Abstract
Purpose
Pyrus boissieriana is a rich source of arbutin and has been used in herbal medicine to treat infectious diseases. This study aimed to investigate the effect of the arbutin-rich fraction of Pyrus boissieriana aerial parts on Toxoplasma gondii In Vitro and In Vivo.
Methods
An arbutin-rich fraction of P. boissieriana was prepared beforehand. Flow cytometry was used to evaluate the effect of different concentrations (1–512 µg/ml) of the P. boissieriana arbutin-rich fraction on Toxoplasma tachyzoites (RH strain). The cytotoxicity of the concentrations on the macrophage J774 cell line was also investigated by MTT assay. For In Vivo investigation, 4–6-week-old female mice infected with the RH strain of T. gondii were treated with different doses (16, 32, 64, 256, and 512 mg/kg) of the fraction using gavage.
Results
The highest and lowest lethality of the tachyzoites were 89.6% and 25.9% related to the concentrations of 512 µg/ml and 1 µg/ml, respectively, with an IC50 value of 18.1 µg/ml ± 0.37. The cytotoxicity test showed an IC50 value of 984.3 µg/ml ± 0.76 after 48 h incubation. The mean survival of mice at the lowest treated dose (16 mg/kg) was 6.6 days, and it was 15 days at the highest dose (512 mg/kg). The concentrations of 512, 256, 128, and 64 mg/kg of the fraction compared to the negative control (6.2 days mean survival) significantly increased the survival time of mice (P < 0.001, P = 0.009, P = 0.018, and P = 0.021, respectively).
Conclusion
The results showed that the arbutin-rich fraction of P. boissieriana is effective against T. gondii In Vitro and In Vivo and may be a reliable alternative to conventional treatment for toxoplasmosis, although further studies are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii (T. gondii) is an Apicomplexa protozoan that causes zoonotic toxoplasmosis. Humans and other warm-blooded animals can be infected as intermediate hosts, and approximately 30% of all people around the world are infected with Toxoplasma [1, 2]. Toxoplasma infection in immunocompetent individuals causes asymptomatic or mild disease symptoms. However, in immunocompromised patients or during pregnancy, it may cause a serious disease. If left untreated, it can lead to the death of the host [3]. Sulfonamide, pyrimethamine, and spiramycin are the routine drugs to treat acute toxoplasmosis [4]. These drugs, in addition to increasing the risk of bone marrow system suppressing, cause various complications such as hematologic toxicity, thrombocytopenia, leukopenia, renal complications, and skin rashes [5]. Recently, many studies on herbal medicine have been underway for the non-toxic and effective treatment of toxoplasmosis. The native plants of each region have been the focus of researchers to conduct experiments [6, 7].
The Pyrus biossieriana (P. boissieriana) from the Rosaceae family is widely distributed in the forests of northern Iran [8]. Additionally, the Pyrus genus is found in other regions of the world, including Central, Southern, and Western Europe, Northwest and Southwest Africa, Asia from Anatolia to Turkmenistan, southwestern and northern Afghanistan through northern Pakistan and the Himalayas to China and Japan, and Armenia [9].
The petioles, leaves, bark, and fruits of some Pyrus species contain large amounts of a phenolic glycoside called arbutin [10]. The extract of this plant is used as a disinfectant for the urinary tract in moderate infection and inflammation of the urinary tract and bile, such as cystitis and dysuria [11]. Its bacteriostatic action in alkaline urine is related to hydroquinone glucuronide and hydroquinone sulfate formed after arbutin liver metabolism in the body. The maximum disinfection effect is 3–4 h after consumption [12, 13]. Due to the outstanding properties of arbutin and its applications in medicine and pharmacy, P. boissieriana can be used as a valuable source of arbutin. Also, the antifungal effects (anti-Candida and Cladosporium), antibacterial, and anti-larval effects of this plant have been proven [14, 15]. Furthermore, the phenolic compounds in P. boissieriana cause strong antioxidant as well as antimicrobial activity by releasing free radicals [16]. In this study, considering the outstanding properties of phenol glycoside (arbutin) and other effective compounds in Pyrus boissieriana, we evaluated the biological effects of the arbutin-rich fraction of P. boissieriana aerial parts on Toxoplasma gondii In Vitro and In Vivo.
Materials and Methods
Ethics Approval
The study protocol has been approved by the ethics committee of Shiraz University of Medical Sciences (Ethical code: IR.SUMS.MED.REC.1400.038). All experiments conform to institutional guidelines for the care and use of laboratory animals of the Institutional Animal and Ethics Committee, Shiraz University of Medical Sciences.
Plant Materials
Pyrus boissieriana, which grows in the Hyrcanian region of the forests of northern Iran, was obtained from there. The plant was identified by the Medicinal Plants Processing Research Center, Shiraz University of Medical Sciences, Iran, and a voucher specimen was deposited with herbarium number: MPPRC-01-01. The aerial parts of P. boissieriana were dried away from direct sunlight in normal air flow and heat.
Preparation of Arbutin-Rich Fraction
The dried plant was pulverized and then extracted by percolation with hydroalcoholic solution (ratio 4:1). This extract was subjected to column chromatography on silica gel 60 (Merck, Germany) with the methanol: chloroform (3:7) %, v/v, as mobile phase. This system was used previously for arbutin extraction [17]. Then this arbutin-rich fraction was concentrated by a rotary evaporator and then dried and finally powdered.
Tachyzoites Preparation
The virulent RH strain of T. gondii for use in this study was maintained by serial intraperitoneal passages in female BALB/c mice (4–5-day intervals). Purification of tachyzoites was based on a previous study [18]. Briefly, after 4–5 days, the infected mice were killed following ethical guidelines. Then, peritoneal fluid was aspirated from the infected mice in phosphate-buffered saline (PBS), and the tachyzoites were purified by centrifugation. Initially, it was centrifuged at 200g for 10 min to remove host cells and debris. Then, the supernatant containing parasites was collected and centrifuged at 1000g for 10 min. The pellet containing tachyzoites was washed three times with PBS at pH 7.2.
Animal Model
Inbred female BALB/c mice (4–6 weeks old) were obtained from the Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. The weight of the animals was between 20 and 22 g. The animals were kept in cages at a temperature of 22 ± 2 °C and a 12-h light and 12-h dark cycle with 40–50% relative humidity. All animals had convenient access to drinking water and standard food during the experiment.
Cell Culture
J774 murine macrophage cell lines were obtained from the Department of Immunology, Shiraz University of Medical Sciences. DMEM medium (Sigma Company, USA) containing 10% heat-inactivated fetal calf serum (FCS; Gibco Company, USA) and 100 IU/mL penicillin–100 μg/mL streptomycin (Roche Company) was used to maintain and culture the cells at 37 °C with 5% CO2 (Corning Costar UK, UK). The cells were routinely subcultured every 3 days by trypsinization and washing with PBS (pH 7.2).
Cytotoxicity Using MTT Assay
For the MTT assay, 100 μL of the culture media in each well of a 96-well plate (containing 3 × 104 macrophage cells) were incubated at 37 °C with 5% CO2 for 24 h. Then, the cells were exposed to different concentrations of the P. boissieriana fraction (1–512 μg/mL in PBS) for 48 h. All experiments were performed in triplicate. After this incubation, 20 μL of the MTT-PBS solution (5 mg/mL) was added to each well. The plate was then covered with aluminum foil (dark condition) and incubated at 5% CO2 at 37 °C for 4 h. Positive controls were 400 μg/mL of sulfadiazine (a conventional drug for toxoplasmosis), and DMEM was used as a control buffer. Then, the supernatant was removed, and 100 μl of acidic isopropanol was added to each well and shaken for 15 min. The optical density was measured at an absorbance of 570 nm using an ELISA reader (Bio-Rad). The cell viability percentage was calculated using the following formula [19]:
In Vitro Anti-Toxoplasma Activity Using Flow Cytometry
For the flow cytometry assay, 15 × 104 tachyzoites/500 μL were deposited in each well and mixed with different concentrations (1–512 μg/mL in PBS) of the fraction at a final volume of 1000 μL. After a 3-h incubation at room temperature, to stain apoptotic tachyzoites, propidium iodide (PI) was added to each microtube at a final concentration of 50 μg/mL. The microtubes were incubated for half an hour in the dark. Saponin 0.2% and a tube without treatment were used as positive and negative controls, respectively. Parasite death based on staining and fluorescence of PI was estimated by flow cytometry.
In Vivo Anti-Toxoplasma Activity in Mice
For the In Vivo evaluation of anti-Toxoplasma activity, forty female BALB/c mice (4–6 weeks old) were divided into 8 groups of 5 and kept in cages. All mice were infected with 105 tachyzoites by subcutaneous injection. After one day, different concentrations (16, 32, 64, 128, 256, and 512 mg/kg-1/day-1) of the fraction were orally gavaged for 7 days. One group was not given any medication or the fraction as a negative control, and a group was given sulfadiazine (400 mg/L/day of sulfadiazine in drinking water) as a positive control. Also, a group of healthy mice received the highest concentration of the fraction (512 mg/kg) to evaluate the possible side effects of the fraction used. All groups were monitored every 24 h until the death of the last mouse, a maximum of 30 days, and daily deaths were recorded.
Statistical Analysis
Statistical analysis and graphs were performed using SPSS Software version 16 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, USA). All values were expressed as the mean ± SD. For multiple comparisons In Vitro, the Kruskal–Wallis test or one-way ANOVA was performed. The survival time of the In Vivo studied groups was compared using the Kaplan–Meier method, and statistically significant was considered P < 0.05.
Results
Cytotoxicity Activity of P. boissieriana
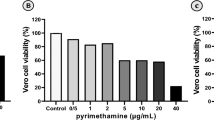
The viability of J774 cells in the presence of different concentrations of the fraction after 48 h shows that 512 μg/mL and 1 μg/mL with 61.3% and 98.1% viability had the highest and lowest toxicity, respectively, with an IC50 value of 984.3 µg/mL ± 0.76 (Fig. 1). It was also found that none of the concentrations was above 50% toxicity, and no significant difference was observed with the negative control group (P > 0.5).
In Vitro Effect of P. boissieriana on T. gondii Tachyzoites
Flow cytometry results showed that the effect of the arbutin-rich fraction of the P. boissieriana aerial parts was directly related to the dose of the fraction and the mortality of tachyzoites. The concentration of 512 μg/mL with 89.6% and 1 μg/mL with 25.9% showed the highest and lowest lethality, respectively. The calculated IC50 value was 18.32 μg/mL ± 0.37. Also, in the unexposed group, the viability of the Toxoplasma tachyzoites was 88.31%, and in the positive control (Tachyzoites that were exposed to 0.2% saponin), the mortality rate was 92.08% (Fig. 2).
In Vivo Effect of P. boissieriana on T. gondii Infection
The survival time of the mice in different groups was recorded (Fig. 3). In the negative control group, the death rate of mice was from day 5 to day 8 (mean survival rate: 6.2 days). In the positive control group (sulfadiazine), the mean survival rate was 25.8 days, and in the toxicity assessment group, the mice did not die until the end of the experiments. In treated groups with different concentrations of the fraction, mortality was observed from day 5 to day 16. The mean survival rate of mice in the groups treated with concentrations of 16, 32, 64, 125, 256, and 512 mg/kg were 6.6 days, 7.4 days, 10.4 days, 12.8 days, 13.6 days, and 15 days, respectively.
There was no significant difference between the survival time of the treatment groups and the positive control group (P > 0.05), although between the negative control group and the concentration of 512 mg/kg (P < 0.001), 256 mg/kg (P = 0.009), 128 mg/kg (P = 0.018), and 64 mg/kg (P = 0.021) of the fraction were significant differences. The comparison between other treatment groups and the control group did not show a significant difference (P > 0.05).
Discussion
P. boissieriana is one of the effective herbal medicines that have been used in traditional medicine; however, there is limited available research on its antiparasitic activity [20]. In the present study, the lethality of Toxoplasma tachyzoites after exposure to different concentrations of P. boissieriana arbutin-rich fraction was between 25.9 to 89.6%. The concentrations of 512, 256, 128, and 64 mg/kg of the fraction significantly increased the survival rate of Toxoplasma-infected mice. Also, our study showed that P. boissieriana arbutin-rich fraction can kill Toxoplasma tachyzoites at a much lower concentration than the toxic concentration for the cell, which can be a very important and good advantage of this fraction (IC50 values 18.32 µg/mL vs. 984.3 µg/mL, respectively).
Various studies have examined the antimicrobial aspects of Pyrus boissieriana. Azadbakht et al. [21], showed that methanolic and dichloromethane extracts of P. boissieriana leaves have antifungal (anti-Candida and Cladosporium), antibacterial (Bacillus subtilis), antioxidant, and anti-larval effects. Guven et al. [14], described the antimicrobial activity of ethyl acetate extract from the fruits of several species of Pyrus (Rosaceae). Jin et al. [22], confirmed the strong antibacterial activity of P. boissieriana extract against Erwinia amylovora. The results of these studies, in line with our study, confirm the antimicrobial activity of Pyrus boissieriana. The presence of compounds such as phenol, arbutin, and benzoquinones (a metabolic product of arbutin) in P. boissieriana can be the reason for this [22]. Arbutin, as a very useful biological substance, is present in large quantities in Pyrus boissieriana, which has proven its antioxidant and antimicrobial activity [8]. The lack of significant adverse effects of arbutin and plant extracts containing it makes them a valuable medical agent [23]. In some investigations, the anti-inflammatory, antioxidant, and antibacterial potential of arbutin have each been documented [24, 25]. Also, Adeyemi et al. [26], showed a mild suppressive action of arbutin against the In Vitro growth of T. gondii (IC50: 17.20 µg/mL). Arbutin has been shown to inhibit tyrosinase activity through different mechanisms of action [27]. Tyrosinase is an important and vital enzyme of Toxoplasma that is essential in tyrosine metabolism. Inhibition of this enzyme by disturbing tyrosine metabolism has been shown to reduce parasite growth as well as its pathogenicity [28]. Phenolic compounds in P. boissieriana have also been shown to be involved in its antioxidant and antimicrobial activity by releasing free radicals [16].
Conclusion
The results of this study showed that an arbutin-rich fraction of P. boissieriana aerial parts dose-dependently increases the mortality of Toxoplasma tachyzoites and significantly increases survival time in mice infected with acute toxoplasmosis.
Data sharing statement
The authors confirmed that all the data for this manuscript are available; if someone wants to request the data, they can contact the corresponding author.
References
Bahreini MS, Zarei F, Dastan N, Sami Jahromi S, Pourzargham P, Asgari Q (2020) The relationship between Toxoplasma gondii infection in mothers and neonate’s gender. J Matern Fetal Neonatal Med 35(22):4263–4267. https://doi.org/10.1080/14767058.2020.1849103
Omidian M, Asgari Q, Bahreini MS, Moshki S, Sedaghat B, Adnani Sadati SJ (2022) Acute toxoplasmosis can increase serum dopamine level. J Parasit Dis 46(2):337–342. https://doi.org/10.1007/s12639-021-01447-1
Dubey JP (2008) The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol 55(6):467–475. https://doi.org/10.1111/j.1550-7408.2008.00345.x
Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG (2018) Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev 31(4):e00057-e117. https://doi.org/10.1128/cmr.00057-17
Ghanadian M, Khamesipour F, Hejazi SH, Razavi SM, Sadraei H, Namdar F (2022) In Vitro and In Vivo anti-toxoplasma activities of Dracocephalum kotschyi extract in experimental models of acute toxoplasmosis. Acta Parasitol 67(1):487–495. https://doi.org/10.1007/s11686-021-00491-4
Asgari Q, Mikaali F, Ahmadi B, Bahraini MS (2019) In Vitro and In Vivo study of the effects of Lawsonia inermis on Toxoplasma gondii. Armaghane danesh 24(1):31–42. https://doi.org/10.52547/armaghanj.24.1.31
Cheraghipour K, Masoori L, Ezzatpour B, Roozbehani M, Sheikhian A, Malekara V, Niazi M, Mardanshah O, Moradpour K, Mahmoudvand H (2021) The experimental role of medicinal plants in treatment of Toxoplasma gondii infection: a systematic review. Acta Parasitol 66(2):303–328. https://doi.org/10.1007/s11686-020-00300-4
Azadbakht M, Ramzani M (2003) Identification and determination of phenyl glycoside in the leaf of Pyrus boissieriana buhse by two methods of HPLC and spectrophotometery. J Maz Univ Med Sci 13(41):1–8
Browicz K (1993) Conspect and chorology of the genus Pyrus L. Arboretum Kórnickie 38:17–33
Petricic J, Apostolski R, Srepel B (1981) The leaf of the wild pear-tree as an Arbutinic herb
Couteau C, Coiffard LJ (2000) Photostability determination of arbutin, a vegetable whitening agent. II. Farmaco 55(5):410–413. https://doi.org/10.1016/S0014-827X(00)00049-5
Stambergova A, Supcikova M, Leifetova I (1985) Evaluation of phenolic substances in Arctostaphylos uva ursi, determination of arbutin, methylarbutin and hydroquinone in the leaves by HPLC, pp 179–182
Nabeel Yehya N (2014) Identification of some cultivars of Pyrus communis L. planted in Mosul city using morpholgical and chemical characters. Coll Basic Educ Res J 13(2):953–976
Güven K, Yücel E, Cetintaş F (2006) Antimicrobial activities of fruits of Crataegus. and Pyrus. species. Pharm Biol 44(2):79–83. https://doi.org/10.1080/13880200600591253
Elahimanesh F, Hassanzadeh D, Sayfzadeh N, Abdolmohammadi J, Abdolmohammadi J (2015) The effects of Pyrus Biossieriana Bushe leaf extract on the. S J Nurs Midwifery Paramed Fac 1(1):48–55. https://doi.org/10.29252/sjnmp.1.1.48
Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Fazelian M, Eslami B (2009) In Vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharmacog Mag 5(18):122–126
Alam P, Alqasoumi S, Shakeel F, Abdel-Kader M (2011) HPTLC densitometric analysis of arbutin in bulk drug and methanolic extracts of Arctostaphylos uva-ursi. Nat Prod Res 25(17):1671–1675. https://doi.org/10.1080/14786419.2010.529447
Bahreini MS, Iraji A, Edraki N, Arab Monfared A, Asgari Q (2022) Synthesis and anti-Toxoplasma activity of indole-triazole compounds on tachyzoites of RH strain. Ann Med Surg 74:103245. https://doi.org/10.1016/j.amsu.2022.103245
Bahreini MS, Yazdi AR, Jowkar F, Motamedi M, Mikaeili F (2022) Cytotoxic screening and In Vitro effect of sodium chlorite against Leishmania major promastigotes. J Parasit Dis 46(4):945–951. https://doi.org/10.1007/s12639-022-01511-4
Bilia A, Rubio MD, Alvarez ML, Morelli I, Gonzalez JM (1994) New benzyl alcohol glycosides from Pyrus bourgaeana. Planta Med 60(06):569–571. https://doi.org/10.1055/s-2006-959574
Azadbakht M, Ramezani M, Marston A, Hostettmann K, Jahromi Moghaddam M (2004) Biological activity of leaf extract and phenolglycoside arbutin of pyrus boissieriana buhse. J Med Plant 3(10):9–14
Jin S, Sato N (2003) Benzoquinone, the substance essential for antibacterial activity in aqueous extracts from succulent young shoots of the pear Pyrus spp. Phytochemistry 62(1):101–107. https://doi.org/10.1016/S0031-9422(02)00444-2
Migas P, Krauze-Baranowska M (2015) The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem Lett 13:35–40. https://doi.org/10.1016/j.phytol.2015.05.015
Busetto GM, Giovannone R, Ferro M, Tricarico S, Del Giudice F, Matei DV, De Cobelli O, Gentile V, De Berardinis E (2014) Chronic bacterial prostatitis: efficacy of short-lasting antibiotic therapy with prulifloxacin (Unidrox®) in association with saw palmetto extract, lactobacillus sporogens and arbutin (Lactorepens®). BMC Urol 14(1):1–9. https://doi.org/10.1186/1471-2490-14-53
Zhao W, Wang S, Qin T, Wang W (2019) Arbutin attenuates hydrogen peroxide-induced oxidative injury through regulation of microRNA-29a in retinal ganglion cells. Biomed Pharmacother 112:108729–108729. https://doi.org/10.1016/j.biopha.2019.108729
Adeyemi OS, Atolani O, Awakan OJ, Olaolu TD, Nwonuma CO, Alejolowo O, Otohinoyi DA, Rotimi D, Owolabi A, Batiha GE (2019) In Vitro screening to identify anti-Toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J Biol Med 92(3):369–383
Jin YH, Lee SJ, Chung MH, Park JH, Park YI, Cho TH, Lee SK (1999) Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch Pharm Res 22(3):232. https://doi.org/10.1007/BF02976355
Bekier A, Węglińska L, Paneth A, Paneth P, Dzitko K (2021) 4-Arylthiosemicarbazide derivatives as a new class of tyrosinase inhibitors and anti-Toxoplasma gondii agents. J Enzyme Inhib Med Chem 36(1):1145–1164. https://doi.org/10.1080/14756366.2021.1931164
Acknowledgements
This study was undertaken as an MD degree thesis for Meysam Gholami and Mojtaba Habibollahi and the work was supported by Shiraz University of Medical Sciences.
Funding
The study was financially supported by the office of the vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 21764).
Author information
Authors and Affiliations
Contributions
QA, AH, and APL Conceived and designed the experiments. MSB, SFP, MGh, and MH Performed the experiments. MSB and QA Analyzed and interpreted the data. QA Contributed reagents, materials, analysis tools, or data. The first draft of the manuscript was written by MSB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics and consent statement
The current study was approved by the Ethical Committee of Shiraz University of Medical Sciences, Shiraz, Iran (ethical code: IR.SUMS.MED.REC.1400.038).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahreini, M.S., Pourmohammadi, S.F., Gholami, M. et al. Anti-Toxoplasma In Vitro and In Vivo Activity of Pyrus boissieriana Arbutin-Rich Fraction. Acta Parasit. 69, 567–573 (2024). https://doi.org/10.1007/s11686-023-00759-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00759-x