary Devi Lal University, Sirsa in the form of University Research Scholarship to Anil Kumar Siroha is deeply acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors also declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Siroha, A.K., Sandhu, K.S. Effect of heat processing on the antioxidant properties of pearl millet (Pennisetum glaucum L.) cultivars. Food Measure 11, 872–878 (2017). https://doi.org/10.1007/s11694-016-9458-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9458-1