Abstract
Theileriosis continues to threaten the livestock industry worldwide, but comprehensive epidemiological surveys for this disease have not been conducted in the Tibet Plateau Region, China. In this study, we screened 154 cattle blood samples from the Tibet Plateau Region (Lhasa, Lhoka, and Tianzhu), China, for detection of Theileria pathogens by polymerase chain reaction (PCR) with species-specific primers. The results revealed that the prevalence was 6.9 % (2/29) for Theileria orientalis and 27.6 % (8/29) for Theileria sinensis in Lhasa, 0 % (0/30) for T. orientalis and 26.7 % (8/30) for T. sinensis in Lhoka, and 0 % (0/95) for T. orientalis and 30.5 % (29/95) for T. sinensis in Tianzhu. Interestingly, Theileria luwenshuni, which was a previously reported pathogenic Theileria sp. in sheep and goats, was detected in blood samples from cattle and yaks for the first time, with a prevalence of 10 % (3/30) in Lhoka and 1.1 % (1/95) in Tianzhu. No other Theileria sp. was detected in these samples. T. sinensis and T. orientalis infections were detected in cattle and yaks, and T. luwenshuni was discovered for the first time in cattle and yaks in the Tibet Plateau Region, China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine theileriosis is a tick-borne disease caused by Theileria spp., causing clinical infections in domestic and wild bovine species that lead to heavy economic losses (Dolan 1989). Of all bovine Theileria spp., only three (Theileria annulata, Theileria orientalis, and Theileria sinensis) have been found in China (Bai et al. 2002; Luo and Lu 1997; Liu et al. 2010). T. annulata is the most pathogenic etiological agent of theileriosis worldwide (Bilgic et al. 2010). It is prevalent in the Ningxia, Xinjiang, Inner Mongolia, and Gansu province (Luo and Lu 1997; Lü et al. 1995), and its average infection rate is up to 57.7 %. T. orientalis has caused outbreaks of mild theileriosis in many countries (McFadden et al. 2011; Eamens et al. 2013); it is distributed throughout Henan, Hebei, Yunnan, Guizhou, Jiangsu, Hunan, Liaoning, Jilin, Guangdong, Guangxi, Qinghai, Gansu, Heilongjiang, etc. (Liu et al. 2010, 2013; Lü et al. 1995). T. sinensis has been previously reported in cattle and yaks in Gansu, China, but its level of pathogenicity is unclear (Bai et al. 2002).
There are many reports providing epidemiological data on bovine theileriosis in China, but there are no data on bovine theileriosis in the Tibet Plateau Region, China. The aim of this study was to detect and identify the Theileria spp. infecting cattle and yaks in Tibet Plateau Region, China.
Material and methods
Blood sampling and DNA extraction
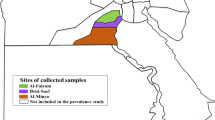
A total of 154 blood samples were collected into Vacutainer tubes with ethylene diamine tetraacetic acid (EDTA), from cattle and yaks in three regions (Lhasa (29° 58′ 12″ N, 91° 6′ 36″ E), Lhoka (29° 14′ 24″ N, 91° 46′ 12″ E), and Tianzhu (37° 14′ 24″ N, 102° 50′ 24″ E)) of Tibet Plateau, China in September 2014. Genomic DNA samples were extracted using a genomic DNA extraction kit (Qiagen, Hilden, Germany).
Identification using species-specific primers
Theileria spp. were detected via polymerase chain reaction (PCR) using species-specific primers; information on the PCR primers is shown in Table 1. Six positive Theileria genomic DNA samples (from Lanzhou Veterinary Research Institute) were used as the positive controls, while the genomic DNA of healthy bovines was used as the negative control.
PCR amplification and sequence analysis
The PCR products were purified using a DNA gel extraction kit (Axygen, Hangzhou, China), ligated into the pGEM-T Easy vector (Invitrogen, Carlsbad, CA, USA), and subjected to sequencing. All results of sequencing were compared with sequences in the GenBank database (www.ncbi.nlm.nih.gov/genbank/; National Center for Biotechnology Information, NCBI), and the alignments of multiple sequences were executed in Florence Corpet (http://multalin.toulouse.inra.fr/). The final high homology sequences have been deposited in the GenBank database, and phylogenetic trees of major piroplasm surface protein genes and 18S rDNA genes of Theileria were constructed using other sequences from NCBI.
Results
In this study, out of 154 blood samples from cattle and yaks in the Tibet Plateau Region (Lhasa, Lhoka and Tianzhu), China, the prevalence of T. sinensis in cattle in Lhasa was 27.6 % (8/29) (Table 2) and the appropriate accession numbers are KU518036, KU518037, and KU518038. The prevalence was 6.9 % (2/29) for T. orientalis in cattle in Lhasa, and the accession numbers are KU247950 and KU518033. The prevalence was 26.7 % (8/30) for T. sinensis in cattle in Lhoka, and the accession numbers are KU247951, KU518034, and KU518035. The prevalence was 30.5 % (29/95) for T. sinensis in yaks in Tianzhu (Table 2), and the accession numbers are KU518039, KU518040, and KU518041. Interestingly, T. luwenshuni, which is a Theileria sp. previously reported to be pathogenic for sheep and goats, was detected from 10 % (3/30) of cattle blood samples in Lhoka (accession numbers KU247948, KU518031, and KU518032) and in 1.1 % (1/95) of yak blood samples in Tianzhu (accession number KU247949) (Table 2).
The phylogenetic tree based on the major piroplasm surface protein showed that a Theileria sp. obtained from Lhasa was T. orientalis (group 1) and that the Theileria sp. from Lhasa, Lhoka, and Tianzhu was T. sinensis (group 2) (Fig. 1). Another phylogenetic tree constructed in this study showed that the newly isolated Theileria sp. from the Tibet Plateau was in the same clade as T. luwenshuni (group 1) (Fig. 2). No other Theileria sp. was detected in these samples.
Discussion
In this study, only two bovine Theileria spp., T. orientalis and T. sinensis, were detected in cattle and yaks in the Tibet Plateau Region of China. Interestingly, T. luwenshuni, which has been previously reported to be pathogenic to sheep and goats, was detected for the first time in blood samples from cattle and yaks in the Tibet Plateau. No other Theileria sp. was detected in this study.
Although the pathogenicity of T. orientalis is lower than that of T. annulata, it is widely distributed in most provinces in China and also, to some extent, has limited the development of the cattle industry. The prevalence of T. orientalis in cattle is up to 72 % in northeast China (Jin et al. 2007). T. sinensis differs from all other known Theileria spp. It was first found in Gansu province (Bai et al. 2002), transmitted by Haemaphysalis qinghaiensis (Yin et al. 2004); the adult H aemaphysalis japonica ticks also transmit T. sinensis (Yin et al. 2004). A previous report revealed that T. sinensis was distributed only in Gansu province (Yin et al. 2004), but Li et al. (2015) reported that the prevalence of T. sinensis in cattle was 3.3 % in Jiangxi, China. Generally, the distribution of Theileria spp. closely matches the distribution of their vectors. H. qinghaiensis ticks were first reported in Qinghai province in 1978. They are distributed in Qinghai, Gansu, Ningxia, Sichuan, Yunnan, and Tibet; these regions are 1600–4200 m above sea level (Teng and Jiang 1991). We can conclude that T. sinensis may be extensively distributed in these areas of China.
Theileria luwenshuni was first reported in sheep and goats; it is widely distributed in most parts of China (Yin et al. 2007, 2008; Li et al. 2007, 2014b; Chen et al. 2014; Yang et al. 2014) and is transmitted by both H. qinghaiensis and Haemaphysalis longicornis (Li et al. 2007). There is a lack of relevant information about T. luwenshuni infection in other animals. In the past, T. luwenshuni was considered to be a parasite of only sheep and goats. However, T. luwenshuni has been detected recently in roe, sika, and red deer (Li et al. 2014b), hedgehogs (Chen et al. 2014), and Mongolian gazelle (Li et al. 2014c) in China. Therefore, Li et al. concluded that T. luwenshuni is a multi-host parasite and that at least seven mammals, including sheep, goats, roe deer, sika deer, red deer, hedgehogs, and Mongolian gazelle, can be infected by the parasite (Li et al. 2014a, 2014b, 2014c). In this study, T. luwenshuni was also detected in blood samples taken from cattle and yaks in the Tibet Plateau Region of China. Given that T. luwenshuni is highly pathogenic in sheep and goats and its infection of cattle and yaks was previously unknown, the influence of T. luwenshuni on cattle and yaks requires further research. The results of this study are very important to herders in the Tibet Plateau Region, where the main livestock breeding industry involves cattle, yaks, sheep, and goats.
Our results provide important data that extend our understanding of the epidemiology of theileriosis in cattle and yaks and should facilitate the implementation of measures to control the transmission of Theileria parasites among ruminants in the Tibet Plateau, China.
References
Allospp BA, Baylis HA, Allsopp MT, Cavalier-Smith T, Bishop RP, Carrington DM, Sohanpal B, Spooner P (1993) Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitol 107(Pt2):157–165
Altay K, Dumanli N, Holman PJ, Aktas M (2005) Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol 127(2):99–104
Bai Q, Liu GY, Yin H, Zhao QZ, Liu DK, Ren JX, Li X (2002) Theileria sinensis sp nov: a new species of bovine Theileria-classical taxonomic studies. Acta Vet Zootech Sin 33(1):73–77 (in Chinese)
Bilgic HB, Karagenç T, Shiels B, Tait A, Eren H, Weir W (2010) Evaluation of cytochrome b as a sensitive target for PCR based detection of T. annulata carrier animals. Vet Parasitol 174(3-4):341–347
Chen Z, Liu Q, Jiao FC, Xu BL, Zhou XN (2014) Detection of piroplasms infection in sheep, dogs and hedgehogs in central China. Infect Dis Poverty 3:18
d’Oliveira C, van der Weide M, Habela MA, Jacquiet P, Jongejan F (1995) Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol 33(10):2665–2669
Dolan TT (1989) Theileriasis: a comprehensive review. Rev Sci Tech Off Int Des Epizooties 8(1):11–78
Eamens GJ, Gonsalves JR, Jenkins C, Collins D, Bailey G (2013) Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet Parasitol 191(3-4):209–217
Jin CM, Xu YT, Zhang SF, Wang XY, Yu LZ (2007) Establishment of PCR method for detection Theileria sergenti infection in cattle. Anim Husb Vet Med 39(5):46–48 (in Chinese)
Li YQ, Luo JX, Liu ZJ, Guan GQ, Gao JL, Ma ML, Dang ZS, Liu AH, Ren QY, Lu BY, Liu JL, Zhao HP, Li JJ, Liu GY, Bai Q, Yin H (2007) Experimental transmission of Theileria sp. (China 1) infective for small ruminants by Haemaphysalis longicornis and Haemaphysalis qinghaiensis. Parasitol Res 101(3):533–538
Li YQ, Zhang X, Liu ZJ, Chen Z, Yang JF, He HN, GuanGQ LAH, Ren QY, Niu QL, Liu JL, Luo JX, Yin H (2014a) An epidemiological survey of Theileria infections in small ruminants in central China. Vet Parasitol 200(1-2):198–202
Li YQ, Chen Z, Liu ZJ, Liu JL, Yang JF, Li Q, Li YQ, Cen SQ, Guan GQ, Luo JX, Yin H (2014b) Molecular identification of Theileria parasites of northwestern Chinese Cervidae. Parasit Vectors 7:225
Li YQ, Chen Z, Liu ZJ, Liu JL, Yang JF, Li Q, Li YQ, Ren QY, Niu QL, Guan GQ, Luo JX, Yin H (2014c) First report of Thrileria and Anaplasma in the Mongolian gazelle, Procapra gutturosa. Parasit Vectors 7:614
Li YQ, Li YQ, Li Q, Yang ST, Luo JX, Yin H (2015) Epidemiologic survey for detection of Theileria-infected cattle in Gaoan prefecture in Jiangxi province. China Dairy Cattle 3(4):34–36 (in Chinese)
Liu AH, Guan GQ, Liu ZJ, Liu JL, Leblanc N, Li YQ, Gao JL, Ma ML, Niu QL, Ren QY, Bai Q, Yin H, Luo JX (2010) Detecting and differentiating Theileria sergenti and Theileria sinensis in cattle and yaks by PCR based on major piroplasm surface protein (MPSP). Exp Parasitol 126(4):476–481
Liu AH, Guan GQ, Du PF, Gou HT, Zhang J, Liu ZJ, Ma ML, Ren QY, Liu JL, Yang JF, Li YQ, Niu QL, Bai Q, Yin H, Luo JX (2013) Rapid identification and differentiation of Theileria sergenti and Theileria sinensis using a loop-mediated isothermal amplification (LAMP) assay. Vet Parasitol 191(1-2):15–22
Lü WX, Lü WS, Zhang QC, Luo JX, Yin H, Dou HF (1995) Survey of the species of tick-borne haemoprotozoan of cattle and feature in china. Chin J Vet Sci Tech 25(8):13–16 (in Chinese)
Luo J, Lu W (1997) Cattle theileriosis in China. Trop Anim Health Prod 29(4Suppl):4S–7S
McFadden AM, Rawdon TG, Meyer J, Makin J, Morley CM, Clough RR, Tham K, Mullner P, Geysen D (2011) An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N Z Vet J 59(2):79–85
Teng GF, Jiang ZJ (1991) Chinese economic insect fauna, the thirty-ninth copies Acari: Ixodidae. Science Press, Beijing (in Chinese)
Yang JF, Li YQ, Liu ZJ, Liu JL, Guan GQ, Chen Z, Luo JX, Wang XL, Yin H (2014) Molecular evidence for piroplasms in wild Reeves’ muntjac (Muntiacus reevesi) in China. Parasitol Int 63(5):713–716
Yin H, Luo JX, Schnittger L, Lu BY, Beyer D, Ma ML, Guan GQ, Bai Q, Lu CP, Ahmed J (2004) Phylogenetic analysis of Theileria species transmitted by Haemaphysalis qinghaiensis. Parasitol Res 92(1):36–42
Yin H, Schnittger L, Luo J, Seitzer U, Ahmed JS (2007) Ovine theileriosis in China: a new look at an old story. Parasitol Res 101(Suppl 2):S191–S195
Yin H, Liu ZJ, Guan GQ, Liu AH, Ma ML, Ren QR, Luo JX (2008) Detection and differentiation of Theileria luwenshuni and T. uilenbergi infection in small ruminants by PCR. Transbound Emerg Dis 55(5-6):233–237
Acknowledgments
The research was supported by the Natural Science Foundation of China (Nos. 31272556, 31372432, 31101621, 31201899, 31402189, and 31471967), ASTIP, FRIP (2014ZL010), CAAS; “948” (2014-S05), NBCIS (CARS-38), Special Fund for Agro-scientific Research in the Public Research (No. 201303035, 2015CB150300), MOA, 973 Program (2010CB530206), Supporting Program (2013BAD12B03, 2013BAD12B05), Specific Fund for Sino-Europe Cooperation, MOST, China, State Key Laboratory of Veterinary Etiological Biology Project. The research was also facilitated by CRP No. 16198/R0 IAEA, Creative Research Groups of Gansu Province (No. 1210RJIA006). We thank International Science Editing for help in modifying the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Qin, G., Li, Y., Liu, J. et al. Molecular detection and characterization of Theileria infection in cattle and yaks from Tibet Plateau Region, China. Parasitol Res 115, 2647–2652 (2016). https://doi.org/10.1007/s00436-016-5011-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5011-8