Abstract
Purpose
Ticks are dangerous ectoparasites for humans and other animals, and tick-borne pathogens of Bactrian camels have been epidemiologically surveyed in Gansu Province, China. We aimed to determine the current distribution of tick-borne pathogens among Bactrian camels in Gansu during August 2013 using molecular tools.
Methods
All ticks underwent morphological identification. We extracted DNA from the blood samples and ticks, screened them for Theileria, Babesia, Anaplasma, and Ehrlichia using standard or nested PCR with specific primers.
Results
All ticks collected from the skin were identified as Hyalomma asiaticum. The blood and tick samples harbored similar pathogens, including the Theileria species, T. annulata, T. luwenshuni, T. uilenbergi, and T. capreoli, the Anaplasma species A. bovis and uncultured Anaplasma, the Ehrlichia species E. canis and uncultured Ehrlichia, and a new haplotype of Babesia species.
Conclusion
Our findings of anaplasmataceae and piroplasmida in Bactrian camels in Gansu provide a theoretical basis for deeper investigation into the epidemiology of tick-borne pathogens in these camels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
China is home to the most Bactrian camels worldwide, and Zhangye City (Gansu Province, China) is one of their main production sites [1]. Bactrian camels have become an important natural way for local peasants and herdsmen to eliminate poverty, and they also provide local residents with milk, meat, and fur. To date, few studies have investigated the occurrence of tick-borne pathogens in these camels from Gansu [2]. Recently, 125 species of ticks have been identified in China, including 111 hard and 14 soft types [3]. Hyalomma asiaticum is a major tick species that is widespread in Zhangye City. Semi-desert and desert environments are conducive to the survival and reproduction of H. asiaticum, a 3-host tick that parasitizes cattle, goats, camels, sheep, pigs, horses, hedgehogs and humans [4]. It is widely distributed in northern China, Mongolia, and southern Russia [5].
Ticks are vectors of many zoonotic pathogens, that have recently increased the incidence of livestock diseases, and have impacted human health and the development of animal husbandry. H. asiaticum is an important disease vector that serves as a host for various microorganisms. Currently known pathogenic microorganisms carried by H. asiaticum in China include: Crimean-Congo hemorrhagic fever virus (CCHFV), Rift Valley fever virus (RVFV) [6], and Q fever and Tamdy virus [7]. Theileriosis, babesiosis, rickettsioses and anaplasmosis have been investigated in H. asiaticum [8]. Few studies have investigated hemoparasite infection in Bactrian camels in China, despite many species of anaplasmataceae and piroplasmida have been reported in domestic animals and wild animals [9].
Anaplasma and Ehrlichia belong to the anaplasmataceae. Anaplasmosis is caused by obligate intracellular bacteria of the genus Anaplasma. To date, eight Anaplasma species have been identified worldwide [10]. Known Anaplasma species that infect humans include Anaplasma phagocytophilum, Anaplasma ovis and Anaplasma capra [11, 12]. Infection often manifests as fever, thrombocytopenia, leukopenia, and organ dysfunction] [13]. Ehrlichia species are obligate intracellular Gram-negative bacteria [14]. Ten Ehrlichia species have been identified since the first strain was isolated from a canid in 1935 [15]. Piroplasmida that mainly includes Babesia and Theileria are considered parasites with high host specificity that can infect host red blood cells, lymphocytes, or macrophages, causing a series of clinical symptoms such as fever, anemia, and jaundice [16]. Currently, over 100 species of Babesia have been identified, and the prevalence of infection is increasing worldwide [1]. Approximately, 13 species of Theileria have been reported [18, 19]. Rickettsia, Theileria and Babesia are common tick-borne pathogens that infect domestic animals worldwide, but little is known about hemoparasitic infections in Bactrian camels in China [20]. There is limited information on tick-borne diseases in the Gansu and genetic characterization of tick-borne pathogens (TBPs) in that area. Therefore, we aimed to investigate the occurrence and genetic diversity of tick-borne pathogens in Bactrian camels and ticks from Gansu. Our findings provide a scientific reference for the epidemiology of tick-borne diseases in this region of China.
Materials and Methods
Sample Collection
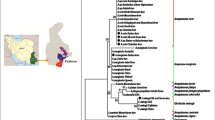
Zhangye city is located in the middle of the Hexi Corridor at the northern base of the Qilian Mountains in Gansu, a long narrow stretch of terrain in northwestern China with a complex landform and a variable climate. A unique desert oasis provides a natural pasture suitable for the survival of Bactrian camels [21]. The study was conducted in dry August 2013 at sites in Zhangye City (Fig. 1). The climate is also conducive to the prevalence of ticks and tick-borne diseases. We collected 45 blood samples and 33 unfed or partially engorged ticks from 45 Bactrian camels. All ticks were morphologically identified according to previous report [22] and stored at − 20℃.
Sampling sites in Zhangye, Gansu Province, China. The map is produced using NB Map (https://www.nbcharts.com/map/map) and WPS
DNA Extraction
Before DNA extraction, each tick was washed three times with 75% alcohol and followed by one wash of normal saline to remove surface impurities. Then, the ticks were homogenized by grinding rod. Seventy-eight genomic DNA was extracted from 45 camel blood samples and 33 ticks using DNeasy Blood & Tissue Kits (Qiagen, Germany) as described by the manufacturer, and stored at − 80 °C.
PCR Amplification
All DNA samples were initially screened by standard PCR-specific primers to detect and amplify piroplasmida, Anaplasma, and Ehrlichia shown in Table 1, and the DNA samples of piroplasmida were amplified by nested PCR. Anaplasma and Ehrlichia and piroplasmida were used for the preliminary genera-specific identification of the samples. A. bovis and A. phagocytophilum were used for the species-specific identification of the positive samples about Anaplasma and Ehrlichia. Fragments were amplified in a total volume of 25 µL in PCR Amplifier (Bio-Rad, Hercules, CA, USA) containing 2.5 µL of PCR buffer (10 ×), 2.0 µL of dNTPs (2.5 mM), 1.25 U of Taq DNA polymerase (Takara Bio Inc., Kusatsu, Japan), 2.0 µL of template DNA, 1.0 µL of each primer (10 pmol) and 16.25 µL of distilled water. Amplicons were separated by 0.5 µg/mL ethidium bromide-stained 1% agarose gel electrophoresis (Proteinsimple Alphalmager HP, USA) and visualized under ultraviolet light.
Sequencing and Phylogenetic Analyses
All positive PCR products derived from the 18S rRNA of piroplasmida and the 16S rRNA of Anaplasma and Ehrlichia were purified using a TaKaRa agarose gel DNA purification kit version 2.0, ligated into a pGEM-T Easy vector (Promega Corp., Madison, WI, USA), and transformed into Escherichia coli JM109 competent cells. Two recombinant clones of each sample were selected for sequencing using BigDye Terminator Mix (GenScript, Nanjing, China). Sequences from recombinant positive clones were compared with those in GenBank using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences were then phylogenetically analyzed using the neighbor-joining algorithm in Molecular Evolutionary Genetics Analysis (MEGA) 7 software [27] and analyzed with the Clustal W method in the MegAlign software (DNAStar, Madison, WI, USA). We then constructed a phylogenetic evolutionary tree of 16S rRNA sequences in Anaplasma and Ehrlichia, and 18S rRNA sequences in Theileria and Babesia using MEGA7.
Results
Tick Identification and PCR Detection of 18S RNA and 16S RNA Using Specific Primer Sets
All ticks used in this study were identified as H. asiaticum as described. The PCR result shows that among anticoagulant blood samples from Bactrian camels, 43 (95.56%) of 45 were positive for piroplasmida, including T. luwenshuni, T. uilenbergi, T. capreoli, T. annulata, uncultured Babesia sp., 11 (24.45%), and 24 (53.33%) of 45 were positive for A. bovis and A. phagocytophilum, respectively. All 33 (100%) tick samples were positive for piroplasmida, including T. luwenshuni, T. uilenbergi, T. capreoli, T. annulata, whereas 7 (21.21%) and 6 (18.18%) were also positive for A. bovis and A. phagocytophilum.
Sequence Alignment
Eleven of 45 and 6 of 33 sequences derived from Bactrian camel blood and from ticks in camel skin differed. We obtained 17 representative sequences and deposited them in GenBank (Table 2).
Phylogenetic Analyses
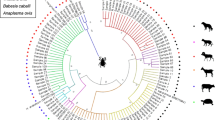
Three phylogenetic trees were constructed based on the 18S rRNA sequences of Babesia and Theileria, and the 16S rRNA sequences of Anaplasma and Ehrlichia determined herein and GenBank (Figs. 2, 3, and 4).
The evolutionary history was inferred using the Neighbor-Joining method (bootstrap replications: 1000). The evolutionary distances were computed using the p-distance method. The scale bar indicated the estimation of evolutionary distance. The optimal tree with the sum of branch length = 0.50053544 is shown. The taxa marked by black triangle and bold font depict the sequences obtained in the study, and GenBank accession number is provided in front of each strain name. Note: All trees were mid-point rooted for clarity only. Bootstrap values (> 50%) were shown for appropriate nodes. Scale bar represents number of nucleotide substitutions per site. The analysis involved 13 nucleotide sequences.
The evolutionary history was inferred using the Neighbor-Joining method (bootstrap replications: 1000). The evolutionary distances were computed using the p-distance method. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The analysis involved 33 nucleotide sequences. Target size of the primer used to generate the phylogenies is 851–1379.
The evolutionary history was inferred using the Neighbor-Joining method (bootstrap replications: 1000). The evolutionary distances were computed using the p-distance method. The scale bar indicated the estimation of evolutionary distance. The taxa marked by black triangle and bold font depict the sequences obtained in the study, and GenBank accession number is provided in front of each strain name. Note: All trees were mid-point rooted for clarity only. Bootstrap values (> 50%) were shown for appropriate nodes. Scale bar represents number of nucleotide substitutions per site.
Discussion
Climate change and global warming have facilitated disease vector spreading into new geographic locations, such diseases are a challenge in the region not only in veterinary medicine but also in human healthcare. Therefore, it is necessary to continuously investigate tick-borne diseases in this region, which is of utmost importance in devising appropriate vector and pathogen control interventions. This is the first study to report the presence of T. luwenshuni, T. uilenbergi, T. capreoli, T. annulata, uncultured Babesia sp., A. platys, A. bovis, E. canis and uncultured Ehrlichia sp. in Bactrian camels blood and in H. asiaticum in Gansu. Our results complement the vacancy of Bactrian camels carrying pathogens in Zhangye, Gansu. H. asiaticum has recently parasitized Bactrian camels in inner Mongolia from China [28].
Anaplasma and Ehrlichia were identified commonly of the tick-borne organisms, also occasionally infect humans. Anaplasma was initially detected in Jordanian dromedary camels [12]. Anaplasma phagocytophilum was originally identified detected in cattle on Yonaguni Island, Okinawa, Japan [29]. In recent years, A. bovis was initially thought to be only the agent of animal ehrlichiosis until it was the etiologic agent of human infection, when the first patient case was reported in 2019 [30]. In China, the report shows two cases of A. bovis infection in humans in Anhui Province [31]. This study shows that Bactrian camels contain A. bovis pathogens and indicates the spread of pathogens among livestock. Therefore, mixing several types of grazing livestock should be avoided. Anaplasma platys has been found mainly infecting dogs, cats, goats, cattle, deer and Bactrian camels [32, 33]. Anaplasma platys is a parasite with tropism for platelets having a wide host range, primarily being the causative agent of canine cyclic thrombocytopenia [34]. Although A. platys and E. canis is best known as a very common dog pathogen around the world, infections have also been described in people and other hosts include cattle, sheep, goats, rodents, and deer [15, 34]. Ehrlichia is a genus closely related to human diseases. Ehrlichia canis has also been detected in Bactrian camels, indicating the importance of epidemiologically detecting tick pathogens in these hosts. These ecological features give a hint about the possibility of transmission of these pathogens among domestic animals. Ehrlichia canis is considered a potential agent of human disease, although human infection cases have never been reported in China[35].
To date, DNA of Theileria equi, T. annulata, T. mutans, T. ovis and B. caballi has been detected in blood of dromedaries [36]. In our study, we found five piroplasmid infections in Bactrian camel bloods and ticks, including T. luwenshuni, T. uilenbergi, T. capreoli, T. annulata, uncultured Babesia sp. Babesia and Theileria have been found not only in Bactrian camels, but also in horses in Gansu Province [37]. Babesia and Theileria have also been found in wild Reeves’ muntjacs (Muntiacus reevesi) in China [26], as well as in horses and Bactrian camels in northeastern Mongolia [38]. Theileria luwenshuni and T. uilenbergi have previously infected small domestic ruminants, but we detected these pathogens in Bactrian camel blood. Theileria luwenshuni and T. uilenbergi, which have been previously reported to petits ruminants, were detected for the first time in blood samples from cattle and yaks on the Tibetan Plateau, and lack of relevant information infection in other animals [39]. Detecting T. luwenshuni and T. uilenbergi in Bactrian camels fortifies their roles as vectors of these economically threatening tick-borne pathogens [40]. Theileria capreoli was recorded in roe deer, red deer, fallow deer, roe deer and Chinese water deer [41]. This was first described in Bactrian camel in China.
Therefore, these bacteria are important for both veterinary and human public health. However, mortality associated with tick-borne diseases in livestock keepers has not been reported in Gansu. We investigated the molecular prevalence and genetic diversity of TBPs in Bactrian camels in Gansu to increase the amount of available epidemiological data on these pathogens in this area of China. Our result may contribute to the current knowledge of the biodiversity of part of tick-borne circulating in this area. The detection of A. platys, A. bovis and E. canis in Bactrian camel blood and ticks indicates that this issue requires more investigation and further measures should be taken to control and prevent of anaplasmataceae transmission between humans and other animals.
Bactrian camel blood and tick collection were not conducted at different times throughout the year, and as a result, the study did not investigate the seasonality of tick-borne diseases in Zhangye. The study is also limited in its selection of sampling sites and, therefore, could not cover all the Gansu ecological zones to provide a good representation of all Bactrian camel blood, tick and tick-borne pathogens in the entire country. Another limitation of this study is that the majority of the infections detected were in Bactrian camel blood samples or tick samples, and therefore, it could not be differentiated whether these represent actual coinfections or whether individual samples among the Bactrian camel blood samples or tick samples each carried multiple pathogens.
Conclusions
The present findings confirm previous results and provide more details about the molecular detection of tick-borne pathogens in blood and ticks from camels in Gansu Province, China, including part of Theileria, Babesia, Anaplasma, and Ehrlichia, while highlighting the importance and relevance of molecular methods. In recent years, with rising global temperature, the range of tick activity has expanded, and our findings reflected the prevalence of tick-borne diseases in Gansu. We showed that Bactrian camel blood and H. asiaticum are carriers of T. luwenshuni, T. uilenbergi, T. capreoli, T. annulata, Babesia, A. platys, A. bovis, E. canis and uncultured Ehrlichia sp. These potential zoonotics suggests that preventive and control measures are needed to avoid transmission between humans and other animals and among other animals. Clarification of the roles of the Bactrian camels and ticks as reservoir hosts for some Theileria, Babesia, Anaplasma, and Ehrlichia species is critical to determine whether ticks contribute to the spread of ruminant piroplasmida and anaplasmataceae in China. Our results provide sufficient epidemiological data to enhance the understanding of the measures required to effectively control theileriosis transmission to Bactrian camels and other ruminants in Gansu.
Availability of Data and Materials
All data generated during this study are included in this published article.
References
Liu CM, Chen HL, Ren ZJ, Zhang CD, Yang XJ (2019) Population genetic analysis of the domestic Bactrian camel in China by RAD-seq. Ecol Evol 9(19):11232–11242
Li YC, Wen XX, Li M, Moumouni PFA, Galon EM, Guo QY, Rizk MA, Liu MM, Li JX, Ji SW, Tumwebaze MA, Byamukama B, Chahan B, Xuan XN (2020) Molecular detection of tick-borne pathogens harbored by ticks collected from livestock in the Xinjiang Uygur Autonomous Region. China Ticks Tick Borne Dis 11(5):101478. https://doi.org/10.1016/j.ttbdis.2020.101478
Zhang YK, Zhang XY, Liu JZ (2019) Ticks (Acari: Ixodoidea) in China: Geographical distribution, host diversity, and specificity. Arch Insect Biochem Physiol 102(3):e21544. https://doi.org/10.1002/arch.21544
Chen Z, Yu ZJ, Yang XJ, Zheng HY, Liu JZ (2009) The life cycle of Hyalomma asiaticum kozlovi Olenev, 1931 (Acari: Ixodidae) under laboratory conditions. Vet Parasitol 160(1–2):134–137. https://doi.org/10.1016/j.vetpar.2008.10.028
Meng K, Li Z, Wang Y, Jing Z, Zhao X, Liu J, Cai D, Zhang L, Yang D, Wang S (2014) PCR-based detection of Theileria annulata in Hyalomma asiaticum ticks in northwestern China. Ticks Tick Borne Dis 5(2):105–106. https://doi.org/10.1016/j.ttbdis.2013.09.006
Teng AY, Che TL, Zhang AR, Zhang YY, Xu Q, Wang T, Sun YQ, Jiang BG, Lv CL, Chen JJ, Wang LP, Hay SI, Liu W, Fang LQ (2022) Mapping the viruses belonging to the order Bunyavirales in China. Infect Dis Poverty 11(1):81. https://doi.org/10.1186/s40249-022-00993-x
Moming A, Shen S, Fang YH, Zhang JY, Zhang YF, Tang S, Li TX, Hu ZH, Wang HL, Zhang YJ, Sun SR, Wang LF, Deng F (2021) Evidence of human exposure to Tamdy Virus. Northwest China Emerg Infect Dis 27(12):3166–3170. https://doi.org/10.3201/eid2712.203532
Zhao GP, Wang YX, Fan ZW, Ji Y, Liu MJ, Zhang WH, Li XL, Zhou SX, Li H, Liang S, Liu W, Yang Y, Fang LQ (2021) Mapping ticks and tick-borne pathogens in China. Nat Commun 12(1):1075. https://doi.org/10.1038/s41467-021-21375-1
Fang LQ, Liu K, Li XL, Liang S, Yang Y, Yao HW, Sun RX, Sun Y, Chen WJ, Zuo SQ, Ma MJ, Li H, Jiang JF, Liu W, Yang XF, Gray GC, Krause PJ, Cao WC (2015) Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis 15(12):1467–1479. https://doi.org/10.1016/S1473-3099(15)00177-2
Rar V, Tkachev S, Tikunova N (2021) Genetic diversity of Anaplasma bacteria: twenty years later. Infect Genet Evol 91:104833. https://doi.org/10.1016/j.meegid.2021.104833
Chochlakis D, Ioannou I, Tselentis Y, Psaroulaki A (2010) Human anaplasmosis and Anaplasma ovis variant. Emerging Infect Dis 16:1031–1032. https://doi.org/10.3201/eid1606.090175
Qablan MA, Sloboda M, Jirků M, Oborník M, Dwairi S, Amr ZS, Hořín P, Lukeš J, Modry D (2012) Quest for the piroplasms in camels: Identification of Theileria equi and Babesia caballi in Jordanian dromedaries by PCR. Vet Parasitol 186:456–460. https://doi.org/10.1016/j.vetpar.2011.11.070
Aubry P, Geale DW (2011) A review of bovine anaplasmosis. Transbound Emerg Dis 58(1):1–30. https://doi.org/10.1111/j.1865-1682.2010.01173.x
Bekker CP, de Vos S, Taoufik A, Sparagano OA, Jongejan F (2002) Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet Microbiol 89:223–238. https://doi.org/10.1016/S0378-1135(02)00179-7
Vieira RF, Biondo AW, Guimarães A, Santos A, Santos R, Dutra LH, Diniz PP, de Morais HA, Messick JB, Labruna MB, Vidotto O (2011) Ehrlichiosis in Brazil. Rev Bras Parasitol Vet 20(1):1–12. https://doi.org/10.1590/s1984-29612011000100002
García AT (2006) Piroplasma infection in dogs in northern Spain. Vet Parasitol 138(1–2):97–102. https://doi.org/10.1016/j.vetpar.2006.01.043
Antunes S, Rosa C, Couto J, Ferrolho J, Domingos A (2017) Deciphering babesia-vector interactions. Front Cell Infect Microbiol 29(7):429. https://doi.org/10.3389/fcimb.2017.00429
Tian ZC, Gao SD, Ren QY, Du JZ, Guan GQ, Liu GY, Luo JX, Yin H (2021) Mitochondrial genome of Theileria uilenbergi endemic in sheep and goats in China. Parasitol Res 120:3429–3436. https://doi.org/10.1007/s00436-021-07304-7
Wang Y, Wang B, Zhang QX, Li Y, Yang ZW, Han SY, Yuan GH, Wang SL, He HX (2021) The common occurrence of Theileria ovis in tibetan sheep and the first report of Theileria sinensis in Yaks from Southern Qinghai. China Acta Parasitol 66(4):1177–1185. https://doi.org/10.1007/s11686-021-00381-9
Li JX, Jian YN, Jia LJ, Galon EM, Benedicto B, Wang GP, Cai QG, Liu MM, Li YC, Ji SW, Tumwebaze MA, Ma LQ, Xuan XN (2020) Molecular characterization of tick-borne bacteria and protozoans in yaks (Bos grunniens), Tibetan sheep (Ovis aries) and Bactrian camels (Camelus bactrianus) in the Qinghai-Tibetan Plateau Area. China Ticks Tick Borne Dis 11(5):101466. https://doi.org/10.1016/j.ttbdis.2020.101466
Hasi S, Yao J, Yu S, Tian Y (2018) Diversity and distribution of CYP gene family in Bactrian camel. Funct Integr Genom 18(1):23–29. https://doi.org/10.1007/s10142-017-0571-y
Apanaskevich DA, Horak IG (2010) The genus Hyalomma. XI. Redescription of all parasitic stages of H. (Euhyalomma) asiaticum (Acari: Ixodidae) and notes on its biology. Exp Appl Acarol 52(2):207–220. https://doi.org/10.1007/s10493-010-9361-0
Kawahara M, Rikihisa Y, Lin Q, Isogai E, Tahara K, Itagaki A, Hiramitsu Y, Tajima T (2006) Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol 72(2):1102–1109. https://doi.org/10.1128/AEM.72.2.1102-1109.2006
Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, Conrad PA (1992) Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol 30(8):2097–2103. https://doi.org/10.1128/jcm.30.8.2097-2103.1992
Olmeda AS, Armstrong PM, Rosenthal BM, Valladares B, del Castillo A, de Armas F, Miguelez M, González A, Rodríguez RJA, Spielman A, Telford SR 3rd (1997) A subtropical case of human babesiosis. Acta Trop 67(3):229–234. https://doi.org/10.1016/s0001-706x(97)00045-4
Yang JF, Li YQ, Liu ZJ, Liu JL, Guan GQ, Chen Z, Luo JX, Wang XL, Yin H (2014) Molecular evidence for piroplasms in wild Reeves’ muntjac (Muntiacus reevesi) in China. Parasitol Int 63(5):713–716. https://doi.org/10.1016/j.parint.2014.06.002
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Batu N, Wang YC, Liu ZC, Huang TP, Bao WD, He HY, Geri L (2020) Molecular epidemiology of Rickettsia sp and Coxiella burnetii collected from Hyalomma asiaticum in Bactrian camels (Camelus bactrianus) in inner Mongolia of China. Ticks Tick Borne Dis 11(6):101548. https://doi.org/10.1016/j.ttbdis.2020.101548
Ooshiro M, Zakimi S, Matsukawa Y, Katagiri Y, Inokuma H (2008) Detection of Anaplasma bovis and Anaplasma phagocytophilum from cattle on Yonaguni Island, Okinawa. Japan VET PARASITOL 154(3–4):360–364. https://doi.org/10.1016/j.vetpar.2008.03.028
Lu M, Li F, Liao Y, Shen JJ, Xu JM, Chen YZ, Li JH, Holmes EC, Zhang YZ (2019) Epidemiology and diversity of rickettsiales bacteria in humans and animals in Jiangsu and Jiangxi provinces. China Sci Rep 9(1):13176. https://doi.org/10.1038/s41598-019-49059-3
Lu M, Chen QQ, Qin XC, Lyu Y, Teng ZQ, Li K, Yu L, Jin XJ, Chang HW, Wang W, Hong DY, Sun Y, Kan B, Gong L (2021) anaplasma bovis infection in fever and thrombocytopenia patients-Anhui Province, China, 2021. China CDC Wkly 4(12):249–253. https://doi.org/10.46234/ccdcw2022.053
Wei W, Li J, Wang YW, Jiang BG, Liu HB, Wei R, Jiang RR, Cui XM, Li LF, Yuan TT, Wang Q, Zhao L, Xia LY, Jiang JF, Qiu YF, Jia N, Cao WC, Hu YL (2020) Anaplasma platys-Like Infection in Goats, Beijing. China Vector Borne Zoonotic Dis 20(10):755–762. https://doi.org/10.1089/vbz.2019.2597
Li YQ, Yang JF, Chen Z, Qin GG, Li YQ, Li Q, Liu JL, Liu ZJ, Guan GQ, Yin H, Luo JX, Zhang L (2015) Anaplasma infection of Bactrian camels (Camelus bactrianus) and ticks in Xinjiang. China Parasit Vectors 8:313. https://doi.org/10.1186/s13071-015-0931-1
Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, Breitschwerdt EB (2014) Case report: Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am J Trop Med Hyg 91(6):1161–1165. https://doi.org/10.4269/ajtmh.14-0372
Zhang JL, Kelly P, Guo WN, Xu CL, Wei LJ, Jongejan F, Loftis A, Wang CM (2015) Development of a generic Ehrlichia FRET-qPCR and investigation of ehrlichioses in domestic ruminants on five Caribbean islands. Parasit Vectors 8:506. https://doi.org/10.1186/s13071-015-1118-5
Alanazi AD, Nguyen VL, Alyousif MS, Manoj RRS, Alouffi AS, Donato R, Sazmand A, Mendoza-Roldan JA, Dantas-Torres F, Otranto D (2020) Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province. Saudi Arabia Parasit Vectors 13(1):110. https://doi.org/10.1186/s13071-020-3973-y
Wang JM, Liu JL, Yang JF, Wang XX, Li Z, Jianlin X, Li X, Xiang QJ, Li YQ, Liu ZJ, Luo JX, Guan GQ, Yin H (2019) The first molecular detection and genetic diversity of Babesia caballi and Theileria equi in horses of Gansu province, China. Ticks Tick Borne Dis 10:528–532. https://doi.org/10.1016/j.ttbdis.2019.01.003
Sloboda M, Jirk M, Lukeová D, Qablan M, Batsukh Z, Fiala I, Hořín P, Modrý D, Lukeš J (2011) A survey for piroplasmids in horses and Bactrian camels in North-Eastern Mongolia. Vet Parasitol 179:246–249. https://doi.org/10.1016/j.vetpar.2011.01.064
Qin GG, Li YQ, Liu JL, Liu ZJ, Yang JF, Zhang L, Liu GY, Guan GQ, Luo JX, Yin H (2016) Molecular detection and characterization of Theileria infection in cattle and yaks from Tibet Plateau Region. China Parasitol Res 115(7):2647–2652. https://doi.org/10.1007/s00436-016-5011-8
Yin H, Liu Z, Guan G, Liu A, Ma M, Ren Q, Luo J (2008) Detection and differentiation of Theileria luwenshuni and T. uilenbergi infection in small ruminants by PCR. Transbound Emerg Dis 55:233–237. https://doi.org/10.1111/j.1865-1682.2008.01031.x
Wang HN, Yang JF, Mukhtar MU, Liu ZJ, Zhang MH, Wang XL (2019) Molecular detection and identification of tick-borne bacteria and protozoans in goats and wild Siberian roe deer (Capreolus pygargus) from Heilongjiang Province, northeastern China. Parasit Vectors 12(1):296. https://doi.org/10.1186/s13071-019-3553-1
Funding
This work was funded by the Ningxia Natural Science Foundation (No.2023AAC02017, 2022AAC03017).
Author information
Authors and Affiliations
Contributions
HZ and XD conceived and designed the study. HZ, XZ and JZ Tao carried out the field work, and HZ and XZ drafted the manuscript. All the authors reviewed the original manuscript and agreed to the final version. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
This study was approved by the Animal Ethics Committee of Ningxia University, NXU (No. NXU 2015–012). The use of these field samples was approved by the Animal Ethics Procedures and Guideline of China.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Hx., Zan, Xq., Tao, Jz. et al. Molecular Characterization of Tick-borne Pathogens in Bactrian Camels and Ticks from Gansu Province, China. Acta Parasit. 69, 343–350 (2024). https://doi.org/10.1007/s11686-023-00752-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00752-4