Abstract
Purpose
Cystic echinococcosis (CE) is a neglected tropical disease prevalent worldwide, particularly in rural areas. Previous studies evaluated immune responses in patients with hepatic CE, however none had assessed Th1, Th2 and Th17 levels simultaneously in pulmonary CE patients. This study aimed to fill this gap in literature by using flow cytometry analysis.
Methods
Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples collected from healthy control (HC) volunteers and patients with active pulmonary CE cysts. The PBMCs were analysed to evaluate Th1, Th2, and Th17 cell levels within the CD3 + CD4 + T-cell population, using antibodies against interferon (IFN)-γ, interleukin (IL)-4, and IL-17, respectively.
Results
Our analysis revealed elevated Th2 levels in CE patients, while Th1 and Th17 cell counts showed no significant difference between HC volunteers and patients with pulmonary CE.
Conclusion
The results indicate an imbalanced Th1/Th2/Th17 cell regulation in the pathogenesis of pulmonary CE. Future studies are recommended to compare immune responses between pulmonary and hepatic CE to confirm these findings and evaluate any potential difference in the immunopathology associated with the two clinical forms of CE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human echinococcosis, classified by the World Health Organization (WHO) as a neglected tropical disease, affects over one million people globally, resulting in 19,300 deaths and around 871,000 disability-adjusted life-years (DALYs) each year. The disease manifests in four forms: (1) cystic, (2) alveolar, (3) neotropical and (4) unicystic echinococcosis. Among these, cystic echinococcosis (CE), caused by Echinococcus granulosus sensu lato, is the one which is globally distributed. It primarily affects rural areas in which animals are kept in close proximity to households, and can be found in every continent except for Antarctica [1].
The parasite is transmitted via fecal-oral route and has life cycle that includes definitive (e.g. dogs) and intermediate hosts (e.g. humans). Upon oral ingestion, E. granulosus eggs hatch in the small intestine, releasing oncosphere larvae. The larvae are then carried to primary target organs via lymphatic or vascular system, where cysts develop. In humans, the liver is the most common site for cyst development (50–75%) as most E. granulosus larvae gets trapped in the hepatic sinusoids. However, due to its small size (< 0.3 mm in diameter) or by entering lymphatics in the small intestine or liver, larvae can also escape from the hepatic filter and reach the lungs, where cyst develop in 5–25% of cases [2]. Additionally, cysts can rupture, releasing protoscolices that can generate secondary cysts in other parts of the body [3].
E. granulosus is known to manipulate immune response, particularly by skewing immune response from a protective Th1-type response to a non-protective or permissive Th2-type response which was demonstrated to facilitate the establishment of the infection [4,5,6]. Furthermore, production of IL-17 A, a cytokine associated with Th17 cells, was detected in CE patients and suggested to participate in host’s defence mechanisms against the parasite [7]. A recent study evaluating Th1/Th2/Th17 profile demonstrated the predominance of Th1/Th17-type and Th2-type cytokines in hepatic CE patients with inactive and active cysts, respectively [8].
Nevertheless, to our knowledge, no study has yet assessed Th1/Th2/Th17 balance exclusively in pulmonary CE subjects. The aim of our study is to fill this gap in the literature by monitoring peripheral levels of CD4 + T-cells expressing interferon (IFN)-γ (Th1), IL-4 (Th2) and IL-17 (Th17) cytokines in PBMC samples isolated from pulmonary CE patients using flow cytometry. The immune response triggered during pulmonary CE warrants more attention in literature, especially given previous reports that suggest pulmonary cysts are associated with lower seropositivity rates, and faster growth compared to hepatic cysts [2, 9]. Evaluating the T-cell profile in pulmonary CE patients would also provide valuable data that can influence future treatment strategies and vaccine development [10].
Materials and Methods
Volunteers
The study included two groups of volunteers. The first group (CE group) included eight pulmonary CE patients with active (CE1 and CE2) cysts admitted to the thoracic and general surgery services of Van Yuzuncu Yil University, while the second group (healthy control [HC] group) included ten medical stuff whose ages and sexes were matched with CE patients. The CE patients received treatment with albendazole for three months following initial diagnosis, prior to inclusion in the study. The rates of female and male volunteers were 50% for both groups. The ages of the volunteers ranged from 19 to 68 years with mean average age of 39.2 ± 17.4 years, and from 20 to 65 years with mean average age of 37.6 ± 14.5 years in CE and HC groups, respectively. Pulmonary CE diagnosis was based on clinical symptoms, imaging techniques (ultrasound, X-ray, or computed tomography [CT] scan), and serology. The hydatid cysts were all classified according to the WHO Informal Working Group on Echinococcosis [11]. The study was approved by Van Yuzuncu Yil University Faculty of Medicine Clinical Research Ethics Committee (Decision date and number: 19.03.2019-05).
Isolation, Freezing and Thawing of PBMCs
The PBMC isolation from collected peripheral blood (20 ml) samples was performed using the Ficoll separation method, as previously described [12]. Briefly, peripheral blood samples were diluted 1:1 with 1X PBS (Gibco, USA) and then added to an equal volume of Ficoll solution (Sigma Aldrich, Germany) at a slow rate without mixing. Samples were then centrifuged at 540 x g for 30 min, with minimum deceleration. After centrifugation, the cloudy layer was collected and mixed with 50 ml RPMI-1640 (Gibco, USA) wash media containing 2% fetal bovine serum (FBS, HyClone, USA), followed by centrifugation again at the same speed for 10 min. Following the same washing step, the cells were then suspended in FBS (HyClone, USA) containing 10% DMSO (Sigma Aldrich, Germany) to be stored in liquid nitrogen until to be used.
On the day of use, frozen PBMC samples were thawed by warming cryovials in a 37 ºC water bath for 10 min. The cells were then suspended in 10 ml warm RPMI 1640 (Gibco, USA) media supplemented with 10% FBS (HyClone, USA) (R-10) and centrifuged at 300 x g for 5 min. Following centrifugation, the supernatants were removed and cell pellets were re-suspended in 10 ml R-10. After the same centrifugation step and removal of the supernatant, cell pellets were mixed in RPMI-1640 (Gibco, USA) media supplemented with 20% FBS. The cells were left for incubation at 37 °C for 2 h and then centrifuged. The supernatants were removed and cell pellets were mixed with 1 ml R-10 media.
Detection of Th1, Th2 and Th17 Cells
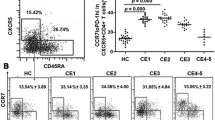
Frequencies of Th1, Th2 and Th17 cells were determined as previously described [13]. The PBMCs were first incubated with human recombinant IL-2 (Biolegend, USA) for a week in order to support the viability of T-cells. Following the incubation period, 5 × 106 cells were mixed with 10 ml 1X PBS (Gibco, USA) and centrifuged at 540 x g for 10 min. After the removal of supernatants, the cells were suspended and left for incubation for 6 hours at 37 ºC in 1 ml of stimulation solution that was prepared by addition of stimulation cocktail (TonboBio, USA) at a ratio of 1:100 in RPMI-1640 (Gibco, USA) containing 10% FBS (HyClone, USA). This step was then followed by fixation, permeabilization and staining with an antibody cocktail containing anti-human CD3 conjugated with CF Blue (Immunostep), anti-human CD4 conjugated with FITC (Immunostep), anti-human IFN-γ conjugated with PerCP/Cyanine 5.5 (Biolegend, USA), anti-human IL-4 conjugated with APC (TonboBio, USA) and anti-human IL-17 conjugated with PE (Biolegend, USA), at room temperature for 30 min. For flow cytometry analysis cells were gated as indicated in Fig. 1.
Statistical Analysis
Descriptive statistics including arithmetic mean, standard deviation, median, minimum and maximum values were calculated. Mann-Whitney U test was applied for the comparison of two independent groups since the data did not fit to parametric assumptions. All statistical analysis was performed with GraphPad (Version 9.5.1 for Mac) software. Data yielding p < 0.05 was considered statistically significant. Data in the text were given as mean ± standard deviation (SD).
Results
Elevated Th2 but Unaltered Th1 and Th17 Levels in CE Patients
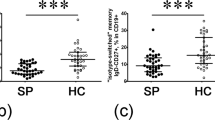
The PBMC samples isolated from the collected blood samples were used to evaluate and compare Th1, Th2, and Th17 cell frequencies between CE patients and HC volunteers. Our results revealed CE patients with statistically significantly higher rates of Th2 cells than HC volunteers (3.4%±1.0 vs. 8.14%±2.3; p-value: 0.0013). In contrast, neither Th1 (10.2%±3.3 vs. 12.2%±4.4; p-value: 0.237) nor Th17 counts (1.0%±0.5 vs. 1.2%±0.7; p-value: 0.5569) exhibited statistically significant difference between the two subject groups (Fig. 2).
Discussion
The CE is characterized by an elongated asymptomatic phase with cysts evolving, growing, and proliferating over many years. While CE pathogenesis is strictly controlled by mixed Th1/Th2 response [14,15,16], the establishment of the infection was associated with a shift from a protective Th1- to a non-protective/permissive Th2-type response [5, 6, 17]. Elevated levels of Th1 and Th2 cells/cytokines were correlated with inactive and active hepatic cysts, respectively [16, 18,19,20,21,22]. On the other hand, Th17 responses are also thought to confer protection against E. granulosus since higher levels of both Th1-type IL-2 and Th17-type IL-17 A cytokines were detected in patients with early stage of hepatic cyst development (CE1) [23]. Moreover, Yasen et al. demonstarted Th1/Th2/Th17 cytokine profile with predominance of Th1/Th17-type and Th2-type cytokines in CE patients with inactive and active cysts, respectively [8].
However, most of the studies evaluating CE-mediated immune response utilized cytokine profiling, and none was able to provide data on Th17 cell levels in CE patients. Furthermore, there has not yet been any study assessing CD4 + T-cell phenotypes in patients with only pulmonary CE which is associated with lower seropositivity rates and faster cyst growth in comparison to hepatic CE [2, 9]. In an attempt to fill this gap in the literature, our study compared peripheral Th1, Th2 and Th17 frequencies between pulmonary CE patients and HC individuals.
Our analysis demonstrated CE patients with statistically significantly elevated Th2 cell levels and unaltered Th1 and Th17 percentages. The results were in correlation with literature suggesting that Th2 responses permit parasite reproduction and cyst development, however they conflict with previous data showing co-existence of Th1 and Th2 responses in CE patients [14,15,16]. On the other hand, to our knowledge, our study is the first to report unaltered peripheral frequencies of Th17 cells in CE patients, which contrasts with previous studies that demonstrated higher or reduced Th17 responses in volunteers with CE cysts [7, 8, 23, 24].
The contradictory data on Th1 and Th17 cell levels can be attributed to the manifestation of different E. granulosus antigens during hepatic and pulmonary CE [9], as well as the low statistical power because of small sample size in our study. Alternatively, the developmental stage of cysts in CE patients may also account for our inconsistent results, since both Th1- and Th17-type cytokine production were previously reported to drop significantly as hepatic cysts proceed from early to late activation stages [23]. Therefore, future animal studies with consideration of cyst development stage and larger sample size are required to obtain more conclusive and informative data on the role played by CD4 + T-cell subsets, including Th17 cells, in CE pathogenesis.
Due to financial constraints, it was not also possible to include hepatic CE patient samples in our analysis, which is another limitation of our study. Currently, most of the literature on immune response triggered by E. granulosus is derived from studies using hepatic CE patient samples. Therefore, comparison analysis between pulmonary and hepatic CE samples is crucial to elucidate the immune evasion mechanisms utilised by the parasite during pulmonary CE. Additionally, it was not possible to include E. granulosus genotype to our analysis which could have provided further insights into our results as the parasite genotype can both determined the organ(s) to be affected and influence clinical symptoms in CE [25].
To sum up, for the first time in the literature, our results demonstrated pulmonary CE induces Th2 responses without affecting Th1 and Th17 counts. Future studies involving both hepatic and pulmonary CE patient groups are highly recommended to confirm these findings and investigate any potential difference in the immune responses triggered by the two clinical forms of CE.
Gating strategy used to identify Th1, Th2 and Th17 cells. Following the selection of lymphocytes based on CD3/side scatter dot plot, the total helper T-cell population was selected as CD3 + CD4 + T cells. Polarization of CD3 + CD4 + T-cells was further investigated by using IFN-γ, IL-4 and IL-17 expression levels; Th1, Th2 and Th17 cells were represented as CD3 + CD4 + IFN-γ+, CD3 + CD4 + IL-4 + and CD3 + CD4 + IL-17+, respectively
Comparison of Th1, Th2 and Th17 cell levels between CE and HC subjects. PBMC samples isolated from peripheral blood samples were subjected to T-cell expansion before the evaluation of helper T-cell subset levels by flow cytometry. The percentages of Th1 (A), Th2 (B), and Th17 (C) cells in CD3 + CD4 + T-cell population were determined by monitoring IFN-γ, IL-4, and IL-17 expression, respectively. The comparison analysis revealed unaltered Th1 and Th17 levels and significantly higher Th2 percentages in CE patients
Data Availability
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
WHO– World Health Organization (2018) Echinococcosis. In: Facts Sheets. http://www.who.int/news-room/fact-sheets/detail/echinococcosis
Sarkar M, Pathania R, Jhobta A et al (2016) Cystic pulmonary hydatidosis. Lung India 33. https://doi.org/10.4103/0970-2113.177449
CDC (2016) CDC - DPDx - Echinococcosis. Glob. Heal.– div. Parasit. Dis. Malar
Grubor NM, Jovanova-Nesic KD, Shoenfeld Y (2017) Liver cystic echinococcosis and human host immune and autoimmune follow-up: a review. World J Hepatol 9:1176–1189
Mourglia-Ettlin G, Marqués JM, Chabalgoity JA, Dematteis S (2011) Early peritoneal immune response during Echinococcus granulosus establishment displays a biphasic behavior. PLoS Negl Trop Dis 5. https://doi.org/10.1371/journal.pntd.0001293
Rostami-Rad S, Jafari R, Yousofi Darani H (2018) Th1/Th2-type cytokine profile in C57 black mice inoculated with live Echinococcus granulosus protoscolices. J Infect Public Health 11. https://doi.org/10.1016/j.jiph.2018.06.007
Mezioug D, Touil-Boukoffa C (2012) Interleukin-17A correlates with interleukin-6 production in human cystic echinococcosis: a possible involvement of IL-17A in immunoprotection against echinococcus granulosus infection. Eur Cytokine Netw 23. https://doi.org/10.1684/ecn.2012.0314
Yasen A, Li W, Aini A et al (2021) Th1/Th2/Th17 cytokine profile in hepatic cystic echinococcosis patients with different cyst stages. Parasite Immunol 43. https://doi.org/10.1111/pim.12839
Amni F, Hajizadeh M, Elmi T et al (2021) Different manifestation of Echinococcus Granulosus immunogenic antigens in the liver and lungs of intermediate host. Comp Immunol Microbiol Infect Dis 74:101573. https://doi.org/10.1016/j.cimid.2020.101573
Zhang W, Wen H, Li J et al (2012) Immunology and immunodiagnosis of cystic echinococcosis: An update. Clin. Dev. Immunol. 2012
Macpherson CNL, Vuitton DA, Gharbi HA et al (2003) International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. In: Acta Tropica
Gazi U, Yapar D, Karasartova D et al (2018) The role of Treg population in pathogenesis of Crimean Congo hemorrhagic fever. Virus Res 250:1–6. https://doi.org/10.1016/j.virusres.2018.04.003
Karasartova D, Gazi U, Tosun O et al (2017) Anti-pneumococcal vaccine-induced cellular immune responses in post-traumatic splenectomized individuals. J Clin Immunol 37:388–396. https://doi.org/10.1007/s10875-017-0397-3
Naik MI, Tenguria RK, Haq E (2016) Detection of serum cytokines before and after pharmacological and surgical treatment in patients with cystic echinococcosis. J Helminthol 90. https://doi.org/10.1017/S0022149X15000085
Biranvand E, Rafiei A, Beiromvand M et al (2020) Cytokine profiles in peripheral blood mononuclear cells from patients with cystic echinococcosis. Comp Immunol Microbiol Infect Dis 70. https://doi.org/10.1016/j.cimid.2020.101469
Riganò R, Buttari B, De Falco E et al (2004) Echinococcus granulosus-specific T-cell lines derived from patients at various clinical stages of cystic echinococcosis. Parasite Immunol 26:45–52. https://doi.org/10.1111/j.0141-9838.2004.00682.x
Amri M, Mezioug D, Touil-Boukoffa C (2009) Involvement of IL-10 and IL-4 in evasion strategies of Echinococcus granulosus to host immune response. Eur Cytokine Netw 20:63–68. https://doi.org/10.1684/ecn.2009.0154
Piccoli L, Meroni V, Genco F et al (2012) Serum cytokine profile by ELISA in patients with echinococcal cysts of the liver: a stage-specific approach to assess their biological activity. Clin Dev Immunol 2012. https://doi.org/10.1155/2012/483935
Tamarozzi F, Meroni V, Genco F et al (2010) Ex vivo assessment of serum cytokines in patients with cystic echinococcosis of the liver. Parasite Immunol 32. https://doi.org/10.1111/j.1365-3024.2010.01236.x
Petrone L, Vanini V, Petruccioli E et al (2015) Polyfunctional specific response to Echinococcus Granulosus associates to the biological activity of the cysts. PLoS Negl Trop Dis 9. https://doi.org/10.1371/journal.pntd.0004209
Fratini F, Tamarozzi F, Macchia G et al (2020) Proteomic analysis of plasma exosomes from cystic echinococcosis patients provides in vivo support for distinct immune response profiles in active vs inactive infection and suggests potential biomarkers. PLoS Negl Trop Dis 14. https://doi.org/10.1371/journal.pntd.0008586
Petrone L, Vanini V, Petruccioli E et al (2015) IL-4 specific-response in whole blood associates with human cystic echinococcosis and cyst activity. J Infect 70. https://doi.org/10.1016/j.jinf.2014.10.009
Li ZD, Mo XJ, Yan S et al (2020) Multiplex cytokine and antibody profile in cystic echinococcosis patients during a three-year follow-up in reference to the cyst stages. Parasites Vectors 13. https://doi.org/10.1186/s13071-020-4003-9
Tuxun T, Wang JH, Lin RY et al (2012) Th17/Treg imbalance in patients with liver cystic echinococcosis. Parasite Immunol 34. https://doi.org/10.1111/j.1365-3024.2012.01383.x
Sadjjadi SM, Mikaeili F, Karamian M et al (2013) Evidence that the Echinococcus granulosus G6 genotype has an affinity for the brain in humans. Int J Parasitol 43:875–877. https://doi.org/10.1016/j.ijpara.2013.06.008
Acknowledgements
We would like to thank Thorvacs (Ankara/Turkey) for the technical support.
Funding
We would like to thank Hitit University Scientific Research Projects Coordination Unit / Corum / Turkey for the financial funding (TIP19001.20.003). The funder had no roles in the study design, data analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Study conception and design: Umut Gazi, Aysegul Taylan-Ozkan; data collection: Yunus Emre Beyhan, Djursun Karasartova, Ufuk Cobanoglu; analysis and interpretation of results: Umut Gazi, Ozgur Tosun; draft manuscript preparation: Umut Gazi. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by Van Yuzuncu Yil University Faculty of Medicine Clinical Research Ethics Committee (Decision date and number: 19.03.2019-05).
Patient Consent for Publication
A written informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gazi, U., Beyhan, Y.E., Tosun, O. et al. Evaluation of Th1/Th2/Th17 Balance in Pulmonary Cystic Echinococcosis Patients. Acta Parasit. (2024). https://doi.org/10.1007/s11686-024-00907-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11686-024-00907-x