Abstract
A high proportion of patients with drug-resistant temporal lobe epilepsy (TLE) show focal relative hypometabolism in the region of the epileptogenic zone on [18F]-Fluorodeoxyglucose positron emission tomography (FDG PET). However, whether focal (hypo)metabolism changes over time has not been well studied. We analysed repeated [18F]-FDG PET scans of patients with TLE to determine longitudinal changes in glucose metabolism. Adults (n = 16; 9 female, 7 male) diagnosed with drug resistant chronic TLE were assessed. Each patient had two [18F]-FDG PET scans that were 2–95 months apart. Region-of-interest analysis was performed on MR images onto which PET scans were coregistered to determine the relative [18F]-FDG uptake (normalised to pons) in the bilateral hippocampi and temporal lobes. Statistical Parametric Mapping analysis investigated global voxel-wise changes in relative metabolism between timepoints. Normalised [18F]-FDG uptake did not change with time in the ipsilateral (baseline 1.14 ± 0.03, follow-up 1.19 ± -0.04) or contralateral hippocampus (baseline 1.18 ± 0.03, follow-up 1.19 ± 0.03). Uptake in the temporal neocortex also remained stable (ipsilateral baseline 1.35 ± 0.03, follow-up 1.30 ± 0.04; contralateral baseline 1.38 ± 0.04, follow-up 1.33 ± 0.03). The was no relationship between change in uptake on the repeated scans and the time between the scans. SPM analysis showed increases in metabolism in the ipsilateral temporal lobe in 2/16 patients. No areas of decreased metabolism concordant to the epileptogenic zone were identified. [18F]-FDG uptake showed no significant changes over time in patients with drug-resistant TLE. This suggests that repeating FDG-PET scans in patients with subtle or no hypometabolism is of low clinical yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temporal lobe epilepsy (TLE) is the most common form of focal epilepsy in adults and is estimated to account for the highest proportion of drug-resistant cases (Stephen et al., 2001). 70–89% of patients with drug-resistant TLE have focal glucose hypometabolism in the region of the epileptogenic zone detected on [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) (Benedek et al., 2006; Gaillard et al., 1995; O'Brien et al., 2001, 2008; Spencer, 1994). [18F]-FDG PET is particularly useful in cases of MRI negative focal epilepsy, and depending on the sensitivity and scanner resolution this method can detect focal and regional hypometabolism in 80% of patients (Carne et al., 2004; Engel, 1984; Engel et al., 1990; Gaillard et al., 1995; O'Brien et al., 2001, 2008; Theodore et al., 1983), which is predictive of a good outcome following resective epilepsy surgery (Carne et al., 2004; O'Brien et al., 2001, 2008), thus reducing the need for invasive intracranial EEG recording (Theodore et al., 1983). The extent of [18F]-FDG-PET hypometabolism can also be used to predict surgical outcome (Vinton et al., 2007). Despite the central role [18F]-FDG PET imaging plays in the diagnosis and the pre-surgical evaluation of focal epilepsy, there are limited data regarding the progression of these metabolic abnormalities over time.
It has been shown that increasing epilepsy duration is strongly associated with a reduction in the volumes of temporal lobe structures (Goncalves Pereira et al., 2005), a trend that can be augmented in patients with familial mesial TLE (Conz et al., 2011). Conflicting evidence exists for whether these changes are dependent on seizure frequency (Conz et al., 2011; Theodore, 2003). Although the serial MRI findings indicate progressive structural atrophy, few studies have investigated whether similar evolution of brain metabolism can be observed in patients with chronic TLE. The reasons for this are manifold, and include limited access to PET, risks associated with radiation exposure and financial considerations, and patients proceeding to resective epilepsy surgery following the initial pre-surgical PET scan.
Several studies have suggested that metabolic abnormalities observed in epilepsy are associated with repeated seizures. Jokeit and colleagues demonstrated a positive correlation between epilepsy onset, duration and hypometabolism (Jokeit et al., 1999). Furthermore, only a small number of patients with newly diagnosed focal epilepsy had glucose metabolism abnormalities (Gaillard et al., 2002) and greater hypometabolism in the ipsilateral temporal lobe is associated with longer epilepsy duration in unilateral TLE (Vivash et al., 2013). A longitudinal PET study in children with non-lesional focal epilepsy demonstrated progressive cortical glucose hypometabolism, which was moderately related to seizure frequency (Benedek et al., 2006). However, to our knowledge there are no longitudinal studies of PET in adult epilepsy. Therefore, this study aimed to investigate glucose metabolism longitudinally in adults with chronic drug resistant TLE, to ascertain whether repeating an FDG-PET scan at a later time point yields additional information upon reinvestigation.
Methods
Subjects
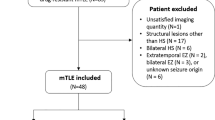
Retrospective data were collected from patients who were admitted to the Comprehensive Epilepsy Program Video EEG Monitoring (VEM) Unit at Royal Melbourne Hospital between 2004 and 2014. Inclusion criteria were patients with chronic drug resistant TLE that had undergone two [18F]-FDG scans and at least one epilepsy protocol MRI as part of their evaluation for potential epilepsy surgery. Localisation of the epileptogenic zone was determined in accordance with International League Against Epilepsy guidelines by the consensus of the consultant epileptologists on the basis of the ictal and interictal VEM findings, unequivocal patient history, and without any psychogenic events captured (Nightscales et al., 2020). In cases of bilateral TLE the hemisphere with the greater proportion of seizures was considered ipsilateral. The study was approved by the Melbourne Health Human Ethics Research Committee (QA2012044).
[18F]-FDG PET acquisition
Interictal [18F]-FDG PET scans were acquired at the Peter MacCallum Cancer Centre on the Discovery STE PET/CT scanner (GE healthcare) or PENN PET 300H Tomograph scanner, and image acquisition was conducted as previously described (O'Brien et al., 2001, 2008). Patients were scanned on the same scanner in all but two cases (those with the longest inter-scan intervals). Patients fasted for 4 h prior to the scan and rested for 15–20 min in a darkened and quiet room. Subsequently, [18F]-FDG (250 MBq) was administered intravenously and scanning was undertaken 1 h after radiotracer administration. The patient was in a single bed position for at least 20 min and tape was used to immobilise the head. Voxel size was 2.73 × 2.73 × 3.27 mm, FOV 350 mm and matrix size 128 × 128 × 47.
MRI acquisition
MR imaging was conducted using a 3 T clinical whole-body scanner (Sigma Horizon SE120, GE Healthcare). A standardised TLE protocol was undertaken, including volumetric T1, FLAIR and high resolution T2 through the temporal lobes (Vivash et al., 2013). The T1-weighted volumetric MPRAGE (1.5mm3, FOV 22 × 22 cm, matrix size 128*128*208) was used for image analysis.
ROI Analysis
An operator blinded to all clinical variables and demographics conducted all image processing. Manual region of interest (ROI) tracing was performed on volumetric MPRAGE images using Analyze v10.0 (Mayo Clinic, MO), delineating the hippocampi, temporal lobes and pons. Hippocampal delineation was conducted in the coronal plane, followed by corrections in the sagittal plane. The temporal lobes, including the hippocampal, limbic, and neocortical structures, were delineated as previously described (Vinton et al., 2007). The pons was traced manually, where the tracing reached the edge of the pons, in the sagittal plane and corrected in the coronal plane. Intracranial volume was estimated using the tissue segmentation tool within SPM12 (Matlab 2017b).
PET scans were coregistered to their corresponding MRI using the 3D voxel registration tool in Analyze, and the ROIs applied producing values of uptake for all ROIs. Uptake in the hippocampi and temporal lobes were normalised to uptake in the pons.
SPM analysis
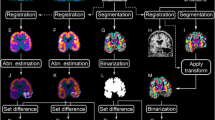
In addition to the ROI analysis, whole-brain voxel-wise changes in hypometabolism were investigated individually in each patient using SPM12 (Matlab 2017b). The second PET scan was realigned to the first PET scan using linear registration. These scans were masked with SPM’s MNI intracranial volume (ICV) mask as follows: the two aligned PET scans were averaged and the average image registered to the inbuilt SPM PET template, followed by inversion of the MNI intracranial volume (ICV) and grey matter (GM) masks back into subject space. The PET images were proportionally scaled to their global mean, to account for between subject uptake variability. The mean activity in each scan within the ICV mask was calculated and voxels with activity < 0.125 of the mean discounted and the mean recalculated. All remaining voxels were then divided by the global mean and multiplied by 50, giving a mean signal of 50. Images of percentage change were then created: % change = ((T2-T1)/T1)*100.
These % change images were then masked using the GM mask (threshold > 0.1). This image contains the relative change from T1 to T2 in each voxel independently. To measure meaningful change over time the percentage change was initially set at 20%, which resulted in two reductions in hypometabolism at T2, but no increases in hypometabolism. The percentage change was then adjusted to 10%, which was too noisy, detecting multiple areas of increased and decreased hypometabolism in each patient that were neither observable on visual inspection of the image pairs, nor pathologically or clinically meaningful. Thus 15% threshold was selected, considerably reducing the false detection of noise, without missing potentially pathological alterations. Images were extent thresholded, with clusters smaller than 300mm3 removed. The resulting thresholded difference images were visually inspected overlaid on the realigned, normalised PET images to ensure findings were real and not due to a processing error.
Statistical analysis
The normalised [18]F-FDG uptake values were compared between the two scans using Jamovi (version 1.1.9.0). Median and SEM per group are reported within the text and a two-tailed Wilcoxon Signed Rank test was used to assess differences between regions, and Spearman’s correlation was used to assess relationship with time. Mixed models were also used to investigate whether covarying for seizure frequency affected the association with time. Statistical analyses were re-run excluding the two bilateral TLE cases, this did not change the significance of any of the results described. p < 0.05 was considered significant.
Results
Patient characteristics
Sixteen patients with chronic drug resistant TLE (9 females and 7 males) were included in this study. Median age was 35 years (range 18–64 years), and median age of epilepsy onset 27 years (range 4–58 years). Median epilepsy duration at scan 1 was 4.5 years, (range 0–41 years), and at scan 2 was 7 years (range 1–52 years). Six patients had MRI lesions (n = 3 hippocampal or mesial temporal sclerosis, n = 1 focal cortical dysplasia, n = 1 tuberous sclerosis, and n = 1 cavernoma), ten patients had non-lesional TLE. Clinical characteristics are detailed in Table 1.
ROI analysis—MRI
Hippocampal and temporal lobe volumes are shown in Fig. 1. Ipsilateral hippocampal volume (0.207 ± 0.015 %ICV, 2792 ± 200.1 mm3) was less than contralateral (0.225 ± 0.01 %ICV, 3051 ± 130.2 mm3) hippocampal volume (p = 0.01, Fig. 1A). Temporal lobe volumes were similar between the ipsilateral (6.474 ± 0.268 %ICV, 90,856 ± 3370 mm3) and contralateral (6.72 ± 0.233 %ICV, 91,258 ± 3033 mm3) regions (p = 0.89) (Fig. 1B). Exclusion of the two bilateral TLE cases does not affect the hippocampal asymmetry (0.207 ± 0.015 %ICV vs 0.225 ± 0.01 %ICV, p = 0.01), nor the similarity of temporal lobe volumes observed (6.528 ± 0.289 %ICV vs 6.860 ± 0.251 %ICV, p = 0.95).
Volumes and normalised FDG uptake in the hippocampi and temporal lobes. (A) Hippocampal and (B) temporal lobe volumes (expressed as %ICV). Blue squares represent the regions ipsilateral to the epileptogenic temporal lobe, and red squares the contralateral hemisphere. (C) Hippocampal and (D) temporal lobe FDG uptake (normalised to the pons). Closed circles represent baseline measurements, and open circles follow-up measurements. Error bars show median and inter-quartile range. * p < 0.05
ROI analysis – FDG-PET
Figure 1C shows [18F]-FDG uptake in the hippocampal ROIs on the serial scans, and Fig. 1D temporal lobe ROIs. Normalised FDG uptake was lower in the ipsilateral than contralateral hippocampus at baseline (1.14 ± 0.03 vs 1.18 ± 0.03, p = 0.04), but interestingly not at follow-up (1.19 ± 0.04 vs 1.19 ± 0.03, p > 0.19). Normalised FDG uptake did not differ between ipsilateral and contralateral temporal lobes at baseline or follow-up (p > 0.16). No change in normalised FDG uptake over time was observed in the ipsilateral (baseline 1.14 ± 0.03, follow-up 1.19 ± 0.04, p = 0.25; Fig. 1C) or contralateral (baseline 1.18 ± 0.03, follow-up 1.19 ± 0.03, p = 0.94) hippocampi. Similarly, normalised FDG uptake in the temporal lobes did not change over time (ipsilateral baseline 1.35 ± 0.03, follow-up 1.30 ± 0.04, p = 0.63; contralateral baseline 1.38 ± 0.04, follow-up 1.33 ± 0.03, p = 0.82; Fig. 1D). Exclusion of the two bilateral cases did not affect the overall results of the normalised FDG uptake (baseline hippocampal FDG uptake 1.15 ± 0.04 vs 1.18 ± 0.04, p = 0.04, all other comparisons non-significant).
Median change in normalised FDG uptake between baseline and follow-up was 0.056 ± 0.031 in the ipsilateral hippocampus, 0.014 ± 0.035 in the contralateral hippocampus and -0.002 ± 0.032 and -0.003 ± 0.037 in the ipsilateral and contralateral temporal lobes respectively. No differences were detected between regions and none of these were different from 0 (p > 0.53). Given the wide range of time between scans, and annualised rates of change were also calculated which were 0.013 ± 0.040, 0.003 ± 0.041 for the ipsilateral and contralateral hippocampus, and -0.001 ± 0.039, 0.001 ± 0.057 for the ipsilateral and contralateral temporal lobes. No differences were detected between regions and none of these were different from 0 (p > 0.3).
Correlation with clinical characteristics
Figure 2 shows change in FDG uptake plotted against time. No correlations were found between time between scans and change in FDG uptake in any of the regions (Spearman’s rho < 0.37, p > 0.16). Including seizure frequency as a covariate in mixed models showed no effect of time on uptake in any region.
The relationship between FDG uptake and epilepsy duration was also investigated. No correlation was found in the ipsilateral hippocampus or temporal lobes bilaterally (Spearman’s rho < 0.13, p > 0.49), however a positive correlation was observed in the contralateral hippocampus (Spearman’s rho = 0.41, p = 0.02). Covarying for seizure frequency did not change these results.
The change in FDG uptake was also investigated relative to the inter-scan duration as a proportion of total epilepsy duration (Fig. 3). Positive correlations were observed between change in FDG uptake and proportion of total epilepsy duration for the ipsilateral hippocampus (Spearman’s rho = 0.67, p < 0.01, Fig. 3A) and bilateral temporal lobes (Spearman’s rho > 0.53, p < 0.04, Fig. 3B), the contralateral hippocampus was approaching significance (Spearman’s rho = 0.48, p = 0.06). The data shows that for patients where this proportion is low (i.e. their epilepsy was long-standing at the time of the first PET scan) FDG uptake tended to decrease, suggesting disease progression, whereas for patients where the proportion was at or approaching 1 (i.e. they were newly diagnosed at the time of the first PET scan) FDG uptake did not change or increased marginally.
Change in FDG uptake between baseline and follow-up scans plotted against inter-scan duration as a proportion of epilepsy duration. Change in hippocampal ROI (A) and temporal lobe ROI (B) relative FDG uptake between scans plotted against inter-scan duration as a proportion of epilepsy duration in the ipsilateral (blue) and contralateral (red) hemispheres
SPM analysis
Of the 16 subjects, 4 had supra-threshold increases in FDG-PET signal, and 3 had supra-threshold decreases. The most common locations of signal increases were the cerebellum and temporal lobes, and areas of decrease the frontal lobes. Figure 4 shows example images of areas of increased and decreased metabolism between scans. 3/4 patients showed increases in the cerebellum, and 2/4 showed increases in the ipsilateral temporal lobe (and 1/4 in the contralateral temporal lobe). 2/3 patients had decreases in the frontal lobe, one contralateral and one bilateral, the other patient had a decrease in the cerebellum. No areas of decreased metabolism colocalised with the epileptogenic zone.
Example SPM images showing increases (top row) and decreases (bottom row) in FDG uptake between scans. The left panel show baseline images, the middle panel the follow-up images and the right panel the specific region of changed signal between the two images. The increased metabolism in both temporal lobes between baseline and follow-up suggests a resolution of hypometabolism (top row, patient 15), whereas the decreased metabolism in the left frontal lobe is suggestive of a misalignment during processing, and not a real change (bottom row, patient 4)
Discussion
This study investigated whether brain glucose metabolism, as measured on [18F]-FDG PET imaging, changes over time in adults with chronic drug resistant TLE. The main finding was that there was no evidence that [18F]-FDG uptake evolved over time in this cohort of patients studied. This is therefore of significance in the clinical setting, suggesting repeating an FDG-PET scan in a patient who has previously had a negative or equivocal scan, will not yield different information.
Previous studies in an animal model of epilepsy showed acute hypometabolism early after an epileptogenic insult, with an increase in the severity of hypometabolism during chronic epileptogenesis and the development of spontaneous recurrent seizures (Jupp et al., 2012). In traumatic brain injury, hypometabolism occurred acutely following injury, which remained stable at later time points (Y. R. Liu et al., 2010). However, both these animal studies focused on the epileptogenic period, rather than chronic epilepsy.
We expected an increase in the hypometabolism to be observed on the FDG-PET scan over time in our patients, which was not the case. Patients included in the current study showed either subtle hypometabolism, or no hypometabolism, rather than the typical severe ipsilateral hypometabolism seen in the majority of TLE cases, which is the reason for repeating the PET scan and thus inclusion in the current study. This suggests the patients included in this study may be considered “complex” or “atypical” cases, which is why they had not proceeded to epilepsy surgery, but rather had an FDG-PET repeated, and thus the findings may not necessarily be generalisable to the wider TLE population.
Clinical studies of longitudinal imaging are scarce in patients with epilepsy, one study reported progressive volume loss in the ipsilateral hippocampus in TLE patients with ongoing seizures, which was not seen in those who were seizure free (Fuerst et al., 2003). However, another study found early volume loss does not evolve over time, even with recurrent seizures (R. S. Liu et al., 2002). More recent studies report progressive atrophy in focal epilepsies (most commonly TLE). Temporal and extratemporal atrophy are observed in non-lesional TLE and TLE with hippocampal sclerosis both independent of, and associated with, seizures (Alvim et al., 2016; Bernhardt et al., 2009, 2013). Additionally, widespread cortical thinning is reported in focal epilepsies (TLE and extra-TLE) in both the ipsilateral and contralateral hemispheres (Galovic et al., 2019).
The only previously published studies investigating longitudinal FDG PET have been in paediatric epilepsy populations. One study reported a significant reduction in the spatial extent of cortical glucose hypometabolism ipsilateral to the epileptic zone over time in paediatric patients with “intractable” non-lesional focal epilepsy. Another study investigated patients with West syndrome, an infantile epileptic encephalopathy that can be associated with focal cortical dysplasia in some patients. Of four patients, two had hypometabolism at baseline which had resolved on repeated imaging, one had hypermetabolism which resolved on repeated imaging, and one had normal metabolism at baseline which evolved to hypometabolism at follow-up (Sakaguchi et al., 2018). These, however, may not be suitable comparators for several reasons. Firstly, the aetiology of adult TLE and paediatric nonlesional focal epilepsy are different (Shukla & Prasad, 2012). Additionally, paediatric epilepsy will also be influenced by higher cortical glucose metabolism, a trait which occurs during early development of the brain and may have underlying consequences on epilepsy related glucose hypometabolism (Chugani et al., 1987).
This study did observe a positive correlation between contralateral hippocampal FDG uptake and epilepsy duration. This finding is contrary to our expectations, as previous work by our group has shown epilepsy duration negatively correlated with ipsilateral but not contralateral hippocampal FDG uptake in patients with TLE (Vivash et al., 2013). Further, this change in FDG uptake on ROI analysis is not supported by the SPM analysis and should therefore be interpreted with caution.
An interesting finding was the relationship between changes in FDG uptake as related to total epilepsy duration. Here, the results demonstrate that for patients whose epilepsy was long-standing at the time of their first FDG-PET scan, the hypometabolism increased on the scan. However, for those who were newly diagnosed at the time of their first FDG-PET scan there was no change in hypometabolism over the repeat scans. This suggests that hypometabolism may worsen after many years of chronic TLE, but may not evolve in the first few years after a diagnosis of TLE.
This study has several limitations. Firstly, due to the reticence to rescan patients without a strong clinical justification, and the fact that a proportion of patients proceed to resective epilepsy surgery following their FDG-PET, the sample size is relatively small, and heterogenous with respect to epilepsy duration, MRI lesions, seizure frequency and interscan interval. Secondly, none of the patients included in this study had a typical pattern of severe unilateral hypometabolism often seen in TLE. For this reason they may be considered complex or atypical TLE cases. Lastly, changes in PET acquisition and reconstruction parameters may have impacted image quality and SUV quantification through improvements in signal to noise, which have the potential to impact differences in measures for the cases of long inter-scan intervals who were scanned on two different scanners.
Conclusions
This study indicates that [18F]-FDG PET does not significantly change in patients with chronic drug resistant TLE who had subtle or normal focal temporal hypometabolism on the first scan. Therefore, rescanning these patients is unlikely to provide clinically useful new information. While a larger cohort study is required to confirm these findings, the results could impact clinical care by reducing radiation exposure and lessening economic burden on the health care system of repeating FDG-PET scans in these patients.
Data availability
Data included in this study is patients’ clinical data and is therefore not publicly available.
Code availability
Not applicable.
References
Alvim, M. K., Coan, A. C., Campos, B. M., Yasuda, C. L., Oliveira, M. C., Morita, M. E., & Cendes, F. (2016). Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia, 57(4), 621–629. https://doi.org/10.1111/epi.13334
Benedek, K., Juhasz, C., Chugani, D. C., Muzik, O., & Chugani, H. T. (2006). Longitudinal changes in cortical glucose hypometabolism in children with intractable epilepsy. Journal of Child Neurology, 21(1), 26–31. https://doi.org/10.1177/08830738060210011101
Bernhardt, B. C., Kim, H., & Bernasconi, N. (2013). Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology, 81(21), 1840–1847. https://doi.org/10.1212/01.wnl.0000436069.20513.92
Bernhardt, B. C., Worsley, K. J., Kim, H., Evans, A. C., Bernasconi, A., & Bernasconi, N. (2009). Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology, 72(20), 1747–1754. https://doi.org/10.1212/01.wnl.0000345969.57574.f5
Carne, R. P., O'Brien, T. J., Kilpatrick, C. J., MacGregor, L. R., Hicks, R. J., Murphy, M. A., . . . Cook, M. J. (2004). MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain, 127(Pt 10), 2276-2285. https://doi.org/10.1093/brain/awh257
Chugani, H. T., Mazziotta, J. C., Engel, J., Jr., & Phelps, M. E. (1987). The Lennox-Gastaut syndrome: Metabolic subtypes determined by 2-deoxy-2[18F]fluoro-D-glucose positron emission tomography. Annals of Neurology, 21(1), 4–13. https://doi.org/10.1002/ana.410210104
Conz, L., Morita, M. E., Coan, A. C., Kobayashi, E., Yasuda, C. L., Pereira, A. R., . . . Cendes, F. (2011). Longitudinal MRI volumetric evaluation in patients with familial mesial temporal lobe epilepsy. Frontiers in Neurology, 2, 5. https://doi.org/10.3389/fneur.2011.00005
Engel, J., Jr. (1984). The use of positron emission tomographic scanning in epilepsy. Annals of Neurology, 15(Suppl), S180-191. https://doi.org/10.1002/ana.410150735
Engel, J., Jr., Henry, T. R., Risinger, M. W., Mazziotta, J. C., Sutherling, W. W., Levesque, M. F., & Phelps, M. E. (1990). Presurgical evaluation for partial epilepsy: Relative contributions of chronic depth-electrode recordings versus FDG-PET and scalp-sphenoidal ictal EEG. Neurology, 40(11), 1670–1677. https://doi.org/10.1212/wnl.40.11.1670
Fuerst, D., Shah, J., Shah, A., & Watson, C. (2003). Hippocampal sclerosis is a progressive disorder: A longitudinal volumetric MRI study. Annals of Neurology, 53(3), 413–416. https://doi.org/10.1002/ana.10509
Gaillard, W. D., Bhatia, S., Bookheimer, S. Y., Fazilat, S., Sato, S., & Theodore, W. H. (1995). FDG-PET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology, 45(1), 123–126. https://doi.org/10.1212/wnl.45.1.123
Gaillard, W. D., Kopylev, L., Weinstein, S., Conry, J., Pearl, P. L., Spanaki, M. V., . . . Theodore, W. H. (2002). Low incidence of abnormal (18)FDG-PET in children with new-onset partial epilepsy: a prospective study. Neurology, 58(5), 717-722. https://doi.org/10.1212/wnl.58.5.717
Galovic, M., van Dooren, V. Q. H., Postma, T., Vos, S. B., Caciagli, L., Borzi, G., . . . Koepp, M. J. (2019). Progressive Cortical Thinning in Patients With Focal Epilepsy. JAMA Neurology. https://doi.org/10.1001/jamaneurol.2019.1708
Goncalves Pereira, P. M., Insausti, R., Artacho-Perula, E., Salmenpera, T., Kalviainen, R., & Pitkanen, A. (2005). MR volumetric analysis of the piriform cortex and cortical amygdala in drug-refractory temporal lobe epilepsy. AJNR. American Journal of Neuroradiology, 26(2), 319–332.
Jokeit, H., Ebner, A., Arnold, S., Schuller, M., Antke, C., Huang, Y., . . . Witte, O. W. (1999). Bilateral reductions of hippocampal volume, glucose metabolism, and wada hemispheric memory performance are related to the duration of mesial temporal lobe epilepsy. Journal of Neurology, 246(10), 926-933. https://doi.org/10.1007/s004150050484
Jupp, B., Williams, J., Binns, D., Hicks, R. J., Cardamone, L., Jones, N., . . . O'Brien, T. J. (2012). Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia, 53(7), 1233-1244. https://doi.org/10.1111/j.1528-1167.2012.03525.x
Liu, R. S., Lemieux, L., Bell, G. S., Sisodiya, S. M., Bartlett, P. A., Shorvon, S. D., . . . Duncan, J. S. (2002). The structural consequences of newly diagnosed seizures. Annals of Neurology, 52(5), 573-580. https://doi.org/10.1002/ana.10338
Liu, Y. R., Cardamone, L., Hogan, R. E., Gregoire, M. C., Williams, J. P., Hicks, R. J., . . . Bouilleret, V. (2010). Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. Journal of Nuclear Medicine, 51(11), 1788-1795. https://doi.org/10.2967/jnumed.110.078626
Nightscales, R., McCartney, L., Auvrez, C., Tao, G., Barnard, S., Malpas, C. B., . . . O'Brien, T. J. (2020). Mortality in patients with psychogenic nonepileptic seizures. Neurology, 95(6), e643-e652. https://doi.org/10.1212/WNL.0000000000009855
O'Brien, T. J., Hicks, R. J., Ware, R., Binns, D. S., Murphy, M., & Cook, M. J. (2001). The utility of a 3-dimensional, large-field-of-view, sodium iodide crystal--based PET scanner in the presurgical evaluation of partial epilepsy. Journal of Nuclear Medicine, 42(8), 1158-1165
O’Brien, T. J., Miles, K., Ware, R., Cook, M. J., Binns, D. S., & Hicks, R. J. (2008). The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. Journal of Nuclear Medicine, 49(6), 931–937. https://doi.org/10.2967/jnumed.107.048207
Sakaguchi, Y., Kidokoro, H., Ogawa, C., Okai, Y., Ito, Y., Yamamoto, H., . . . Natsume, J. (2018). Longitudinal Findings of MRI and PET in West Syndrome with Subtle Focal Cortical Dysplasia. AJNR. American Journal of Neuroradiology, 39(10), 1932-1937. https://doi.org/10.3174/ajnr.A5772
Shukla, G., & Prasad, A. N. (2012). Natural history of temporal lobe epilepsy: Antecedents and progression. Epilepsy Research and Treatment, 2012, 195073. https://doi.org/10.1155/2012/195073
Spencer, S. S. (1994). The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia, 35(Suppl 6), S72-89. https://doi.org/10.1111/j.1528-1157.1994.tb05990.x
Stephen, L. J., Kwan, P., & Brodie, M. J. (2001). Does the cause of localisation-related epilepsy influence the response to antiepileptic drug treatment? Epilepsia, 42(3), 357–362. https://doi.org/10.1046/j.1528-1157.2001.29000.x
Theodore, W. H. (2003). Magnetic Resonance Imaging of Familial Temporal Lobe Epilepsy. Epilepsy Currents, 3(2), 42–43. https://doi.org/10.1046/j.1535-7597.2003.03203.x
Theodore, W. H., Schulman, E. A., & Porter, R. J. (1983). Intractable seizures: Long-term follow-up after prolonged inpatient treatment in an epilepsy unit. Epilepsia, 24(3), 336–343. https://doi.org/10.1111/j.1528-1157.1983.tb04897.x
Vinton, A. B., Carne, R., Hicks, R. J., Desmond, P. M., Kilpatrick, C., Kaye, A. H., & O’Brien, T. J. (2007). The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain, 130(Pt 2), 548–560. https://doi.org/10.1093/brain/awl232
Vivash, L., Gregoire, M. C., Lau, E. W., Ware, R. E., Binns, D., Roselt, P., . . . O'Brien, T. J. (2013). 18F-flumazenil: a gamma-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. Journal of Nuclear Medicine, 54(8), 1270-1277. https://doi.org/10.2967/jnumed.112.107359
Funding
This project was not supported by any specific grant or other external party. PK is supported by a Medical Research Future Fund Fellowship (MRF1136427).
Author information
Authors and Affiliations
Contributions
CS drafted the manuscript. CS and BS were responsible for data collection, analysis and interpretation. RH supervised the FDG-PET image acquisitions and contributed to data interpretation. PK and TOB contributed to data analysis and interpretation. LV conceived the study and contributed to data analysis and interpretation. All authors edited the manuscript and approved the final submitted version.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Melbourne Health Human Research Ethics Committee (QA2012044). The study was conducted in accordance with this approval and ICH GCP.
Consent to participate
In accordance with the HREC approval, specific consent to participate was not sought from patients.
Consent for publication
Not applicable.
Conflict of interest
The authors have no conflicts to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharpe, C., Sinclair, B., Kwan, P. et al. Longitudinal changes of focal cortical glucose hypometabolism in adults with chronic drug resistant temporal lobe epilepsy. Brain Imaging and Behavior 15, 2795–2803 (2021). https://doi.org/10.1007/s11682-021-00576-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-021-00576-8