Abstract
Purpose
Species of Hepatozoon Miller, 1908 (Hepatozoidae) are blood protozoans with a cosmopolitan distribution and are reported to parasitize a range of vertebrate hosts including mammals, birds, reptiles, and amphibians. The present study aimed to describe a new species of Hepatozoon (Apicomplexa: Adeleorina: Hepatozoidae) found infecting the sleep snake Dipsas mikanii (Schlegel, 1837) (Squamata: Colubridae: Dipsadinae).
Methods
The snake was collected in 2017 at the municipality of Britânia, Goiás State, Brazil. Blood smears were made in order to find blood gametocytes and PCR was performed targeting the 18S rRNA gene.
Results
Microscopy screening of blood smears revealed the presence of intraerythrocytic gamont stages of Hepatozoon sp. in the peripheral blood with a parasitemia of 0.25%. Furthermore, meronts and monozoic cysts were observed in histological sections of the liver from the infected individual. The interspecific divergence of 18S rRNA sequences fragments isolated from D. mikanii had differences (2.39–11.3%) as compared to other sequences of species of Hepatozoon from snakes.
Conclusions
Based on morphological and molecular data, a new species of Hepatozoon infecting D. mikanii from Brazil is described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haemogregarines are a group of adeleorinid coccidia (Apicomplexa: Adeleorina) subdivided into four families: Dactylosomatidae Jakoeska and Nigrelli, 1955; Karyolysidae Labbé, 1984; Haemogregarinidae Léger, 1911, and Hepatozoidae Miller, 1908. Hepatozoidae includes only species of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina: Hepatozoidae) with a cosmopolitan distribution, reported parasitizing the erythrocytes or leukocytes of reptile, amphibian, bird, and mammal vertebrate hosts [1].
Hepatozoon spp. are heteroxenous with a life-cycle involving an obligatorily vertebrate and invertebrate (hematophagous) host. Up until 1996, more than 120 species were identified globally [1]. According to Úngari et al. [2], the number of species has increased with at least 18 additional species described. Species of Hepatozoon are commonly reported in snake hosts, with transmission occurring either via ingestion of an infected invertebrate vector, by the ingestion of an intermediate vertebrate host serving as prey or through vertical transmission [3,4,5].
The description of Hepatozoon spp. in the recent past was mainly based on morphological characteristics [1, 6, 7], that alone are not always reliable in identifying species of Hepatozoon due to the gamont plasticity and low host specificity [8, 9]. Currently, with advances in molecular tools and techniques, it is possible to distinguish between species of Hepatozoon and compare their phylogenetic relationships [10].
Therefore, the main goal of this study was to describe a new species of Hepatozoon infecting the Brazilian sleep snake, Dipsas mikanii (Schlegel, 1837) using morphological and molecular characterisation. This is the first report of haemogregarine parasites infecting the snake D. mikanni.
Material and Methods
Snake Collection
In August of 2017, reptiles were collected as part of a larger project on the biodiversity of parasites in Brazilian reptiles, a single individual of the snake Dipsas mikanii was collected from the municipality of Britânia, Goiás State, Brazil (14°57′08,4″ S 51°06′30,7″ W) and examined. The blood collection was by puncture of the cervical paravertebral sinus [11, 12] using sterile and disposable syringes and needles. The sex (male/female) and age of the specimen was estimated. After the blood collection, three blood smears were made and the remaining blood sample was stored in EDTA tubes and frozen at −10ºC for further molecular work. For histopathological analysis, the sleep snake was euthanized using 50 mg/kg Tiopentax®, a commercial anaesthetic administered intracerebrally, following the guidelines of Sebben [13] and the Animal Ethics Committee of Veterinary Medicine. The liver, spleen, heart, and kidney were fixed in 4% buffered neutral formalin and stained with haematoxylin–eosin [14].
All applicable international, national, and institutional guidelines for the care and use of animals were followed (IBAMA license #60640-1; CEUA-UNESP #1061). The access to the genetic data was authorized by the Brazilian Ministry of Environment (Sisgen A627064).
Morphological and Morphometric Analysis
Blood smears were fixed with absolute methanol and stained with 10% Giemsa Methylene Blue Eosin Merck® diluted in distilled water (pH 7.0 for 50 min) [15]. For morphological analysis of mature gamonts, digital images were taken at 1000×magnification, using a light microscope and the Leica software application suite LAS V3.8 (Leica Microsystems).
Measurements in micrometres (µm) comprised the parasite’s length and width, with mean and standard deviation (means ± standard deviation). Parasitaemia was calculated as the percentage of infected erythrocytes observed in 10,000 cells [16].
The effect of the parasite on the erythrocytes was evaluated by the comparison of infected and non-infected parameters using the non-parametric Mann–Whitney Test. The significance level was 5%.
Molecular Analysis
DNA extraction from whole blood was carried out using the Illustra blood genomicPrep Mini Spin Kit (GE Healthcare), following the manufacturer’s instructions. An 18S rDNA fragment (600 bp) was amplified using the primers HepF300 (5′-GTTTCTGACCTATCAGCTTTCGACG-3′) and Hep900 (5′-CAAATCTAAGAATTTCACCTCTGAC-3′) [17]. PCR reaction was carried out in a final volume of 25 µL, including 1 µL of each primer (5 pmol), 12.5 µL of the MyFi™ Mix Bioline ® Master Mix, and 5 µL of extracted DNA, with nuclease-free water accounting for the remaining volume; under conditions established by O´Dwyer et al. [18]. Amplifications were performed on a Peltier 200 Thermocycler (MJ Research, Watertown, MA), with initial denaturation at 94 ºC for 3 min, followed by 35 cycles of 94 ºC for 45 s, 50 ºC for 60 s, and 72 ºC for 60 s, followed by a final extension at 72 ºC for 7 min.
DNA was extracted from liver tissue, following the blood protocol of the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Partial 18S rRNA gene fragments (1120 bp) were amplified using the primer set 4558 (5′-GCTAATACATGAGCAAAATCTCAA-3′) and 2733 (5′-CGGAATTAACCAGACAAAT-3′) [19]. For PCR amplification, reactions were carried out in a final volume of 25 µL, containing 1 µL of each primer (10 pmol), 12.5 µL of the MyFi™ Mix Bioline® Master Mix, and 5 µL of the extracted DNA, with nuclease-free water accounting for the remaining volume. PCR amplification was performed on a Peltier 200 Thermocycler (MJ Research, Watertown, MA), with initial denaturation at 94 °C for 3 min; followed by 40 cycles of 94 °C for 1 min, 56° for 1 min, and 72 °C for 90 s; and final extension for 7 min at 72 °C, with 4 °C hold.
The amplified PCR products were subjected to electrophoresis at 80 V in a 1.5% agarose gel, stained with GelRed, and observed using an ultraviolet transilluminator. The products of interest were purified by adding 2 µL of ExoSAP-IT® (Affymetrix, Santa Clara, CA, USA) to 5 µL of PCR product according to the manufacturer’s recommendations. Amplicons were then sequenced in both directions using the PCR primer sets on a 3500 Genetic Analyzer capillary sequencer (Applied Biosystems) and after BigDye Terminator Cycle Sequencing Ready Reaction Kit v.3.1 (Applied Biosystems) according to the manufacturer’s recommendations.
Resultant sequences were assembled and chromatogram-based contigs generated using Geneious version 7.1.3 [16]. The two resultant sequences from the present study of 589 bp (HepF300/Hep900) and 891 bp (4558/2733) were identical for the overlapping sections. The longer sequence fragment (891 pb) was aligned with haemogregarine sequences downloaded from GenBank, using the MUSCLE algorithm implemented from within Geneious version 7.1.3 (Bomatters, www.geneious.com). Phylogenetic analyses were performed using Bayesian inference (BI) and maximum likelihood (ML). For maximum-likelihood analysis, JModelTest v.2.1.10 [20] was used to identify the best evolutionary model. Based on Akaike information criterion (AIC) the TVM + G model was selected. The phylogenetic analysis was inferred using PhyML v.3.0 [21] with 1000 replicate bootstraps (> 50%).
For Bayesian inference (BI), MrBayes analysis was carried out using computational resource CIPRES [22]. The Bayesian analysis was run with the nucleotide substitution model GTR + I + G. To search with the Markov chain Monte Carlo method, chains were run with 10,000,000 generations, saving one tree every 1000 generations. On the burn-in, the first 25% of generations were discarded, and the consensus trees were estimated using the remaining trees. Bayesian posterior probabilities’ (BPP) cut-off was considered > 50%.
The resultant phylogenetic trees for both BI and ML analysis were edited in FigTree v1.4 [23]. Klossia equi (MH211602), Adelina dimidiata (DQ096835), and Adelina grylli (DQ096836) were used as out-groups. A pair-wise distance (p distance) matrix was used to compare the interspecific divergence between species of Hepatozoon sequences isolated from snake hosts performed on MEGA7 [24].
Results
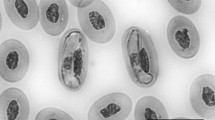
The Brazilian free-living sleep snake D. mikanii was found infected with a species of Hepatozoon. The parasitized individual with a parasitaemia of 0.25% was an adult male measuring 48.3 cm (snout–vent length) and weighing 42 g. Mature gamonts were observed infecting the snake peripheral blood erythrocytes, and meronts and monozoic cysts were observed in histological sections of the snake’s liver (Figs. 1, 2). The association of the morphological and morphometric results together with the molecular and phylogenetic analysis (Fig. 3; Table 1, 2; Supplementary materials 1, 2, 3) revealed a new species of Hepatozoon.
Species Description
Phylum Apicomplexa Levine, 1970.
Class Conoidasida Levine, 1988.
Subclass Coccidia Leuckart, 1879.
Order Eucoccidiorida Léger, 1911.
Suborder Adeleorina Léger, 1911.
Family Hepatozoidae Wenyon, 1926.
Genus Hepatozoon Miller, 1908.
Hepatozoon quagliattus Úngari, Netherlands, Silva and O´Dwyer sp. nov.
Taxonomic Summary
Type-host: sleep snake Dipsas mikanii (Schlegel, 1837) (Squamata: Colubridae: Dipsadinae).
Type-locality: Municipality of Britânia, Goiás State, Brazil (18°53′09.4″ S 48°15′29.5″ W).
Site of infection: Blood erythrocytes and liver.
Vector: Unknown.
Etymology: The species, Hepatozoon quagliattus sp. nov., is named after Prof. André Luiz Quagliatto Santos, to honour his contribution to the parasites of Brazilian wildlife. Professor Quagliatto has dedicated his work to studying and preserving Brazilian wildlife, especially in regards to reptiles.
Parasitemia: The parasitemia was 0.25%.
Material deposited: Hapantotypes, one blood smear from D. mikanii and one histological slide of the liver were deposited in the collection of the Nacional Institute of Amazonian Research (INPA), Manaus, Brazil [INPA23].
Gene sequence: The 18S rRNA gene sequences obtained from the blood and liver of a sleep snake D. mikanii were deposited in GenBank under accession numbers [MW591556 / MW591599].
Morphological and Morphometric Analysis
Gamonts (Fig. 1): Gamonts were found infecting only the host’s erythrocytes. Mature gamonts are characterised as full-bodied and elongated with thin parasitophorous vacuole (PV); however, in some cases, the PV is not evidenced; both ends rounded; barely noticeable, but slightly curved at one end with cytoplasm stained purplish blue. Gamont nucleus ovoid in shape with dense chromatin stained dark purple, and slightly displaced to one side of the parasite. Gamonts measured (mean ± standard deviation) 13.58 ± 0.62 µm long and 6.22 ± 0.41 µm wide, with an area of 72.24 ± 5.94 µm2; parasite nucleus measured 6.21 ± 0.32 µm long and 4.18 ± 0.54 µm wide, with an area of 19.16 ± 1.04 µm2 (n = 25) (Table 1).
Effects of host cell: Gamonts cause displacement of the host cell nucleus with morphological changes. Mean cell length, width and area measures were higher in infected cells (p ≤ 0.001). In some cases, the parasite causes the rupturing of the erythrocyte membrane, destroying it in the process. Infected erythrocytes measured 20.17 ± 1.23 µm long and 10.67 ± 1.45 µm wide with an area of 168.72 ± 0.85 µm2 (n = 15). Non-infected erythrocytes measured 18.38 ± 0.54 µm long and 9.54 ± 0.39 µm wide with an area of 131.39 ± 1.78 µm2 (n = 50) (Table 1).
Tissue developmental stages: From the histological analysis (liver, heart, spleen, and lung sections), four meronts and two monozoic cysts were observed in the snake’s liver sections (Fig. 2).
Meronts: Characterised by a thick-walled and robust capsule. Meronts measured (excluding capsule) 4.96 ± 0.32 µm long and 4.96 ± 0.49 µm wide with an area of 19.58 ± 1.35 µm2 (n = 4); measurements including the membrane capsule measured 9.24 ± 0.58 µm long and 10.95 ± 0.79 µm wide with an area of 74.82 ± 1.20 µm2 (n = 4) (Table 1).
Monozoic cysts: Characterised by a thick-wall encapsulating a single macromerozoite. Monozoic cysts measured 9.81 ± 0.72 µm long and 13.04 ± 1.02 µm wide with an area of 92.92 ± 0.85 µm2. Cysts including the membrane measured 10.35 ± 0.81 µm long and 2.14 ± 0.30 µm wide with an area of 20.80 ± 0.12 µm2. Nuclei measured 2.15 ± 0.50 µm by 1.49 ± 0.32 µm with an area of 4.87 ± 0.41 µm2 (Table 1).
Remarks
With regards to species of Hepatozoon described from Brazilian snakes, there are currently 35 recognised species, of which 14 have been described from the subfamily Diapsadinae (Supplementary material 1). To date, there have been no reports on the occurrence of species of Hepatozoon in D. mikanii. Furthermore, Hepatozoon musa Borges-Nojosa, Borges-Leite, Maia, Zanchi-Silva, da Rocha Braga and Harris, 2017 described from Philodryas nattereri Steindachner, 1870 is the only species of Hepatozoon described from the Diapsadinae using morphological and molecular tools. In comparison to H. quagliattus sp. nov., gamonts of H. musa are longer and thinner, measuring 18.95 ± 0.93 µm long and 3.76 ± 0.34 µm wide with an area of 77.60 ± 8.50 µm2 [25]. In addition, to the morphometric differences of Hepatozoon quagliattus sp. nov., gamonts of H. musa are curved at both ends with a centrally placed nucleus. Molecular data support the morphological findings with an interspecific divergence of between 7.2 and 7.5% among isolates of H. musa and the new species. Gamonts of Hepatozoon philodryasi Carini, 1910 described from the Patagonia green racer Philodryas patagoniensis (Girard, 1858) are smaller in size compared to H. quagliattus sp. nov. measuring 11.00–13.00 µm long and 2.00–3.50 µm wide [26]. Furthermore, gamonts of H. philodryasi do not alter host cell morphology. Hepatozoon carinicauda Pessôa and Cavalheiro 1969 described from Helicops carinicaudus (Wied-Neuwied, 1825) have larger gamonts measuring 23.00–25.00 µm long by 5.00–6.00 µm wide as compared to the new species (Table 2).
Through molecular analysis, Hepatozoon quagliattus sp. nov. have shown nucleotide identity with Hepatozoon sipedon Smith, Desser and Martin, 1994 infecting the northern water snake, Nerodia sipedon sipedon (Linnaeus, 1758). Thus, comparing the gamonts morphology of both species, H. sipedon has longer and slender gamonts, with central roughly ovoid nuclei, and the morphometric analysis revealed 19.00 µm long and 3.70 µm wide [27], differing from the present study (Table 2).
With regards to the other developmental stages, meronts and monozoic cysts were observed in the host's liver and spleen tissues, which presented unique characteristics with reduced size, but with great membrane thickness, measuring almost the size of the meront itself. In comparison to species of Hepatozoon from other snakes, the meront’s capsule size is thicker than the other species. Nevertheless, morphometric analyses have shown total measurements significantly smaller as compared with other species from snakes. Macromeronts of H. sipedon measures 49.00 ± 4.00 µm long by 40.30 ± 5.50 µm wide, and micromeronts measure 55.10 ± 3.10 µm long by 44.60 ± 4.00 µm wide, both meronts measuring larger than Hepatozoon quagliattus sp. nov. Another well-defined species is Hepatozoon terzii (Sambon and Seligman, 1907). According to Paperna and Lainson [28], the meronts of H. terzii found in the snake Boa constrictor Linnaeus, 1758, measured 26.00 ± 5.70 µm long by 21.30 ± 4.90 µm wide and dizoic and tetrazoic cysts measured 19.00 ± 4.00 µm long by 13.80 ± 2.30 µm wide, both larger than meronts of Hepatozoon quagliattus sp. nov. (Table 2).
Hepatozoon strigatus (Pessôa, 1967) described from the Brazilian snake Thamnodynastes strigatus (Günther, 1858) contains gamonts with a prominent recurved tail (14.00–18.00 µm long by 6.00–8.00 µm wide) and nucleus occupying the second quarter and extend to the first quarter of the gamont, differing from Hepatozoon quagliattus sp. nov. Regarding tissue merogony, only data for micromeronts in the liver and lung are reported, measuring up to 50 µm in diameter and containing hundreds of micromerozoites (Table 2) [29].
Therefore, the meronts and monozoic cysts of Hepatozoon quagliattus sp. nov., encapsulated within a thick-walled membrane, differ in comparison to Hepatozoon spp. described from other Brazilian snakes. In addition to the difference in gamont size, the main characteristics separating Hepatozoon quagliattus sp. nov., from other species of Hepatozoon described from Brazilian snakes, are the effects caused to the host cell, including hypertrophy (enlargement) of the host cell and displacement of the host cell nucleus (Table 2).
Molecular and Phylogenetic Analysis
Bayesian inference (BI) and the maximum-likelihood (ML) method resulted in identical topologies with supported nodes values in the clades (Fig. 3). The phylogenetic analysis included isolates of adeleorinid parasites (Haemogregarinidae, Hepatozoidae, Karyolysidae, and Dactylosomatidae) available from GenBank (Supplementary material 2). From the trees, the Haemogregarinidae Clade forms a sister group to the large well-supported clade comprising species from the Dactylosomatidae, Karyolysidae, and Hepatozoidae. Species of Dactylosoma form a monophyletic group, sister to a large-clade comprising species of Hemolivia, Karyolysus, and Hepatozoon. Species of Hepatozoon were polyphyletic forming two separated clades according to their vertebrate host species. Species of Hepatozoon isolated from large mammals formed a sister group to the Karyolysidae clade comprising species of Karyolysus. With regards to the reptile, anuran and marsupial Hepatozoidae clade, Hepatozoon quagliattus sp. nov. [GenBank number] grouped with Hepatozoon sipedon Smith, Desser and Martin, 1994 [JN181157], Hepatozoon angeladavesae Cook, Netherlands, van As and Smit, 2018 [MG519501], and Hepatozoon cecilhoarei Cook, Netherlands, van As and Smit, 2018 [MG519504], sister to a clade comprising species of Hepatozoon from anuran hosts (Fig. 3). The interspecific divergence between H. sipedon and Hepatozoon quagliattus sp. nov. was 2.4%. In comparison, the species of Hepatozoon from snakes varied between 88.7 and 93.7% as compared to the isolate from the present study. Moreover, the pair-wise distance among Hepatozoon species in snakes and the new species varied from 0.024 to 0.099.
Consensus phylogram of haemogregarines based on 18S rRNA sequences (492 nt). The topology trees were inferred by Bayesian (BI) and maximum-likelihood (ML) methods (represented by ML tree). The isolates Adelina dimidiata (DQ096835), Adelina grylli (DQ096836), and Klossia helicina (HQ224955) were used as an out-group
Discussion
Brazil is considered rich in reptile biodiversity [30]. According to Costa and Bérnils [31], Brazil is listed as the third-highest biodiversity hotspot for reptiles, currently with 795 recognised species, of which 405 species are snakes. The genus Dipsas Laurenti, 1768 (Squamata: Colubridae: Dipsadinae) comprises 53 species worldwide and 14 of them occur in Brazil [32, 33]. One of the species, D. mikanii (Schlegel, 1837), is nocturnal, with terrestrial or semi-arboreal habits [34, 35]. Interestingly, this neotropical snake feeds primarily on land snails and slugs, specialising on slugs of the Veronicellidae [36].
In the literature, there are some reports of greater abundance of D. mikanii snakes during the rainy season, which must be related to the period of greater availability of prey. This snake show activity stimulated during wetter periods (under or after rains) to search for molluscs, and at times of drought, it is possible that they remain at rest [37, 38]. However, leeches that remain a potential vector for species of Hepatozoon [5] share similar body morphology with slugs. Also, the dipsadinae snake Atractus reticulatus (Boulenger, 1885) has been reported to readily feed on earthworms and leeches from the savannahs and grasslands of southern Brazil [39].
Species of Hepatozoon are considered the most commonly reported haemoparasites found in snakes [7]. In Brazil, species of Hepatozoon from snake hosts are also commonly reported. Pessoa et al. [40] conducted a large-scale study on the prevalence of Hepatozoon species in Brazilian snakes, screening approximately 2100 snakes, belonging to 24 genera and 45 species, with a prevalence of up to 70% depending on the host species. However, there are no previous reports of haemogregarines infecting D. mikanii.
In the current study, the infected D. mikanii had a low parasitaemia with only 25 mature gamonts counted from the three blood smears prepared. The low parasitaemia could be related to the chronic and cyclic merogonic phases, which maintain the infection for long periods of time [41]. For example, De Biase et al. [42] reported a long-term infection of species of Hepatozoon in a Brazilian snake with the parasitaemia decreasing after 8 years. Santos et al. [43] also observed a tendency to parasitaemia decrease without complete elimination. According to Tenter et al. [44], species of Hepatozoon infections may be similar to Toxoplasma gondii, with a persistent infection throughout the hosts’ life.
According to Conradie et al. [45], the infection duration of Hepatozoon theileri (Laveran, 1905) and Hepatozoon ixoxo Netherlands, Cook and Smith, 2014 in its frog host may be related to gamont morphology and the parasites effect on the its host cell. Hepatozoon theileri and Hepatozoon quagliattus sp. nov. share several characteristics as both are rather large, displace the host cell nucleus, cause erythrocyte distortion, and possess a thin or non-evident capsule. Using electron microscopy, these authors provide the possible relationship between certain characteristics observed in H. theileri and the infection period within the host. These characteristics include the absence of a dense capsule and high pathogenicity to the host cell, which, instead of preserving the host’s erythrocyte, utilise all the resources from the infected cell before the gamont moves on to a new host cell [45]. When compared to H. ixoxo, the dense PV encasing the gamont possibly enables the parasite to remain for long periods of time in the same erythrocyte, thus associated with longevity.
In the past, haemogregarines were identified and new species were described based solely on the morphological comparison of the developmental stages in the vertebrate host, the host species, or the geographic location [5]. However, morphological analysis alone is not always effective in differentiating between species, due to the low host specificity of certain species and plasticity of gamont morphology [8, 9]. Thus, molecular tools have greatly assisted in the classification or identification of species and genera [10].
Based on the 18S rRNA gene, the closest relative of Hepatozoon quagliattus sp. nov., with an interspecific divergence of 2.4%, is H. sipedon (JN181157) described infecting the northern water snake, Nerodia sipedon sipedon (Linnaeus, 1758) [3]. This proximity was supported in the ML/BI phylogenetic analyses, with Hepatozoon quagliattus sp. nov. and H. sipedon forming a monophyly sister to H. angeladaviesae and H. cecilhoarei.
Hepatozoon sipedon and Hepatozoon quagliattus sp. nov. were identified in different host species with a great geographic distance, being N. sipedon sipedon reported from Canada. Moreover, N. sipedon sipedon is an aquatic snake from the family Colubridae and subfamily Natricinae, feeding on small fish, frogs, worms, leeches, salamanders, small birds, and mammals.
To date, the life-cycle of only a few species of Hepatozoon from snake hosts has been successfully elucidated. Due to the lack of biological data for this group, the life history and transmission mechanisms of Hepatozoon quagliattus sp. nov. in D. mikanii are difficult to conclude. Since D. mikanii do not feed on other vertebrate animals, such as frogs and fish, transmission via an intermediate host is unlikely to occur, as in the case of the genetically close related species H. sipedon (based on the 18S rRNA gene). Hepatozoon sipedon is characterised by an infection pathway requiring three hosts: an intermediate vertebrate host (snake), a second intermediate vertebrate host (anuran), and a final or definitive invertebrate host/vector (mosquitoes) [3]. These findings are in contrast to the hypothesis that host diet and phylogenetic relationships represent the life-history trends of species of Hepatozoon [40, 45, 46]. Furthermore, although unlikely, it is possible that D. mikanii may accidentally prey on leeches confused for slugs (due to morphological similarities); however, leeches have not yet been reported as vectors for any species of Hepatozoon nor have there been any reports of leeches feeding on D. mikanii. The most likely transmission scenario is accidental ingestion of mites or ticks feeding on D. mikanii.
Therefore, with the combination of morphological and molecular tools, it is possible to clearly differentiate between H. sipedon and the new species here described as Hepatozoon quagliattus sp. nov.
Conclusions
Although Brazil is a hotspot for snake species, there is a lack of information on the biodiversity of haemoparasites infecting these hosts, and this number is even lower if related to the use of the right techniques. Thus, the present study reports the first haemogregarine infecting the sleep snake Dipsas mikanii, with the description of a new species, Hepatozoon quagliattus sp. nov., based on molecular and morphological diagnostics.
Future work should include molecular characterisation with variable markers, such as mitochondrials, to increase the knowledge of phylogenetic position on species of Hepatozoon infecting snakes. Notwithstanding, electron microscopy screening may provide further insights into the cytopathogenicity of Hepatozoon quagliattus sp. nov. Furthermore, studies focusing on elucidating life-cycle, describing possible vectors, and determining routs of transmission are important for future research on Hepatozoon quagliattus sp. nov.
References
Smith TG (1996) The genus Hepatozoon (Apicomplexa: adeleina). J Parasitol 82:565–585. https://doi.org/10.2307/3283781
Úngari LP, Santos ALQ, O´Dwyer LH, Silva MRL, Santos TCR, Cunha MJR, Pinto RMC, Cury MC (2018) Molecular characterization and identification of Hepatozoon species Miller, 1908 (Adeleina: Hepatozoidae) in captive snakes from Brazil. Parasitol Res 117:3857–3865. https://doi.org/10.1007/s00436-018-6092-3
Smith TG, Desser SS, Martin DS (1994) The development of Hepatozoon sipedon sp. nov. (Apicomplexa: Adeleina: Hepatozoidae) in its natural host, the Northern water snake (Nerodia sipedon sipedon), in the culicine vectors Culex pipiens and C. territans, and in an intermediate host, the Northern leopard frog (Rana pipiens). Parasitol Res 80:559–568. https://doi.org/10.1007/BF00933003
Tomé B, Maia JPMC, Harris DJ (2012) Hepatozoon infection prevalence in four snake genera: influence of diet, prey parasitemia levels, or parasite type? J Parasitol 98:913–917. https://doi.org/10.1645/GE-3111.1
Kauffman KL, Sparkman A, Bronikowski AM, Palacios MG (2017) Vertical transmission of Hepatozoon in the garter snake Thamnophis elegans. J Wildl Dis 53(1):121–125. https://doi.org/10.7589/2016-03-056
Desser SS (1993) The Haemogregarinidae and Lankesterellidae. In: Levine N (ed) Parasitic protozoa, vol 4. Academic Press, New York, pp 247–272
Telford SR Jr (2009) Hemoparasites of the Reptilia: Color Atlas and text. CRC Press Taylor and Francis Group, Boca Raton, Florida
Telford SR (2010) Three new Hepatozoon species (Apicomplexa: Hepatozoidae) infecting the Florida kingsnakes, Lampropeltis getula floridana. J Parasitol 96:162–169. https://doi.org/10.1645/GE-2161.1
Harris DJ, Maia JPMC, Perera A (2011) Molecular characterization of Hepatozoon species in reptiles from the Seychelles. J Parasitol 97:106–110. https://doi.org/10.1645/GE-2470.1
O’Donoghue P (2017) Haemoprotozoa: making biological sense of molecular phylogenies. Int J Parasitol Parasites Wildl 6:241–256. https://doi.org/10.1016/j.ijppaw.2017.08.007
Zippel KC, Lillywhite HB, Mladinich CR (2001) New vascular system in reptiles: anatomy and postural hemodynamics of the vertebral venous plexus in snakes. J Morphol 250:173–184. https://doi.org/10.1002/jmor.1063
Sebben A (2007) Microdissecação fisiológica a fresco: uma nova visão sobre a anatomia de anfíbios e répteis. In: Nascimento, LB, Oliveira ME (ed) Herpetologia no Brasil II. 1ed. Belo Horizonte—MG, Sociedade Brasileira de Herpetologia, 1 pp 311–325
Soares HS, Marcili A, Barbieri ARM, Minervino AHH, Moreira TR, Gennari SM, Labruna MB (2017) Novel piroplasmid and Hepatozoon organisms infectin the wildlife of two regions of the Brazilian Amazon. Int J Parasitol: Parasit Wildl 6:115–121. https://doi.org/10.1016/j.ijppaw.2017.05.002
Eisen RJ, Schall JJ (2000) Life history of malaria parasite (Plasmodium mexicanum): independent traits and basis for variation. Proc Biol Sci 267:793–799. https://doi.org/10.1098/rspb.2000.1073
Cook CA, Smit NJ, Davies AJ (2009) A redescription of Haemogregarinafitzsimonsi Dias,1953 and some comments on Haemogregarinaparvula Dias, 1953 (Adeleorina: Haemogregarinidae) from southern African tortoises (Cryptodira: Testudinidae), with new host data and distribution records. Folia Parasitol 56:173–179. https://doi.org/10.14411/fp.2009.021
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Ujvari B, Madsen T, Olsson M (2004) High prevalence of Hepatozoon spp. (Apicomplexa, Hepatozoidae) infection in water pythons (Liasis fuscus) from tropical Australia. J Parasitol 90:670–672. https://doi.org/10.1645/GE-204R
O’Dwyer LH, Moço TC, Paduan KS, Spenassatto C, Silva RJ, Ribolla PEM (2013) Description of three new species of Hepatozoon (Apicomplexa, Hepatozoidae) from Rattlesnakes (Crotalus durissus terrificus) based on molecular, morphometric and morphologic characters. Exp Parasitol 135:200–207. https://doi.org/10.1016/j.exppara.2013.06.019
Mathew JS, Van Den Bussche RA, Ewing SA, Malayer JR, Latha BR, Panciera RJ (2000) Phylogenetic relationships of Hepatozoon (Apicomplexa: Adeleorina) based on molecular, morphologic, and life-cycle characters. J Parasitol 86:366–372. https://doi.org/10.1645/0022-3395(2000)086[0366:PROHAA]2.0.CO;2
Darriba D, Toboada GL, Doallo R, Posada D (2012) JModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. https://doi.org/10.1080/10635150390235520
Miller MA, Pfiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for Inference of Large phylogenetic Trees. 2010 Gateway Computing Environments Workshop
Rambaut A (2012) FigTree v1.4. Molecular evolution, phylogenetics and epidemiology. http://tree.bio.ed.ac.uk/software/figtree/05/2017. Accessed 24 April 2017
Kumar S, Stecher G, Tamura K (2016) Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Borges-Nojosa DM, Borges-Leite MJ, Maia JP, Zanchi-Silva D, Braga RR, Harris DJ (2017) A new species of Hepatozoon Miller, 1908 (Apicomplexa: Adelerina) from the snake Philodryas nattereri Steindachner (Squamata: Dipsadidae) in Northeastern Brazil. Syst Parasitol 94:65–72. https://doi.org/10.1007/s11230-016-9676-2
Carini A (1910) Sobre uma haemogregarina de Philodryas schotti. Rev Med S Paulo 23:339–340
Smith TG, Desser SS (1998) Ultrastructural features of cystic and merogonic stages of Hepatozoon sidepon (Apicomplexa: Adeleorina) in Northern leopard frogs (Rana pipiens) and Nothern water snakes (Nerodia sipedon) from Ontario, Canada. J Euk Microbiol 45:419–425. https://doi.org/10.1111/j.1550-7408.1998.tb05093.x
Paperna I, Lainson R (2003) Ultrastructural studies on the sporogony of Hepatozoon spp. In Culex quinquefasciatus Say, 1823 fed on infected Caiman crocodilus and Boa constrictor from Northern Brazil. Parasitology 127:147–154. https://doi.org/10.1017/s0031182003003482
Pessoa SB (1967) Notas sobre hemogregarinas de serpentes brasileiras. I: hemogregarinas de algumas espécies de serpentes da família Colubridae. Rev Bras Biol 27:33–46
Silvano DL, Segalla MV (2005) Conservação de anfíbios no Brasil. Megadiversidade 1:79–86
Costa HC, Bérnils RS (2018) Répteis do Brasil e suas Unidades Federativas: Lista de espécies. Herpetol Bras 7:11–57
Uetz P, Hallermann J (2020) Zoological Museum Hamburg (new species and updates). The Reptile database. https://reptile-database.reptarium.cz/species?genus=Dipsas&species=mikanii&search_param=%28%28search%3D%27Dipsas%27%29%29. Accessed 23 September 2020
Peters J (1960) The snakes of the subfamily Dipsadinae. Misc Publ Mus Zool Univ Chicago 114:1–252
Marques OAV, Sazima I (2004) História natural dos répteis da Estação Ecológica Juréia-Itatins. In: Marques OAV, Duleba W (eds) Estação Ecológica Juréia-Itatins—ambiente físico, flora e fauna. Ribeirão Preto, Holos, pp 257–277
Laporta-Ferreira IL, Salomão MG, Sawaya P (1986) Biologia de Sibynomorphus (Colubridae, Dipsadinae)—Reprodução e hábitos alimentares. Rev Bras Biol 46:793–799
Barbo FE, Marques OAV, Sawaya RJ (2011) Diversity, natural history and distribution of snakes in the municipality of São Paulo. South Am J Herpetol 6:15–160. https://doi.org/10.2994/057.006.0301
Agudo-Padron AI (2012) Brazilian snail-eating snakes (Reptilia, Serpentes, Dipsadidae) and their alimentary preferences by terrestrial molluscs (Gastropoda, Gymnophilia and Pulmonata): a preliminary overview. Biol Evid 2:2–3
Balestrin RL, Di-Bernardo M, Moreno AG (2007) Feeding ecology of the neotropical worm snake Atractus reticulatus in Southern Brazil. Herpetol J 17:62–64
Pessoa SB, De Biasi P, Puorto G (1974) Nota sobre a frequência de hemoparasitas em serpentes do Brasil. Mem Inst Butantan 38:69–118
Tomé B, Maia JPMC, Harris DJ (2013) Molecular assessment of apicomplexan parasites in the snake Psammophis from North Africa: do multiple parasite lineages reflect the final vertebrate host diet? J Parasitol 99:883–887. https://doi.org/10.1645/12-95.1
De Biasi P, Cardodo RP Jr, Santos SMA (1989) Presença de Hepatozoon plimmeri (Sambon, 1909)—Coccidia, Haemogregarinidae—em exemplar de Bothrops jararaca (Wied, 1824)—Serpentes. Viperidae, Critalinae—mantido em cativeiro. Mem Inst Butantan 55:117–121
Santos MMV, O’Dwyer LH, Silva RJ (2005) Seasonal variation of Hepatozoon spp. (Apicomplexa, Hepatozoidae) parasitemia from Boa constrictor amarali (Serpentes, Boidae) and Hydrodynastes gigas (Serpentes, Colubridae). Parasitol Res 97:94–97. https://doi.org/10.1007/s00436-005-1385-8
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258. https://doi.org/10.1016/S0020-7519(00)00124-7
Conradie R, Cook CA, du Preez LH, Jordaan A, Netherlands EC (2017) Ultrastructural comparion of Hepatozoon ixoxo and Hepatozoon theileri (Adeleorina: Hepatozoidae), parasiting South African anurans. J Eukaryot Microbiol 64:193–203. https://doi.org/10.1111/jeu.12351
Cook CA, Netherlands EC, As JV, Smit NJ (2018) Two new species of Hepatozoon (Apicomplexa: Hepatozoidae) parasiting species of Philothamnus (Ophidia: Colubridae) from South Africa. Folia Parasit 65:2–11. https://doi.org/10.14411/fp.2018.004
Barta JR, Ogedengbe JD, Martin DS, Smith TG (2012) Phylogenetic position of the adeleorinid Coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rRNA sequences. J Eukaryot Microbiol 59:171–180. https://doi.org/10.1111/j.1550-7408.2011.00607.x
Acknowledgements
We are grateful to FAPESP (São Paulo State Research Support Foundation) (2018/00754-9; 2018/09623-4), FAPEMIG (Research Foundation of the State of Minas Gerais), and CNPq (National Council for Scientific and Technological Development) (309125/20147-0; 440496/2015-2) for providing a research fellowship. We thank the team of the Laboratory for Teaching and Research in Wild Animals (LAPAS) and Non-Governmental Organization for the Preservation of Brazilian Wild Animals (ONG PAS).
Funding
RJS is supported by FAPESP (2016/50377-1), CNPq (309125/2017-0), and CNPq-PROTAX (440496/2015-2). LPU is supported by FAPESP (2018/00754-9; 2018/09623-4). LHO is supported by FAPESP (2018/09623-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (IBAMA license 60640-1; CEUA-UNESP 1061).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Úngari, L.P., Netherlands, E.C., de Alcantara, E.P. et al. Description of a New Species Hepatozoon quagliattus sp. nov. (Apicomplexa: Adeleorina: Hepatozoidae), infecting the Sleep Snake, Dipsas mikanii (Squamata: Colubridae: Dipsadinae) from Goiás State, Brazil. Acta Parasit. 66, 871–880 (2021). https://doi.org/10.1007/s11686-021-00355-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00355-x