Abstract
Background
The problem of resistance to antiparasitic drugs, associated with their side effects, suggest exploring other alternatives, including medicinal plants. Dracocephalum kotschyi (D. kotschyi), for example, from Lamiaceae family, is a plant widely used in Iran and in many countries, and to which interesting pharmacological properties have been attributed. This study aimed to investigate in vitro and in vivo anti-Toxoplasma activities of D. kotschyi extract in experimental models of acute toxoplasmosis.
Methods
Anti-Toxoplasma activity of the extracts in vitro was performed on Vero Cell, using the MTT test. Vero cell were infected with (3 × 105 tachyzoites/well) following treatment with Dichloromethane (F1), dichloromethane: methanol (F2), methanol (F3), methanol: water (F4), and deionized water (F5) extracts of D. kotschyi, and pyrimethamine and sulfadiazine (positive control). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was used to measure cell viability. Effects of extracts on tachyzoites viability were evaluated by trypan blue exclusion method, followed by light microscopy. For in vivo test, 18 groups of 8–10-week-old Inbred Balb/c mice weighing 18–20 g, were intraperitoneally infected with 2 × 103 tachyzoites and then treated with sterile PBS (negative control), pyrimethamine (25 mg/kg) and sulfadiazine (500 mg/kg) as positive controls, and F1 to F5 extracts (at doses 50, 100 and 200 mg/kg).
Results
The 50% Inhibitory Concentration of F1 extract, F2 extract, Sulfadiazine (Positive control) and Pyrimethamine (Positive control) were 8.77 µg, 7.1 µg 391.18 µg, and 84.20 µg, respectively, while the selectivity indices were 15.667, 30.197, 1.552 and 4.064, respectively. In vivo anti-Toxoplasma activity test showed that Methanol: water (F-4) 50 extract was more active than the positive control.
Conclusions
Indeed, the extract allowed a survival rate of 10% of the mice, compared to 0% for all the other groups. D. kotschyi has the potential to be valorized in the management of toxoplasmosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii (T. gondii) is an obligate intracellular parasite of a large number of homeothermic animals including humans [1]. The most common routes of transmission are the consumption of food or drink (usually water) contaminated with cat feces (containing the parasite's oocytes) or the ingestion of undercooked meat containing the parasite's cysts [2]. In a pregnant woman, T. gondii can cross placental barriers, infecting the fetus, causing death in most cases. If the fetus survives, it can suffer from severe birth defects, growth retardation, mental retardation, eye disease, and many other clinical manifestations [3]. Estimates report that up to one-third of the world's human population has been exposed to the parasite [4]. The overall annual incidence of congenital toxoplasmosis has been estimated at 190,100 cases [5].
The therapeutic management of toxoplasmosis involves antiparasitic drugs, such as Sulfadiazine and Pyrimethamine, but problems of ineffectiveness, parasite resistance, toxicity, and side effects have been reported [6, 7]. Among the possibilities to be explored or alternatives to antiparasitic drugs, natural substances from medicinal plants occupy a prominent place. Indeed, several medicinal plants have been reported in the traditional treatment of toxoplasmosis. Also, several pharmacological studies demonstrated the ability of medicinal plant extracts to inhibit in vitro and in vivo the growth of T. gondii [1, 7,8,9,10,11].
Dracocephalum kotschyi (D. kotschyi), a member of the Lamiaceae family, is vulgarly called Zarrin-giah and Badrandjboie-Dennaie in Iran [12]. The species is widely used in Iran for its many medicinal properties, due to the presence of bioactive compounds [13], especially high content and diversity of polyphenols and flavonoids. Also, it's antispasmodic [12], immunomodulatory [14] properties have been demonstrated. Unfortunately, very little data are available on its antiparasitic properties, in vivo and in vitro, in particular against T. gondii. Indeed, no scientific study has focused on the scientific valorization of D. kotschyi in the search for an alternative to the insufficiencies of classical antiparasitic drugs against toxoplasmosis. This study aims to investigate in vitro and in vivo anti-Toxoplasma activities of D. kotschyi extract in experimental models of acute toxoplasmosis. This study is based on the hypothesis that given the very high use of D. kotschyi, its numerous biological and pharmacological properties and its high content of bioactive compounds, this medicinal plant is likely to be valued in the fight against toxoplasmosis. The ability of several extracts of D. kotschyi to inhibit in vitro and in vivo the tachyzoites of T. gondii are explored using standard and appropriate methodologies to provide scientific data on the anti-Toxoplasma properties of the medicinal plant.
Materials and Methods
Parasite
Tachyzoites from the RH strain of T. gondii were provided by the Toxoplasmosis Research Center (TRC) in Iran.
Vero Cells Culture
In this experimental study, samples were run in triplicate to avoid any biased error. The kidney cell lines "Vero" was initiated from a green monkey kidney and were obtained from the National Cell Bank of Iran (NCBI, Pasteur Institute of Iran, Tehran, Iran). Vero cells were cultured at 37 °C with 5% CO2, in an RPMI-1640 (Gibco) medium supplemented with 100 μg/ml streptomycin, 2 raM l-glutamine, 10% fetal bovine serum (FBS), and 100 units/ml penicillin.
Plant Material
Dracocephalum kotschyi aerial parts were collected from Semirom, Isfahan province, Iran, in October 2019. It was authenticated by Professor of Pharmacognosy: Mustafa Ghanadian, Department of Pharmacognosy, Isfahan University of Medical Sciences, Isfahan, Iran, where a voucher specimen was deposited (No. 1519).
Animal Model and Ethics
In vivo experiments were conducted on 8–10-week-old Inbred Balb/c mice weighing 18–20 g. The animal protocols used in the study were approved by the Ethics Committee before starting the study. All mice were kept in cages under standard laboratory conditions including an average temperature (20–25 °C), humidity (60 ± 10%), light (12 h per day), given drinking water, and regular diet. The study protocol was approved by the ethical committee of the Shiraz University (IR.Shirazu.REC.1399.1214). Guidelines of Animal Ethics Committees (AECs) from Iran, have been used.
Chemicals and Reagents
Chemicals and reagents used in this study include methanol (Chromasolv, Sigma-Aldrich), dichloromethane (Chromasolv, Sigma-Aldrich) and distilled water (Water for chromatography LiChrosolv).
Plant Extraction
The extraction of the plant material was done by direct extraction from raw materials using different solvents in the order of increasing polarity. The fine pulverized plant material (200 g) was extracted with dichloromethane (1.2 L, 11 g, F-1); dichloromethane: methanol 80:20 (1.2 L, 13 g, F-2); methanol (1.2 L, 12 g, F-3); methanol: water 60:40 (1.2 L, 8 g, F-4); and deionized water (1.2 L, 7 g, F-5). Each extraction was done using the maceration method with continuous shaking for 48 h. Each extract was then filtered through Whatman® Grade 1 filter paper, and concentrated under reduced pressure using a rotary evaporator. Concentrated extracts were lyophilized using a freeze dryer, placed into a sterile glass container, and kept at – 20 °C until use.
Phytochemical Analysis, Identification of Bioactive Compounds and NMR Spectroscopy
Chromatographed on a SC6 polyamide column (20 × 300 mm) using gradient mixtures. Major fractions were selected and injected to recycle HPLC pump using Shimpak-RP C18 column (250 × 20 mm) and acetonitrile: DMSO: water (69:9:22) as mobile phase to yield compounds, as the main bioactive compounds. 1H-NMR spectra of bioactive isolated compounds were taken by a regular 5 mm diameter probe using Bruker 400 MHz (Bruker Biospin, Rheinstetten, Germany), operating at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR spectrometry.
Anti-Toxoplasma Activity of the Extracts In Vitro
Cytotoxicity Assessment
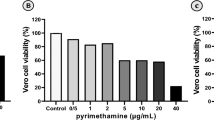
All extracts and control drugs (pyrimethamine and sulfadiazine) were dissolved in complete culture medium RPMI-1640 containing dimethyl sulfoxide (DMSO) less than 1% to improve the dilution. Vero cells were cultured (2 × 104 cell/well/180 μl/ml) in 96 well plates for 24 h. After that, the cells were exposed to the agents at a final concentration of 1000, 500, 100, 50, 10, and 1 μg. After 24 h, cell viability was measured by adding 20 μl of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). Then, the 50% cytotoxic concentration (CC50) was measured using Graph Pad Prism 6.0 software [15]. Then, the selectivity index (SI) was determined (CC50/IC50 = SI). All data points represent the average of three independent experiments [16].
Effects of Extracts on Tachyzoites Viability by Trypan Blue Exclusion
The tachyzoite viability test was performed, in vitro using the method proposed by Cover and Gutteridge [17]. Six concentrations (1, 10, 50, 100, 500, and 1000 µg) of D. kotschyi extracts were incubated for 30, 90, and 180 min. The trypan blue stain was used to assess the viability of the tachyzoites. 45 μl of tachyzoite suspension containing 106 cells/ml and D. kotschyi extracts at six different concentrations (1, 10, 50, 100, 500, and 1000 µg) in 96-well microplates are brought together. The whole is incubated at 37 °C. After 30-, 90-, and 180-min incubation in 5% CO2 at 37 °C, a trypan blue dye exclusion test for tachyzoites was carried out under the microscope. Results were expressed as % viability. Positive controls containing pyrimethamine and sulfadiazine, while PBS were the negative controls. The plates were then spread on a glass slide followed by an examination under an optical microscope. The experiments were repeated three times.
Light Microscopy of Tachyzoites in Cell Lines
Vero cell culture (2 × 105 cells/ml) was carried out on a glass slide in a 35 mm cell culture dish up to the confluence and then infected with 1 × 106 tachyzoites/box. After 4 h incubation, the monolayers were washed with Hanks' Balanced Saline Solution (HBSS; Gibco Inc., USA), followed by the addition of the extracts contained in RPMI-1640. The glass lids were removed from the dishes at 48 h after adding the extracts, pyrimethamine, sulphadiazine, and 1% DMSO (negative control). All glass slides were washed with HBSS and fixed with methanol before staining with Giemsa (Sigma Inc., USA). The samples were observed under an oil-immersion objective lens by an optical microscope.
Anti-Toxoplasma Activity of the Extracts in vivo
BALB/c mice (n = 180), were housed into 18 groups (10 mice each). One group was kept as an uninfected control. 18 groups were intraperitoneally infected with 2 × 104 tachyzoites. The infected control group was administered sterile PBS (negative control), the positive controls received pyrimethamine (25 mg/kg), sulfadiazine (500 mg/kg), and other groups were treated with F1 to F5 extracts (at doses 50, 100, and 200 mg/kg). The mice were monitored daily for mortality and morbidity. The survival periods were recorded daily until all mice died. Initially, for controlling drug side effects, a preliminary experiment was done on Balb/c mice receiving the same dose of drugs, and no mortality or clinically significant toxicity was observed.
Statistical Analysis
Results were analyzed using Graph Pad Prism 6.0 software. Differences between test and control groups were evaluated by analysis of variance (ANOVA) one tailed and Newman–Keuls multiple-comparison test. The P value < 0.05 was considered significant.
Results
In Vitro Anti-Toxoplasma Activity of the Extracts
Cytotoxicity and Antiparasitic Effect
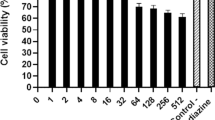
For all extracts, cytotoxicity on Vero cells varied according to the concentrations of the extracts (Fig. 1).
Effects of concentration on cytoxicity of each plants extracts. Vero cell infected with 3 × 105 tachyzoites/well of RH Toxoplasma gondii and treated with Sulfadiazine and Pyrimethamine (Positive control), and + Dichloromethane (F1), dichloromethane: methanol (F2), methanol (F3), methanol: water (F4), and deionized water (F5) extracts of Dracocephalum kotschyi, at different concentration (1, 10, 50, 100, 500, 1000 µg); Negative control = PBS
CC50, IC50, and SI of the extracts and positive controls are shown in Table 1. Antiparasitic assessment of extracts and positive controls showed a high inhibitory activity of Dichloromethane extract and Dichloromethane: Methanol extract of D. kotschyi on tachyzoite with IC50 = 8.77 µg and 7.1 µg, respectively, in comparison to positive control (Sulfadiazine and Pyrimethamine) (IC50 = 391.18 µg and 84.20 µg, respectively).
Dichloromethane: Methanol extract and Dichloromethane extract of D. kotschyi had the highest Selectivity Indices, illustrating better selectivity, with low effect for host cell: 30.197 and 15.667, respectively. On the other hand, the positive control (Sulfadiazine, Positive control) had the lowest selectivity index (1.552). Sulfadiazine was the most toxic treatment for the host cells.
Effects of Extracts on Tachyzoites Viability by Trypan Blue Exclusion
The trypan blue stain was used to assess the viability of the tachyzoites. The results showed that D. kotschyi has acceptable efficacy in vitro, and the parasiticidal effect of F1 (Dichloromethane) and F2 (Dichloromethane: Methanol) extracts were significantly better than positive control in all exposure times (Table 2). Viability was evaluated based on concentration at 30 min, 90 min, and 180 min. It should be noted that cell viability for all extracts and positive controls decreases over time and as the concentration of the extracts or reference molecule increases.
Effects of the Extracts on T. gondii in vivo
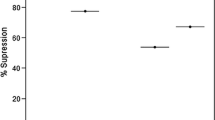
Clinically, the number of mice in the untreated infected group (negative control) began to decrease on day 7 of the study and all mice died until day 9 post-infection.
Mice in the Pyrimethamine group (positive control) began to die on day nine and all died by day eleven. Between the sixth and seventh days, all mice in the Sulfadiazine (positive control) group died (Fig. 2).
Mice in the extract groups also had, for the most part, 100% mortality, except for the methanol: water (F-4) 50 extract group, where mice had a 10% survival rate. Indeed, the mice started to die on day 5 and there was no more death after day 7. At the end of the in vivo test, methanol: water (F-4) 50 had the best in vivo activity compared to the other extracts and positive control.
Phytochemical Analysis and Identification of Bioactive Compounds from Most Active Extract
Antiprotozoal effects of the resulted extracts F1–F5 were compared in Table 1. F2, extracted by dichloromethane: methanol, with more anti-T. gondii activity (IC50 of 7.1 μg/ml) was selected and chromatographed on a SC6 polyamide column (20 × 300 mm) using gradient mixtures of chloroform: methanol as follows: F2.1 (100:0); F.2.2 (96:4); F2.3 (94:6); F2.4 (90:10); F2.5 (88:12); F2.6 (86:14); F2.7 (80:20). Major fractions F2.2 and F2.4 were selected and injected to recycle HPLC pump using Shimpak-RP C18 column (250 × 20 mm) and acetonitrile: DMSO: water (69:9:22) as mobile phase to yield compounds 1–4, respectively, as the main bioactive compounds.
NMR Spectroscopy
1H-NMR spectra of bioactive isolated compounds were taken by a regular 5 mm diameter probe using Bruker 400 MHz (Bruker Biospin, Rheinstetten, Germany), operating at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR spectrometry. Spectra were collected at 25 °C in DMSO-d6. The sugar linkage was detected through Heteronuclear multiple bond correlation (HMBC) between anomeric proton H-1″ of sugar and its location on aglycone structure. The configurations of anomeric proton was deduced to be β-form based on its anomeric large coupling constants (J = 7.0–8.0 Hz). Chemical shifts are reported in delta (δH or δC), after shimming by topshim command in TOPSPIN software. The data were processed using 2018 Mestrelab Research program (www.mestrelab.com). Isolated compounds (Fig. 3) were identified according to data reported in literature [18,19,20,21].
Apigenin: Pale yellow powder, 1H-NMR in pyridine-d6 (400 MHz) ppm: 6.10 (1H, d, J = 2.0 Hz, H-8), 6.36 (1H, d, J = 2.0 Hz, H-6), 6.49 (1H, s, H-3), 6.83 (2H, d, J = 8. 8 Hz, H-6′,2′) and 7.75 (2H, d, J = 8.8 Hz, H-5′,3′); 13C-NMR (100 MHz, pyridine-d6) δC: 183.2 (C4), 166.4 (C2), 163.8 (C7), 163.3 (C5), 163.2 (C4′), 159.0 (C9), 129.4 (C5′, C3′), 117.3 (C6′, C2′), 122.8 (C1′), 106.5 (C10), 104.4 (C3), 100.5 (C6), 95.3 (C8). ESI negative mass m/z 269 [M−1].
Apigenin 4′-O-β-d-glucopyranoside: Pale yellow powder, 1H-NMR in DMSO-d6 (400 MHz) ppm: δ 3.2 to 3.8 (6H, overlapped, H-1″ to H-6″),4.72 (1H, d, J = 7.2 Hz, H-1″), 6.67 (1H, s, H-3), 6.75 (1H, d, J = 2.0 Hz, H-8), 6.81 (1H, d, J = 2.0 Hz, H-6), 6.92 (2H, d, J = 8.8 Hz, H-6′,2′) and 7.90 (2H, d, J = 8.8 Hz, H-5′,3′); 13C-NMR (100 MHz, DMSO-d6) δC: 177.0 (C4), 162.7 (C2), 161.2 (C5), 160.1 (C7), 158.5 (C4′), 158.3 (C9), 128.1 (C6′, C2′), 121.1 (C1′), 115.1 (C5′, C3′), 108.1 (C10), 105.6 (C3), 104.5 (C1″), 104.3 (C6), 98.3 (C8), 77.5 (C5″), 75.5 (C3″), 73.6 (C2″), 69.6 (C4″), 60.8 (C6″). ESI negative mass m/z 431 [M−1].
Apigenin 7-O-β-d-glucopyranoside: Pale yellow powder, 1H-NMR in DMSO-d6 (400 MHz) ppm δ 3.2 to 3.8 (6H, overlapped, H-1″ to H-6″), 5.08 (1H, d, J = 7.6 Hz, H-1″), 6.46 (1H, d, J = 2.0 Hz, H-8), 6.84 (1H, d, J = 2.0 Hz, H-6), 6.88 (1H, s, H-3), 6.94 (2H, d, J = 8.8 Hz, H-6′,2′) and 7.96 (2H, d, J = 8.8 Hz, H-5′,3′); 13C-NMR (100 MHz, DMSO-d6) δC: 181.1 (C4), 164.2 (C2), 162.9 (C5), 162.3 (C7), 161.1 (C9), 156.1 (C4′), 128.5 (C6′, C2′), 120.1 (C1′), 116.0 (C5′, C3′), 105.3 (C10), 103.1 (C3), 99.1 (C1″), 99.5 (C6), 94.1 (C8), 77.1 (C5″), 76.3 (C3″), 73.0 (C2″), 69.5 (C4″), 60.5 (C6″). ESI negative mass m/z 431 [M−1].
Luteolin 7-O-β-d-glucopyranoside: Pale yellow powder, 1H-NMR in DMSO-d6 (400 MHz) ppm δ 3.2 to 3.8 (6H, overlapped, H-1″ to H-6″), 5.08 (1H, d, J = 7.2 Hz, H-1″), 6.44 (1H, d, J = 2.0 Hz, H-8), 6.74 (1H, s, H-3), 6.79 (1H, d, J = 2.0 Hz, H-6), 6.94 (1H, d, J = 8. 8 Hz, H-5′), 7.42 (1H, d, J = 2.0 Hz, H-2′, 7.46 (1H, dd, J = 8.8, 2.0 Hz, H-6′); 13C-NMR (100 MHz, DMSO-d6) δC: 181.8 (C4), 164.6 (C2), 162.9 (C7), 161.1 (C5), 156.9 (C9), 149.7 (C4′), 145.3 (C3′), 120.9 (C1′), 119.3 (C6), 116.0 (C5′), 113.0 (C2′), 105.3 (C10), 103.1 (C3), 99.9 (C1″), 99.7 (C6), 94.7 (C8), 77.1 (C5″), 76.3 (C3″), 73.1 (C2″), 69.5 (C4″), 60.5 (C6″). ESI negative mass m/z 447 [M−1].
Discussion
This study was aimed to investigate in vitro and in vivo anti-Toxoplasma activities of D. kotschyi extract in experimental models of acute toxoplasmosis.
For the investigation, we opted for RH strain of T. gondii, because this strain has been successfully used in several studies devoted to the anti-Toxoplasma activity of natural substances, especially because it produces acute toxoplasmosis [22]. Furthermore, the choice of Vero cells as host cells is due to the fact that this model allows optimal growth of T. gondii with good replication efficiency [23, 24].
One of the problems encountered in the management of toxoplasmosis is the toxicity of chemical drug molecules, hence the need to resort to natural substances. Our study confirmed this observation. Indeed, at the end of thein vitro investigations, it was observed that that Sulfadiazine (reference drugs and positive control) was the most toxic treatment for the host cells (SeIectivity Index = 1.552). Sulfadiazine is one of the molecules used in the treatment of toxoplasmosis [6, 7]. It is, therefore, highly toxic to host cells. Pyrimethamine also presented a low SI (4.064). This constitutes a strategic disadvantage for these reference molecules and justifies the interest in using natural substances. Indeed, we can observe that Dichloromethane: Methanol extract and Dichloromethane extract of D. kotschyi had the highest Selectivity Indices, illustrating better selectivity, with low effect for host cell: 30.197 and 15.667, respectively. The data are consistent with the results of Montazerri et al. [7] who also showed that Pyrimethamine was toxic to the host cell in comparison to Hydroalcoholic Extract of some Brassicaceae Species. This toxicity of the reference drugs is probably linked to their reduced efficacy reported in several studies, which requires the use of high concentrations that are, therefore, toxic for the cells.
About anti-Toxoplasma activity, the 50% Inhibitory Concentration of Dichloromethane extract, Dichloromethane: Methanol, extract, Sulfadiazine (Positive control), and Pyrimethamine (Positive control) were 8.77 µg, 7.1 µg 391.18 µg, and 84.20 µg, respectively. All the plant extracts were more active than the positive control. This confirms that medicinal plants such as D. kotschyi can be used in the management of toxoplasmosis, an alternative to conventional drugs. The extracts of D. kotschyi were distinguished by a strong antiparasitic activity, associated with low toxicity. This is the first report of the anti-Toxoplasma activity of D. kotschyi, but several data confirm the high activity and low toxicity of natural substances from medicinal plants against Toxoplasma. In a study dedicated to anti-Toxoplasma activity of some Brassicaceae, extracts of L. sativum, L. perfoliatum, N. officinale, A. homolocarpum, C. bursa-pastoris presented 50% inhibitory concentrations of 5.1, 14.67, 32.49, 37.31, 71.35 and selectivity indices of 8.06, 2.59, 0.74, 0.78, respectively, in comparison to pyrimethamine (IC50 = 63 μg/ml) [7].
At the organism level (in vivo), the survival test showed high mortality in all groups, illustrating a low activity of extracts and reference molecules. However, it is striking to note that the methanol: water (F-4) 50 extract was more active than the positive control, having allowed a survival rate of 10% of the mice against 0% for all other groups. This result confirms the results of the in vitro tests and attests that D. kotschyi has a better activity in vitro and in vivo against Toxoplasma strains, in comparison with classical antiparasitic agents, such as Sulfadiazine (Positive control) and Pyrimethamine (Positive control). Also, high variability was observed in the activity of the extracts. This may be due to the variability of the solvents used.
The activity of the extract is due to the quality of the active molecules present in the extract, which is related to the affinity that these molecules have for the extraction solvent.
This study was not focus on chemical composition of D. kotschyi; however, the most bioactive extract, F-2, was submitted for phytochemical analysis for identification of its phytochemicals. Four compounds were separated and identified as apigeinin (compound 1), Apigenin-4′-O-β-d-glucopyranoside (compound 2), apigenin-7-O-β-d-glucopyranoside (compound 3) and luteolin-7-O-β-d-glucopyranoside (compound 4). Previous study reported the presence of actives compounds in the plant extract or oil. Shakib et al. [25] found that D. kotschyi essential oils contains Oxygenated Sesquiterpenes, Monoterpene Hydrocarbons, Sesquiterpene Hydrocarbons and Oxygenated Monoterpenes. Flavoinoids such as calycopterin, acacetin 7-O-β-d-glucopyranoside, xanthomicrol, apigenin 4′-O-β-d-glucopyranoside, isokaempferide, luteolin 3′-O-β-d-glucuronide, luteolin, luteolin 7-O-β-d-glucopyranoside, apigenin have also been identified elsewhere [18,19,20,21].
Conclusions
We report for the first-time anti-Toxoplasma activity of D. kotschyi, in vitro, and in vivo. This potential is associated with a low toxicity for the host cell. Supplementary works are necessary to identify actives compounds associated with anti-Toxoplasma activity.
Data Availability
The data sets from the present study are available from the corresponding author upon request.
Change history
13 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11686-021-00516-y
Abbreviations
- D. kotschyi :
-
Dracocephalum kotschyi
- MTT:
-
Thiazolyl blue tetrazolium bromide
- T. gondii :
-
Toxoplasma gondii
- IC50:
-
Half-maximal inhibitory concentration
- PBS:
-
Phosphate-buffered saline
References
Daryani A, Ebrahimzadeh MA, Sharif M, Ahmadpour E, Edalatian S, Esboei BR et al (2015) Anti-Toxoplasma activities of methanolic extract of Sambucus nigra (Caprifoliaceae) fruits and leaves. Rev Biol Trop 63:07–12. https://doi.org/10.15517/RBT.V63I1.14545
Petersen E (2007) Toxoplasmosis. Semin Fetal Neonatal Med 12:214–223. https://doi.org/10.1016/j.siny.2007.01.011
Ambroise-Thomas P, Pelloux H (1993) Toxoplasmosis—congenital and in immunocompromised patients: a parallel. Parasitol Today 9(2):61–63. https://doi.org/10.1016/0169-4758(93)90038-h
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217. https://doi.org/10.1016/s0020-7519(00)00124-7
WHO (2013) OMS|La charge mondiale de la toxoplasmose: une étude systématique. WHO. https://www.who.int/bulletin/volumes/91/7/12-111732-ab/fr/. Accessed 21 Sep 2020
Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS, van Ruyven RL, Klok AM, Hoyng CB, Rothova A (2002) A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol 134(1):34–40. https://doi.org/10.1016/s0002-9394(02)01537-4
Montazeri M, Mirzaee F, Daryani A, Naeimayi R, Karimabad SM, Arjmandi HK et al (2020) Anti-Toxoplasma activities of the hydroalcoholic extract of some Brassicaceae species. Adv Biomed Res 9:5. https://doi.org/10.4103/abr.abr_206_19
Sharif AA, Unyah NZ, Nordin N, Basir R, Wana MN, Alapid Ahmad A et al (2019) Susceptibility of Toxoplasma gondii to ethanolic extract of Tinospora crispa in vero cells. Evid Based Complement Alternat Med 2019:2916547. https://doi.org/10.1155/2019/2916547
Powers JL, Zhang X, Kim CY, Abugri DA, Witola WH (2017) Activity of green algae extracts against Toxoplasma gondii. Med Aromat Plants. https://doi.org/10.4172/2167-0412.1000293
Abdullahi S, Abd Majid R, Unyah N, Nordin N, Basir R, Wana M et al (2018) In vitro anti-toxoplasmal activities of ethanolic extracts from Andrographis paniculata and Tinospora crispa against Toxoplasma gondii parasite. Front Pharmacol. https://doi.org/10.3389/conf.fphar.2018.63.00046
Kavitha N, Noordin R, Chan K-L, Sasidharan S (2012) In vitro anti-Toxoplasma gondii activity of root extract/fractions of Eurycoma longifolia jack. BMC Complement Altern Med 12:91. https://doi.org/10.1186/1472-6882-12-91
Sadraei H, Asghari G, Kasiri F (2015) Antispasmodic effect of Dracocephalum kotschyi hydroalcoholic extract on rat ileum contraction. Res Pharm Sci 10(5):446–452
Heydari P, Yavari M, Adibi P, Asghari G, Ghanadian S-M, Dida GO et al (2019) Medicinal properties and active constituents of Dracocephalum kotschyi and its significance in Iran: a systematic review. Evid Based Complement Alternat Med 2019:9465309. https://doi.org/10.1155/2019/9465309
Amirghofran Z, Azadbakht M, Karimi MH (2000) Evaluation of the immunomodulatory effects of five herbal plants. J Ethnopharmacol 72(1–2):167–172. https://doi.org/10.1016/s0378-8741(00)00234-8
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Park H, Kim MS, Jeon BH, Kim TK, Kim YM, Ahnn J et al (2003) Antimalarial activity of herbal extracts used in traditional medicine in Korea. Biol Pharm Bull 26:1623–1624. https://doi.org/10.1248/bpb.26.1623
Cover B, Gutteridge WE (1982) A primary screen for drugs to prevent transmission of Chagas’s disease during blood transfusion. Trans R Soc Trop Med Hyg 76:633–635. https://doi.org/10.1016/0035-9203(82)90228-0
Saeidnia S, Ar G, Uchiyama N, Ito M, Honda G, Kiuchi F (2004) Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi. Chem Pharm Bull (Tokyo) 52(10):1249–1250. https://doi.org/10.1248/cpb.52.1249
Saeidnia S, Gohari A, Ito M, Kiuchi F, Honda G (2005) Bioactive constituents from Dracocephalum subcapitatum (O. Kuntze) Lipsky. Z Naturforsch C J Biosci 60(1–2):22–24. https://doi.org/10.1515/znc-2005-1-204
Fattahi M, Nazeri V, Torras-Claveria L, Sefidkon F, Cusido R, Zamani Z et al (2013) Identification and quantification of leaf surface flavonoids in wild-growing populations of Dracocephalum kotschyi by LC-DAD-ESI-MS. Food Chem 141(1):139–146. https://doi.org/10.1016/j.foodchem.2013.03.019
Zeng Q, Jin H, Qin J, Fu J, Hu X, Jian L et al (2010) Chemical constituents of plants from the genus Dracocephalum. Chem Biodivers 7(8):1911–1929. https://doi.org/10.1002/cbdv.200900188
Rivera Fernández N, MondragónCastelán M, González Pozos S, Ramírez Flores CJ, Mondragón González R, Gómez de León CT, Castro Elizalde KN, Marrero Ponce Y, Arán VJ, Martins Alho MA, Mondragón FR (2016) A new type of quinoxalinone derivatives affects viability, invasion, and intracellular growth of Toxoplasma gondii tachyzoites in vitro. Parasitol Res 115(5):2081–2096. https://doi.org/10.1007/s00436-016-4953-1
Saadatnia G, Haj Ghani H, Khoo BY, Maimunah A, Rahmah N (2010) Optimization of Toxoplasma gondii cultivation in VERO cell line. Trop Biomed 27:125–130
Khamesipour F, Razavi SM, Hejazi SH, Ghanadian SM (2021) In vitro and in vivo anti-Toxoplasma activity of Dracocephalum kotschyi essential oil. Food Sci Nutr 9(1):522–531. https://doi.org/10.1002/fsn3.2021
Shakib P, Taherikalani M, Ramazanzadeh R (2018) Chemical composition, genotoxicity and antimicrobial activities of Dracocephalum kotschyi Boiss against OXA-48 producing Klebsiella pneumoniae isolated from major hospitals of Kurdistan Province, Iran. Microbiol Res J Int 24:1–8. https://doi.org/10.9734/MRJI/2018/42064
Acknowledgements
The authors also would like to express their special thanks to Dr. Nastaran Sekhavati and all staff for her kind supports and assistance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that there are no conflicts of interest.
Ethics Approval and Consent to Participate
The study protocol was approved by the ethical committee of the Shiraz University (IR.Shirazu.REC.1399.1214).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The copyright holder for this article was incorrectly given as 'The Author(s), under exclusive licence to The Author(s) under exclusive licence to Witold Stefański Institute of Parasitology, Polish Academy of Sciences' but should have been 'The Author(s) under exclusive licence to Witold Stefański Institute of Parasitology, Polish Academy of Sciences'. The original article has been corrected.
Rights and permissions
About this article
Cite this article
Ghanadian, M., Khamesipour, F., Hejazi, S.H. et al. In Vitro and In Vivo Anti-Toxoplasma Activities of Dracocephalum kotschyi Extract in Experimental Models of Acute Toxoplasmosis. Acta Parasit. 67, 487–495 (2022). https://doi.org/10.1007/s11686-021-00491-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00491-4