Abstract
Deficit schizophrenia (DS) is a distinct subtype of schizophrenia characterized by primary and enduring negative symptoms. More severe executive dysfunctions were observed in DS patients, however, the associated neuroimaging characteristics, especially cerebellar functional anomalies in DS, remain largely unknown. We employed resting-state functional and structural MRI data of 106 male participants, including data from 29 DS patients, 39 non-deficit schizophrenia (NDS) patients and 38 healthy controls (HCs). Z-standardized fractional amplitude of low-frequency fluctuation (zfALFF) values were calculated in order to examine spontaneous regional brain activity. Cerebro-cerebellar functional connectivity and changes in the volume of gray matter in the cerebellum were also examined. Relative to the HCs, both DS and NDS patients exhibited decreased zfALFF in the bilateral cerebellar lobules VIII and IX. The zfALFF in the left Crus II was lower in DS patients compared to NDS patients. No significant difference was observed in the volume of cerebellar gray matter among the three groups. Compared with NDS patients, cerebro-cerebellar functional connectivity analysis revealed increased connectivity in the left orbital medial frontal cortex and right putamen regions in DS patients. Reduced zfALFF in the left Crus II in the DS group was significantly positively correlated with Stroop Color and Word scores, while negatively correlated with Trail-Making Test part B scores. The increased functional connectivity in the right putamen in DS patients was significantly positively correlated with Animal Naming Test and semantic Verbal Fluency Test scores. These results highlight cerebellar functional abnormality in DS patients and provide insight into the pathophysiological mechanism of executive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deficit schizophrenia (DS) is characterized by a clinically homogeneous subgroup of patients exhibiting primary and enduring negative symptoms that are trait-like features from the onset and during periods of clinical stability (Carpenter et al. 1988). Evidence suggests that DS can be distinguished from non-deficit schizophrenia (NDS) through clinical symptoms, course of illness, pathophysiological correlates, risk and etiological factors, and response to treatment (Kirkpatrick and Galderisi 2008). In particular, DS patients suffer more severe and widespread neurocognitive impairment associated with the primary negative symptoms than do NDS patients (Yu et al. 2015b; Rethelyi et al. 2012; Cohen et al. 2007a). Studies focusing on the different cognitive components such as executive functioning (Polgar et al. 2010a), ideational fluency domain (Cascella et al. 2008) and sustained attention (Yu et al. 2015a) have provided evidence that DS patients exhibit impoverished cognitive performance. A recent meta-analysis of a large number of patients concluded that global cognition was significantly impaired in DS compared with NDS and that DS patients exhibit severe deficit in social cognition, olfaction, verbal fluency and other speed-based cognitive tasks, suggesting that the evidence is consistent with a relationship between deficit syndrome and patients’ more severe cognitive impairment (Bora et al. 2017). The neuropsychological findings described above emphasize the fact that deficit syndrome might be a specific subgroup within schizophrenia which demands investigation of underlying functional brain features and etiopathogenic mechanisms.
Executive functions are generally referred to as “supervisory” cognitive processes and defined as the ability to utilize higher-level cognitive processes to control and coordinate more elementary cognitive processes (Alvarez and Emory 2006). Since any component can be impaired in schizophrenic patients, executive dysfunctions are considered central features of the cognitive deficit in schizophrenia (Kerns et al. 2008). Early studies reported that deficit performance on executive functions was associated with negative symptoms (Goldberg and Weinberger 1988), with inconsistencies highlighted by Zakzanis who found that positive schizophrenic symptomatology was related to frontal executive tasks rather than negative symptomatology (Zakzanis 1998). These contradictory reports might be due to the heterogeneity of schizophrenia. Research has confirmed the view that DS patients, thought to be a homogeneous group of schizophrenic individuals, suffer a more severe impairment of executive functioning that is sensitive to frontal lobe dysfunction than do NDS patients (Galderisi et al. 2002; Polgar et al. 2010b; Bryson et al. 2001). Interestingly, a neurodevelopmental animal model study suggested that executive functions were disrupted in adult rats when lesions were caused in their developing frontal cortex (Desai et al. 2017), possibly leading to adult-onset negative symptom-like features (Lazar et al. 2008). However, the neural mechanisms underlying executive dysfunction in DS still remain unclear.
Resting-state and task-based functional MRI have been widely used to investigate abnormal spontaneous regional brain activity associated with executive dysfunctions in schizophrenia (Su et al. 2013; Poppe et al. 2016). A number of focal brain regions (Delamillieure et al. 2004) and functional connectivity architectures (Yu et al. 2017) were considered to have critical involvement in executive processes, for instance, deficit in activating the dorsolateral prefrontal cortex (DLPFC) and connectivity abnormalities from DLPFC to the whole brain might contribute to executive dysfunctions in schizophrenia (Su et al. 2013). The cerebellum is traditionally recognized for its involvement in motor coordination and learning, with recent evidence demonstrating its potential role in emotion and cognitive processing (Nguyen et al. 2017; Guell et al. 2018). Abnormalities in the cerebellum and cerebro-cerebellar circuits contribute to the etiology of autism spectrum disorder (ASD) (D'Mello and Stoodley 2015), characterized by a deficit in executive function, suggesting that cerebellar dysfunction is associated with executive function. Intriguingly, the cerebellum and multiple regions of the cerebral cortex with which the cerebellum forms reciprocal connections (S. S. Wang et al. 2014) have been found to be disrupted in subjects suffering neurodevelopmental disorders (Stoodley 2014, 2016; Stoodley and Limperopoulos 2016). These observations raise the intriguing hypothesis that cerebellar anomalies might perform an important role in executive dysfunction in deficit schizophrenia, which is considered an early onset non-progressive developmental process characterized by insufficient acquisition of basic cognitive and social skills (Galderisi et al. 2008). Recent research has reported that reduced cerebellar gray matter volume (GMV) was observed in schizophrenic patients with persistent auditory verbal hallucinations compared to full remission patients (Cierpka et al. 2017), indicating that a subgroup of schizophrenic patients might exhibit characteristic cerebellar anomalies consistent with clinical symptoms. To date, the functional abnormalities of the cerebellum and broader functional connectivity in cerebro-cerebellar circuits across deficit schizophrenia patients remain largely unknown. Furthermore, no studies have reported whether a potential correlation exists between cerebellar alterations and executive dysfunctions in DS patients.
The present study addresses these issues by employing resting-state fMRI and fractional amplitude of low-frequency fluctuations (fALFF) as an indicator to delineate spontaneous regional brain activity. A voxel-based morphometry (VBM) analysis of the cerebellum was performed to exclude the probable impact of comparisons of structural alterations between groups. Functional connectivity (FC) matrices were calculated to explore the altered pattern of cerebro-cerebellar circuits. Our primary hypothesis was that there would be different functional alterations in the cerebellum and cerebro-cerebellar connectivity in DS and NDS patients. Subsequent analyses, dependent on the outcome of the primary hypothesis, explored the proposition that any such differences would be associated with executive dysfunction and psychiatric symptoms in patients.
Materials and methods
Participants
A total of 68 patients with schizophrenia (29 DS and 39 NDS) and 38 healthy subjects participated in the present study. The schizophrenic patients were recruited from the psychiatric rehabilitation unit of Yangzhou Wutaishan Hospital, Jiangsu province, China, based on approval by the Institutional Ethics Committee for clinical research. Inclusion and exclusion criteria are described in the supplementary material. DS and NDS were diagnosed using the Chinese version of the Schedule for Deficit Syndrome (SDS) (X. Wang et al. 2008). Healthy controls were selected from the community by matching patients for age and handedness, excluding individuals with a family history of psychiatric disorders.
Clinical assessments
The Brief Psychiatric Rating Scale (BPRS), which is organized into separate positive, negative, disorganized and affective syndromes based on the scoring of 18 symptoms (Mueser et al. 1997; Cohen et al. 2007b), was used to evaluate the full range of schizophrenic symptomatology. For the purposes of comprehensive reflection of negative symptoms, the Scale for the Assessment of Negative Symptoms (SANS) was utilized (Andreasen 1982). Excluding global items, the SANS currently consists of 19 items, representing 5 rationally-derived domains: Affective Flattening or Blunting, Alogia, Avolition-Apathy, Anhedonia-Asociality and Inattention. The Scale for the Assessment of Positive Symptoms (SAPS) was used to assess the positive symptoms of patients.
Neurocognitive assessments
Neurocognitive performance was assessed using a brief cognitive battery of tests focusing on executive functioning. The following measures were included, corresponding to the various components of executive function (i.e., attention switching, prepotent inhibition, ideational fluency and mental flexibility): Trail-Making Test (TMT), Animal Naming Test (ANT), semantic Verbal Fluency Test (VFT) and Stroop Color-word Test (SCWT). The details of neurocognitive assessments are described in the supplementary material.
Image analysis
MRI data acquisition
All MRI images of patients and HCs were obtained using a 3.0 T MRI scanner (GE HDx) using an 8-channel phased array head coil located in the Subei Hospital of Jiangsu Province, Yangzhou, China. Resting state fMRI images were acquired using a gradient recalled echo echo-planar imaging (GRE-EPI) sequence with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 25 ms; flip angle = 90°; field of view (FOV) = 240 × 240 mm2; matrix size = 64 × 64; thickness = 4 mm without gap; voxel size = 4.0 × 4.0 × 4.0 mm3; slice number = 35; and number of time points = 240. All subjects were required to lie quietly with eyes closed in a wakeful state during MRI scans. In order to stabilize the magnets, each run was preceded with 6-s dummy scans.
T1 weighted structural images were acquired using a three-dimensional brain volume imaging (3D-BRAVO) sequence with high resolution, using the following parameters: TR = 11.94 ms; TE = 5.044 ms; flip angle = 15°; FOV = 240 × 240 mm; matrix size = 256 × 256; slice thickness = 1 mm without gap; voxel size = 1 × 1 × 1 mm3; slice number = 172. Images were checked for artifacts caused by head motions and incomplete scans covering the whole brain volume.
Imaging data preprocessing
Image data preprocessing was performed using Statistical Parametric Mapping version 12 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12) and the Data Processing and Analysis for Brain Imaging (DPABI) toolkit (Yan et al. 2016). The first 10 volumes of each image were excluded to account for magnetization equilibrium. Slice timing was performed to correct for the acquisition time delay of the remaining 230 volumes, with the volumes realigned to the first volume to correct for head motion. The threshold for head motion was set as 3 mm in translation and 3° in rotation. Native T1 images were segmented and co-registered to resting state functional images, then spatially normalized to the standard space of the Montreal Neurological Institute (MNI) using an optimum 12-parameter affine transformation and nonlinear deformations. The functional images were subsequently normalized to MNI space according to the transformation parameters obtained from the structural images and further resampled to an isolated resolution of a 3 × 3 × 3 mm cubic voxel. The resulting functional images were smoothed using a Gaussian kernel with a full-width at half maximum (FWHM) of 4 mm. Any linear drift of the time course for each voxel was removed and a number of nuisance signals (including Friston’s 24 head motion parameters, cerebrospinal fluid signal, white matter signal and global mean signal) were regressed out.
Spontaneous neural activity analysis and gray matter volume analysis
Since low frequency fluctuations between 0.01 Hz and 0.08 Hz are particularly relevant to resting state fMRI (Biswal et al. 1995), the fractional amplitude of low-frequency fluctuations (fALFF) was calculated to examine spontaneous regional brain activity (Zang et al. 2007). After preprocessing, the time series for each voxel was filtered (bandpass, 0.01–0.08 Hz) to remove the effects of low-frequency drift and high-frequency noise. The filtered time series was transformed to a frequency domain using a fast Fourier transform (FFT) to obtain a power spectrum. ALFF was calculated as the sum of amplitudes in the low frequency range, with fALFF calculated from (ALFF/amplitude of the whole frequency range), after image data preprocessing. For standardization purposes, the value of fALFF for each voxel was z-normalized across the full brain for each subject. The zfALFF value within the cerebellum was then extracted for analysis. Calculation of zfALFF and additional intra-group comparisons was restricted to be within a spatially unbiased infratentorial template (SUIT) mask.
Cerebellum-optimized segmentation techniques were employed to investigate differences in the cerebellar gray matter volume (GMV) between the DS, NDS and HC groups. See supplementary material for additional details.
Cerebro-cerebellar resting-state functional connectivity analysis
A seed-based functional connectivity (FC) technique was employed to detect differences in cerebro-cerebellar resting-state FC patterns between the DS, NDS and HC groups. The regions presenting significant group differences in their zfALFF values were selected as regions of interest (ROIs). Seed ROIs were defined as 6 mm radius spheres with their averaged time course extracted for FC analysis using DPABI software. Correlation maps were generated for each seed by calculating the Pearson correlation coefficients between the time course of the ROI and other voxels of the whole brain. A Fisher’s r-to-z transformation was performed to improve normality of the correlation maps thus obtained.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (SPSS Inc., USA). Continuous variables such as demographic, clinical characteristics and neurocognitive assessment were presented as mean ± standard deviation (SD). Differences among DS, NDS and HC groups were calculated through one-way analysis of variance (ANOVA) and post-hoc pairwise comparison analyses. Differences in psychiatric symptoms between the DS and NDS groups were established using two-sample t tests.
To determine the group differences in cerebellar zfALFF, GMV and cerebro-cerebellar resting state FC, nonparametric permutation tests were performed between each pair of the DS, NDS and HC groups (Nichols and Holmes 2002; Winkler et al. 2014). This procedure was accomplished using the Statistical non-Parametric Mapping (SnPM) toolbox (http://warwick.ac.uk/snpm) running under SPM12. Pseudo t-statistic images were constructed using the SnPM toolbox to determine significance using a standard nonparametric multiple comparisons procedure based on permutation testing. The significance level was set at a threshold of 0.01 for cluster-forming with family-wise error (FWE)-corrected clusters considered significant for p < 0.05 and 5000 permutation times for each test. The statistical analyses of cerebellar zfALFF and GMV were performed within the SUIT-mask, with cerebro-cerebellar resting-state FC conducted in the whole brain mask. Age and years of education were treated as covariates in all intra-group statistical analyses. Once significant group differences were observed in cerebellar zfALFF or whole brain FC analyses, the relationships between these image metrics and the neurocognitive variables of executive function were assessed. Pearson’s partial correlation analyses were calculated with age and number of years of education as covariates in the DS and NDS groups, respectively. The statistical significance level was set as p < 0.05.
Results
Demographic and clinical characteristics
The ANOVA analyses demonstrated significant differences in years of education of patients among the DS, NDS and HC groups. The DS group had the shortest period of education with no significant difference detected between the two patient subgroups. The age at onset, duration of illness and CPZ-equivalent dose did not differ significantly between the DS and NDS groups. DS patients demonstrated a more severe syndrome in negative symptoms relative to NDS patients, but not in positive, disorganization or affective syndromes as assessed by BPRS. The SDS and SANS scores were higher in DS patients than NDS patients, and significantly higher in the 5 rationally-derived domains of SANS, namely affective flattening, alogia, avolition-apathy, anhedonia-asociality and inattention (Table 1).
Neurocognitive characteristics
Considering age and years of education as covariates, each neurocognitive test displayed significant differences among the 3 groups. Bonferroni post hoc comparisons indicated that both patient groups performed significantly worse than the HC group on each neurocognitive test. Furthermore, the DS group demonstrated more severe impairment in the majority of the neurocognitive measures (all p’s < 0.05), except the Stroop-interference test (t = −2.076, p = 0.121) (Table 2).
Comparison of cerebellar zfALFF and GMV among three groups
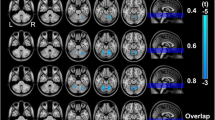
For each neuroimaging analysis comparison, age and education were considered as covariates. All schizophrenic patients exhibited significantly decreased zfALFF relative to healthy controls in the bilateral cerebellar lobule VIII/IX, the left lobule IV/V and vermis IV/V. Further pairwise comparisons showed significantly decreased zfALFF in the bilateral cerebellar lobules VIII/IX and left Crus II in DS patients, and significantly decreased zfALFF in the right lobules VIII/IX, left lobule VIII, left lobules IV/V and vermis IV/V, compared with HCs. The zfALFF in the left Crus II was lower in patients with DS compared to those with NDS (Fig. 1 and Supplementary eTable 1).
Altered cerebellar zfALFF in DS and NDS patients. Between-group differences in zfALFF in the cerebellum. Blue and yellow clusters denote regions with significantly decreased and increased zfALFF. The color bar indicates the T value of comparison within groups. DS: deficit schizophrenia; NDS: non-deficit schizophrenia; HC: healthy control
Compared to HCs, schizophrenia patients demonstrated no significant difference in cerebellar GMV. Moreover, no significant difference in cerebellar gray volume was detected between the DS and NDS groups (Supplementary eFigure 1).
Abnormal cerebro-cerebellar functional connectivity in DS and NDS patients
The cerebellar region demonstrated significant differences between DS and NDS groups, with the left Crus II chosen as the seed for whole brain functional connectivity analysis. Considering age and education as covariates, statistical T-mapping demonstrated that functional connectivity in the left orbital medial frontal cortex and right putamen increased significantly in DS patients relative to NDS patients (Fig. 2a and Supplementary eTable 2).
Altered cerebro-cerebellar functional connectivity and its relationship with executive functions. (a) The pink cluster shows the region chosen as ROI seed. Red clusters denote the regions significantly increased in functional connectivity. The color bar indicates the T value. (b) Scatter plots of cerebro-cerebellar functional connectivity and executive functions (ANT and VFT) in the DS and NDS groups, respectively. DS: deficit schizophrenia; NDS: non-deficit schizophrenia; HC: healthy control; ANT: Animal Naming Test; VFT: semantic Verbal Fluency Test
Correlation between cerebellar functional measurements, clinical symptoms and executive function
After controlling for age and education, partial correlation analysis revealed that zfALFF in the left cerebellar Crus II was significantly positively correlated with the Stroop-color scores (r = 0.502, p = 0.008) in addition to Stroop-word scores (r = 0.386, p = 0.047), and negatively correlated with TMT-B scores (r = −0.488, p = 0.01) in DS patients. There was a significant negative correlation between the left Crus II zfALFF and SANS-inattention subscale scores (r = −0.495, p = 0.016) in the DS patients (Fig. 3). For seed-based cerebro-cerebellar connectivity analysis, increased functional connectivity in the right putamen in DS patients was significantly positively correlated with ANT (r = 0.406, p = 0.036) and VFT (r = 0.416, p = 0.031) scores (Fig. 2b). No significant correlation was found between cerebellar regional changes and clinical or executive function variables in NDS patients.
Correlation between zfALFF and behavioral data in DS and NDS patients. (a) The left Crus II was chosen as an ROI seed to extract zfALFF values. (b) Scatter plots of zfALFF and inattention subscale scores of the Assessment of Negative Symptoms (SANS) in the DS and NDS groups, respectively. (c) Scatter plots of zfALFF and executive functions (TMT-B, Stroop-word and Stroop-color) in the DS and NDS groups, respectively. DS: deficit schizophrenia; NDS: non-deficit schizophrenia; HC: healthy control. TMT-B: Trail-Making Test B
Discussion
In the present study, we investigated abnormalities of functional and structural alterations in the cerebellum in addition to the relationship between cerebellar activity and executive dysfunctions in chronic schizophrenic patients. To conceptualize the primary negative symptoms, we divided schizophrenic patients into DS and NDS subgroups and explored the specific difference in neuro-cognition and cerebellar alterations between these two groups. To our knowledge, this is the first neuroimaging study comparing functional cerebellar activity and cerebro-cerebellar connectivity in DS and NDS patients relative to HCs. We principally found lower zfALFF values in the left Crus II in DS compared with NDS which was significantly correlated with a number of components of executive function (TMT-B, Stroop-color, Stroop-word) and SANS subscale scores (Inattention) only in the DS group. No significant change in cerebellar volume was observed in patients with schizophrenia relative to healthy subjects, and no difference between DS and NDS groups, suggesting that altered cerebellar neural activity is independent of anatomical constraint. Furthermore, we demonstrated that DS patients exhibited increased functional connectivity between the cerebellar seed and cerebral regions in the left orbital medial frontal cortex and right putamen, which might be a salient feature of the cerebro-cerebellar circuit compared with NDS patients.
We observed shared abnormal neural activity in bilateral cerebellum lobules VIII/IX, showing significantly decreased zfALFF in both DS and NDS subgroups relative to HC subjects. Decreased ALFF has previously been reported in the bilateral cerebellar posterior lobes in first episode and treatment-naïve DS and NDS patients (Li et al. 2017). Reliable evidence suggests that functional abnormalities in the cerebellar posterior lobe might be a general feature of schizophrenia. According to cerebellar anatomically-driven and self-organizing map-driven approaches, lobules VIII and IX have been shown to be highly connected with the cerebral default mode network (DMN) (Bernard et al. 2012; Buckner et al. 2011), which constitutes a vital component in the neurobiology of schizophrenia involved in self-referential and stream-of-consciousness processing (Raichle et al. 2001). Given that interaction of cerebellar maps with cerebral association areas has been established (Bernard et al. 2012), this finding of change in functional activity common to cerebellar lobules VIII and IX might be consistent with disruption to DMN connectivities in schizophrenic patients. Our results showed no significant difference in cerebellar GMV in schizophrenic patients compared with healthy subjects, while other studies confirmed the existence of structural abnormality of cerebellum posterior pyramis in the early stages of the disease (Zhang et al. 2017). The lack of significance in this finding might be due to restricted sample size or diffeomorphic anatomical registration (DARTEL-VBM) in assessing changes in cerebellar volume. In addition, the antipsychotic treatment might be a compensatory response to cerebellum structure similar to a study reported previously in a major depressive disorder (Yucel et al. 2013). Another noteworthy observation is the reduction in cerebellar volume in schizophrenia which was highly consistent across all ages, and already present in the youngest patients (Moberget et al. 2018). In this case, it can be seen that alteration of cerebellar grey matter volume in schizophrenia patients compared with controls does not change linearly with age. Stratification analyses with multiple age groups and larger sample sizes might further clarify this divergence. The results above indicate that a decrease in neural activity in posterior cerebellum lobules VIII and IX might be a common feature in schizophrenia and independent of structural change.
Decreased functional activity (zfALFF) in the left cerebellar Crus II was observed in DS patients compared to those with NDS. Furthermore, specific correlations between decreased zfALFF and executive dysfunctions (TMT-B, Stroop-color, Stroop-word) were observed in the DS group but absent in the NDS group. This is consistent with Romanowshi’s research where they found anatomical evidence of volumetric reduction in Crus I/II in schizophrenic patients, where volume deficits in this region were associated with thought disorder and performance in TMT-B (Kuhn et al. 2012). Kim et al., using diffusion tensor imaging and graph theoretical analysis, revealed through cerebellar modular architecture research that Crus II was found to be a particularly altered region with vermis Crus II in schizophrenic patients compared with healthy controls (Kim et al. 2014). It is already known that the region near Crus I/II where Purkinje cells are primarily located in the cerebellum both sends and receives information from the prefrontal cortex associated with higher cognitive functions in Cebus apella monkeys (Kelly and Strick 2003). Concomitantly, the disrupted functional activity in the Crus II region may contribute to the more broad and severe neurocognitive dysfunction that depends on the anatomical substrate of the cortico-cerebellar closed-loop circuit in DS patients. Functional imaging research has established that the cerebellar Crus I/II regions are part of the executive control network (ECN) (Habas et al. 2009), required for the selection and maintenance of relevant information in working memory necessary for the planning of actions (Seeley et al. 2007), in agreement with previous anatomical cortico-cerebellar circuit studies. The present results highlight distinct functional abnormalities in cerebellar Crus II, which might be the neuropathological mechanism that causes more severe executive dysfunctions in DS patients.
DS patients were presented with elevated cerebro-cerebellar resting-state functional connectivity in the cerebral regions of the left orbital medial frontal cortex and right putamen. A pattern similar to that of the cerebellar regions, which exhibit increased functional connectivity with the prefrontal cortex and subcortical nuclei in schizophrenia, has been reported previously, indicating a disruption of the cerebellar-subcortical-cortical loop (Zhuo et al. 2018). The hyper-connectivity in the right putamen exhibited positive correlations with executive function (e.g. ANT and VFT), in agreement with findings by Li (Li et al. 2017), who demonstrated significantly increased ALFF in the right putamen in DS patients relative to NDS patients. Many studies have pointed to increased volume in the caudate, putamen and pallidum in chronic schizophrenic patients (Gur et al. 1998; Mamah et al. 2007; Buchsbaum et al. 2003), suggesting an important role for these structures in the pathophysiology of schizophrenia. The putamen is located in the rostral region of the striatum and considered a relay station between the cortex and basal ganglia nuclei, associating with cognitive functions such as attention, working memory and goal-directed behavior (Middleton and Strick 1994). In the present study, we found that the increased functional connectivity (FC) between the cerebellar Crus II and putamen was positively correlated with executive function in the DS group, which suggests a plausible compensatory response of the cerebro-cerebellar circuit corresponding to functional abnormalities in the cerebellum and executive dysfunctions. The cerebellum is interconnected with the contralateral cerebrum primarily through polysynaptic circuits, possessing complex connectivity with multiple subcortical structures including the vestibular nuclei and basal ganglia (Buckner 2013). Combined with anatomical evidence, our results support the hypothesis that the cerebro-cerebellar circuit plays a vital role in cognitive processing (Alalade et al. 2011). In addition, evidence of intrinsic FC has revealed that the cerebellum was coupled to specific cerebral networks via an emergent property of the closed-loop circuit (Buckner et al. 2011). According to this circuit, the reduction in neural activity in the cerebellar region might be compensated for by enhanced FC with special cerebral regions. Furthermore, the putamen is located in dopamine-rich dorsal striatum in schizophrenia. Previous research has reported dopamine hypofunction in patients with negative symptoms (Pogarell et al. 2012). Therefore, we speculate that elevated FC between the cerebellum and putamen may compensate for the deficit in dopamine metabolism in DS patients.
Several limitations should be considered. Firstly, the recruited patients were all chronic male inpatients, receiving antipsychotic treatment. Because diagnosis of disease (Cui et al. 2016) and receipt of antipsychotic medication (Hu et al. 2016) in schizophrenia patients may contribute to changes in functional neural activity in resting-state MRI, we should consider the influence of these confounding factors. In the present study, clinical status and dosage of antipsychotic drugs were matched stringently in DS and NDS groups, which should strongly increase the reliability of cerebellar functional alterations between the two patient groups. Secondly, due to the cross-sectional study design, we could not determine whether observed cerebellar abnormalities and executive dysfunctions had a causality relationship. A longitudinal study design with multiple neuroimaging measurements such as task functional MRI should be implemented in future studies. Thirdly, because of the inevitable consequence of strict diagnostic criteria for DS and the need to restrict variance by eliminating or minimizing confounders, it should be emphasized that the present sample size was relatively small. The finding in the present results that only the Inattention subscale of SANS was significantly correlated with zfALFF in Crus II might arise from the restricted sample size. Considered as exploratory research, the present study potentially indicates a role of the cerebellum in DS patients. In a future study, we would enlarge the sample size to increase statistical power.
In conclusion, the present study firstly provided evidence for altered spontaneous cerebellar activity in the left Crus II and increased functional connectivity of the cerebro-cerebellar circuit in DS patients, indicating that subjects with deficit syndrome belong to a specific subgroup within schizophrenia. The characteristic changes in cerebellar imaging of DS might involve a neural mechanism of executive dysfunctions. These findings contribute to the development of therapeutic target selection for neurocognition deficit combined with negative symptoms using transcranial magnetic stimulation or direct cortical stimulation.
References
Alalade, E., Denny, K., Potter, G., Steffens, D., & Wang, L. (2011). Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS One, 6(5), e20035. https://doi.org/10.1371/journal.pone.0020035.
Alvarez, J. A., & Emory, E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16(1), 17–42. https://doi.org/10.1007/s11065-006-9002-x.
Andreasen, N. C. (1982). Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry, 39(7), 784–788.
Bernard, J. A., Seidler, R. D., Hassevoort, K. M., Benson, B. L., Welsh, R. C., Wiggins, J. L., Jaeggi, S. M., Buschkuehl, M., Monk, C. S., Jonides, J., & Peltier, S. J. (2012). Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Frontiers in Neuroanatomy, 6, 31. https://doi.org/10.3389/fnana.2012.00031.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using Echo-planar Mri. Magnetic Resonance in Medicine, 34(4), 537–541. https://doi.org/10.1002/mrm.1910340409.
Bora, E., Akdede, B. B., & Alptekin, K. (2017). Neurocognitive impairment in deficit and non-deficit schizophrenia: A meta-analysis. Psychological Medicine, 47(14), 2401–2413. https://doi.org/10.1017/S0033291717000952.
Bryson, G., Whelahan, H. A., & Bell, M. (2001). Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Research, 102(1), 29-37, doi:Doi. https://doi.org/10.1016/S0165-1781(01)00245-1.
Buchsbaum, M. S., Shihabuddin, L., Brickman, A. M., Miozzo, R., Prikryl, R., Shaw, R., & Davis, K. (2003). Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophrenia Research, 64(1), 53–62.
Buckner, R. L. (2013). The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron, 80(3), 807–815. https://doi.org/10.1016/j.neuron.2013.10.044.
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C., & Yeo, B. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(5), 2322–2345. https://doi.org/10.1152/jn.00339.2011.
Carpenter, W. T., Jr., Heinrichs, D. W., & Wagman, A. M. (1988). Deficit and nondeficit forms of schizophrenia: The concept. The American Journal of Psychiatry, 145(5), 578–583. https://doi.org/10.1176/ajp.145.5.578.
Cascella, N. G., Testa, S. M., Meyer, S. M., Rao, V. A., Diaz-Asper, C. M., Pearlson, G. D., & Schretlen, D. J. (2008). Neuropsychological impairment in deficit vs. non-deficit schizophrenia. Journal of Psychiatric Research, 42(11), 930–937. https://doi.org/10.1016/j.jpsychires.2007.10.002.
Cierpka, M., Wolf, N. D., Kubera, K. M., Schmitgen, M. M., Vasic, N., Frasch, K., & Wolf, R. C. (2017). Cerebellar contributions to persistent auditory verbal hallucinations in patients with schizophrenia. Cerebellum, 16(5–6), 964–972. https://doi.org/10.1007/s12311-017-0874-5.
Cohen, A. S., Saperstein, A. M., Gold, J. M., Kirkpatrick, B., Carpenter, W. T., & Buchanan, R. W. (2007a). Neuropsychology of the deficit syndrome: New data and meta-analysis of findings to date. Schizophrenia Bulletin, 33(5), 1201–1212. https://doi.org/10.1093/schbul/sbl066.
Cohen, A. S., Saperstein, A. M., Gold, J. M., Kirkpatrick, B., Carpenter, W. T., Jr., & Buchanan, R. W. (2007b). Neuropsychology of the deficit syndrome: New data and meta-analysis of findings to date. Schizophrenia Bulletin, 33(5), 1201–1212. https://doi.org/10.1093/schbul/sbl066.
Cui, L. B., Liu, K., Li, C., Wang, L. X., Guo, F., Tian, P., Wu, Y. J., Guo, L., Liu, W. M., Xi, Y. B., Wang, H. N., & Yin, H. (2016). Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophrenia Research, 173(1–2), 13–22. https://doi.org/10.1016/j.schres.2016.02.039.
Delamillieure, P., Constans, J. M., Fernandez, J., Brazo, P., & Dollfus, S. (2004). Relationship between performance on the Stroop test and N-acetylaspartate in the medial prefrontal cortex in deficit and nondeficit schizophrenia: Preliminary results. Psychiatry Research-Neuroimaging, 132(1), 87–89. https://doi.org/10.1016/j.pscychresns.2004.06.006.
Desai, S. J., Allman, B. L., & Rajakumar, N. (2017). Infusions of nerve growth factor into the developing frontal cortex leads to deficits in behavioral flexibility and increased perseverance. Schizophrenia Bulletin, 44, 1081–1090. https://doi.org/10.1093/schbul/sbx159.
D'Mello, A. M., & Stoodley, C. J. (2015). Cerebro-cerebellar circuits in autism spectrum disorder. Frontiers in Neuroscience, 9, 408. https://doi.org/10.3389/fnins.2015.00408.
Galderisi, S., Maj, M., Mucci, A., Cassano, G. B., Invernizzi, G., Rossi, A., et al. (2002). Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: A multicenter study. American Journal of Psychiatry, 159(6), 983–990. https://doi.org/10.1176/appi.ajp.159.6.983.
Galderisi, S., Quarantelli, M., Volpe, U., Mucci, A., Cassano, G. B., Invernizzi, G., Rossi, A., Vita, A., Pini, S., Cassano, P., Daneluzzo, E., de Peri, L., Stratta, P., Brunetti, A., & Maj, M. (2008). Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophrenia Bulletin, 34(2), 393–401. https://doi.org/10.1093/schbul/sbm097.
Goldberg, T. E., & Weinberger, D. R. (1988). Probing prefrontal function in schizophrenia with neuropsychological paradigms. Schizophrenia Bulletin, 14(2), 179–183.
Guell, X., Gabrieli, J. D. E., & Schmahmann, J. D. (2018). Triple representation of language, working memory, social and emotion processing in the cerebellum: Convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage, 172, 437–449. https://doi.org/10.1016/j.neuroimage.2018.01.082.
Gur, R. E., Maany, V., Mozley, P. D., Swanson, C., Bilker, W., & Gur, R. C. (1998). Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. The American Journal of Psychiatry, 155(12), 1711–1717. https://doi.org/10.1176/ajp.155.12.1711.
Habas, C., Kamdar, N., Nguyen, D., Prater, K., Beckmann, C. F., Menon, V., & Greicius, M. D. (2009). Distinct cerebellar contributions to intrinsic connectivity networks. The Journal of Neuroscience, 29(26), 8586–8594. https://doi.org/10.1523/JNEUROSCI.1868-09.2009.
Hu, M. L., Zong, X. F., Zheng, J. J., Pantazatos, S. P., Miller, J. M., Li, Z. C., Liao, Y. H., He, Y., Zhou, J., Sang, D. E., Zhao, H. Z., Lv, L. X., Tang, J. S., Mann, J. J., & Chen, X. G. (2016). Short-term effects of risperidone monotherapy on spontaneous brain activity in first-episode treatment-naive schizophrenia patients: A longitudinal fMRI study. Scientific Reports, 6, 34287. https://doi.org/10.1038/srep34287.
Kelly, R. M., & Strick, P. L. (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of Neuroscience, 23(23), 8432–8444.
Kerns, J. G., Nuechterlein, K. H., Braver, T. S., & Barch, D. M. (2008). Executive functioning component mechanisms and schizophrenia. Biological Psychiatry, 64(1), 26–33. https://doi.org/10.1016/j.biopsych.2008.04-027.
Kim, D. J., Kent, J. S., Bolbecker, A. R., Sporns, O., Cheng, H., Newman, S. D., Puce, A., O’Donnell, B. F., & Hetrick, W. P. (2014). Disrupted modular architecture of cerebellum in schizophrenia: A graph theoretic analysis. Schizophrenia Bulletin, 40(6), 1216–1226. https://doi.org/10.1093/schbul/sbu059.
Kirkpatrick, B., & Galderisi, S. (2008). Deficit schizophrenia: An update. World Psychiatry, 7(3), 143–147.
Kuhn, S., Romanowski, A., Schubert, F., & Gallinat, J. (2012). Reduction of cerebellar grey matter in crus I and II in schizophrenia. Brain Structure & Function, 217(2), 523–529. https://doi.org/10.1007/s00429-011-0365-2.
Lazar, N. L., Rajakumar, N., & Cain, D. P. (2008). Injections of NGF into neonatal frontal cortex decrease social interaction as adults: A rat model of schizophrenia. Schizophrenia Bulletin, 34(1), 127–136. https://doi.org/10.1093/schbul/sbm039.
Li, Z., Lei, W., Deng, W., Zheng, Z., Li, M., Ma, X., Wang, Q., Huang, C., Li, N., Collier, D. A., Gong, Q., & Li, T. (2017). Aberrant spontaneous neural activity and correlation with evoked-brain potentials in first-episode, treatment-naive patients with deficit and non-deficit schizophrenia. Psychiatry Research, 261, 9–19. https://doi.org/10.1016/j.pscychresns.2017.01.001.
Mamah, D., Wang, L., Barch, D., de Erausquin, G. A., Gado, M., & Csernansky, J. G. (2007). Structural analysis of the basal ganglia in schizophrenia. Schizophrenia Research, 89(1–3), 59–71. https://doi.org/10.1016/j.schres.2006.08.031.
Middleton, F. A., & Strick, P. L. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science, 266(5184), 458–461. https://doi.org/10.1126/science.7939688.
Moberget, T., Doan, N. T., Alnaes, D., Kaufmann, T., Cordova-Palomera, A., Lagerberg, T. V., et al. (2018). Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega-analysis of 983 patients and 1349 healthy controls. Molecular Psychiatry, 23(6), 1512–1520. https://doi.org/10.1038/mp.2017.106.
Mueser, K. T., Curran, P. J., & McHugo, G. J. (1997). Factor structure of the brief psychiatric rating scale in schizophrenia. Psychological Assessment, 9(3), 196–204. https://doi.org/10.1037/1040-3590.9.3.196.
Nguyen, V. T., Sonkusare, S., Stadler, J., Hu, X., Breakspear, M., & Guo, C. C. (2017). Distinct cerebellar contributions to cognitive-perceptual dynamics during natural viewing. Cerebral Cortex, 27(12), 5652–5662. https://doi.org/10.1093/cercor/bhw334.
Nichols, T. E., & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping, 15(1), 1–25. https://doi.org/10.1002/hbm.1058.
Pogarell, O., Koch, W., Karch, S., Dehning, S., Muller, N., Tatsch, K., et al. (2012). Dopaminergic neurotransmission in patients with schizophrenia in relation to positive and negative symptoms. Pharmacopsychiatry, 45(Suppl 1), S36–S41. https://doi.org/10.1055/s-0032-1306313.
Polgar, P., Rethelyi, J. M., Balint, S., Komlosi, S., Czobor, P., & Bitter, I. (2010a). Executive function in deficit schizophrenia: What do the dimensions of the Wisconsin card sorting test tell us? Schizophrenia Research, 122(1–3), 85–93. https://doi.org/10.1016/j.schres.2010.06.007.
Polgar, P., Rethelyi, J. M., Balint, S., Komlosi, S., Czobor, P., & Bitter, I. (2010b). Executive function in deficit schizophrenia: What do the dimensions of the Wisconsin card sorting test tell us? Schizophrenia Research, 122(1–3), 85–93. https://doi.org/10.1016/j.schres.2010.06.007.
Poppe, A. B., Barch, D. M., Carter, C. S., Gold, J. M., Ragland, J. D., Silverstein, S. M., & MacDonald, A. W., III. (2016). Reduced Frontoparietal activity in schizophrenia is linked to a specific deficit in goal maintenance: A multisite functional imaging study. Schizophrenia Bulletin, 42(5), 1149–1157. https://doi.org/10.1093/schbul/sbw036.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676.
Rethelyi, J. M., Czobor, P., Polgar, P., Mersich, B., Balint, S., Jekkel, E., et al. (2012). General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. European Archives of Psychiatry and Clinical Neuroscience, 262(2), 107–115. https://doi.org/10.1007/s00406-011-0224-4.
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., Reiss, A. L., & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007.
Stoodley, C. J. (2014). Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Frontiers in Systems Neuroscience, 8, 92. https://doi.org/10.3389/fnsys.2014.00092.
Stoodley, C. J. (2016). The cerebellum and neurodevelopmental disorders. Cerebellum, 15(1), 34–37. https://doi.org/10.1007/s12311-015-0715-3.
Stoodley, C. J., & Limperopoulos, C. (2016). Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Seminars in Fetal & Neonatal Medicine, 21(5), 356–364. https://doi.org/10.1016/j.siny.2016.04.010.
Su, T. W., Lan, T. H., Hsu, T. W., Biswal, B. B., Tsai, P. J., Lin, W. C., & Lin, C. P. (2013). Reduced neuro-integration from the dorsolateral prefrontal cortex to the whole brain and executive dysfunction in schizophrenia patients and their relatives. Schizophrenia Research, 148(1–3), 50–58. https://doi.org/10.1016/j.schres.2013.05.005.
Wang, X., Yao, S., Kirkpatrick, B., Shi, C., & Yi, J. (2008). Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Research, 158(2), 195–205. https://doi.org/10.1016/j.psychres.2006.09.007.
Wang, S. S., Kloth, A. D., & Badura, A. (2014). The cerebellum, sensitive periods, and autism. Neuron, 83(3), 518–532. https://doi.org/10.1016/j.neuron.2014.07.016.
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060.
Yan, C. G., Wang, X. D., Zuo, X. N., & Zang, Y. F. (2016). DPABI: Data Processing & Analysis for (resting-state) brain imaging. Neuroinformatics, 14(3), 339–351. https://doi.org/10.1007/s12021-016-9299-4.
Yu, M., Tang, X., Wang, X., Zhang, X., Zhang, X., Sha, W., Yao, S. Q., Shu, N., Zhang, X. Y., & Zhang, Z. J. (2015a). Neurocognitive impairments in deficit and non-deficit schizophrenia and their relationships with symptom dimensions and other clinical variables. PLoS One, 10(9), e0138357. https://doi.org/10.1371/journal.pone.0138357.
Yu, M., Tang, X. W., Wang, X., Zhang, X. R., Zhang, X. B., Sha, W. W., Yao, S. Q., Shu, N., Zhang, X. Y., & Zhang, Z. J. (2015b). Neurocognitive impairments in deficit and non-deficit schizophrenia and their relationships with symptom dimensions and other clinical variables. PLoS One, 10(9). https://doi.org/10.1371/journal.pone.0138357.
Yu, M., Dai, Z. J., Tang, X. W., Wang, X., Zhang, X. B., Sha, W. W., et al. (2017). Convergence and divergence of brain network dysfunction in deficit and non-deficit schizophrenia. Schizophrenia Bulletin, 43(6), 1315–1328. https://doi.org/10.1093/schbul/sbx014.
Yucel, K., Nazarov, A., Taylor, V. H., Macdonald, K., Hall, G. B., & Macqueen, G. M. (2013). Cerebellar vermis volume in major depressive disorder. Brain Structure & Function, 218(4), 851–858. https://doi.org/10.1007/s00429-012-0433-2.
Zakzanis, K. K. (1998). Neuropsychological correlates of positive vs. negative schizophrenic symptomatology. Schizophrenia Research, 29(3), 227–233. https://doi.org/10.1016/S0920-9964(97)00102-3.
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development, 29(2), 83–91. https://doi.org/10.1016/j.braindev.2006.07.002.
Zhang, C., Wang, Q., Ni, P., Deng, W., Li, Y., Zhao, L., Ma, X., Wang, Y., Yu, H., Li, X., Zhang, P., Meng, Y., Liang, S., Li, M., & Li, T. (2017). Differential cortical gray matter deficits in adolescent- and adult-onset first-episode treatment-naive patients with schizophrenia. Scientific Reports, 7(1), 10267. https://doi.org/10.1038/s41598-017-10688-1.
Zhuo, C., Wang, C., Wang, L., Guo, X., Xu, Q., Liu, Y., & Zhu, J. (2018). Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging and Behavior, 12(2), 383–389. https://doi.org/10.1007/s11682-017-9704-0.
Acknowledgements
We sincerely thank Gavin P. Reynold for polishing the article.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) (Nos. 81371474, 81571314, 91132727 and 31671144), National Key Research and Development Program (2016YFC1307002 and 2018YFC1314303), Medical key talent projects in Jiangsu, Province (ZDRCA2016075), the six talent peaks projects in Jiangsu Province (No.2015-WSN-071) and the Shanghai Changning Medical Research Program (CNKW2016Y17).
Author information
Authors and Affiliations
Contributions
All authors reviewed and contributed to the final version of the manuscript. Additional contributions are stated below.
Ju Gao was responsible for data analyses and preparation of the manuscript. Xiaowei Tang and Hongying Zhang performed the experiments. Congjie Wang and Miao Yu contributed to the data analyses and interpretation of findings. Weiwei Sha and Xiaobin Zhang were responsible for obtaining ethical approval and performing neurocognitive assessment. Xiang Wang offered the Chinese version of SDS. Xiangrong Zhang formulated the hypothesis, designed the experimental strategy and obtained funding for the study.
Corresponding authors
Ethics declarations
Ethical statements
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 71 kb)
Rights and permissions
About this article
Cite this article
Gao, J., Tang, X., Wang, C. et al. Aberrant cerebellar neural activity and cerebro-cerebellar functional connectivity involving executive dysfunction in schizophrenia with primary negative symptoms. Brain Imaging and Behavior 14, 869–880 (2020). https://doi.org/10.1007/s11682-018-0032-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-0032-9