Abstract
Deficits in communication are a core feature of autism spectrum disorder (ASD), however, structural language abilities are highly variable, ranging from minimally verbal to superior linguistic skills. Differences in the anatomy of cortical language regions, including anterior and posterior areas, have been found in ASD. It remains unclear, however, if anatomical differences distinguish individuals with impaired expressive language from those without such deficits. In addition, anatomical differences have not been explored in children with extremely low expressive language. This study included 34 boys with ASD, 7–11 years old, including an expressive language impaired group (n = 17) and an average-high language group (n = 17). The language impaired group was subdivided into a low (n = 9) and extremely low (n = 8) language subgroup for exploratory analyses to determine whether children with ASD with extremely low expressive language abilities exhibit distinct anatomy. Gray matter volume of the pars triangularis, pars opercularis, and planum temporale (PT) were measured on MRIs. PT volume was smaller in the ASD group with expressive language impairment relative to those without language deficits. The right PT volume was also positively correlated with language scores. The exploratory analyses revealed differences in the left PT, with smaller volume in the extremely low language subgroup, relative to the average and moderately low language groups. Results suggest that smaller PT volumes in both hemispheres are associated with severe language impairments in ASD. The PT may therefore, be a biomarker of language outcome in young children with ASD, with more studies of PT anatomy necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social interactions and communication with the presence of restricted interests or repetitive behaviors (American Psychiatric Association 2013). Deficits in communication and appropriate use of language are a core feature of ASD, but a range of language abilities is present within the disorder. Some individuals never develop functional language, whereas others demonstrate average or above language skills on standardized language tests (Tager-Flusberg et al. 2005; for review; Groen et al. 2008). It is, however, unclear what differentiates those who develop good language skills from those who have poorer language abilities.
Differences in the anatomy of cortical language regions, including anterior (pars triangularis, PTR, and pars opercularis, POP) and posterior (planum temporale, PT, and posterior superior temporal gyrus, pSTG) areas, have been found in ASD, however, results have been inconsistent (see Knaus et al. 2009; Joseph et al. 2014). One reason for such inconsistencies may be differences in samples and the inclusion of a heterogeneous ASD group. In addition, many prior studies did not examine associations with behavioral measures. A few studies, however, have investigated these associations and found some relationships between language region anatomy and behavior. For example, in a study of school-aged children with ASD, we found that the right PTR volume was negatively correlated with language scores, indicating better language function associated with a smaller right PTR size (Knaus et al. 2009). Joseph et al. (2014) examined asymmetries of language-related cortex and the microstructure of the arcuate fasciculus in younger children with ASD (4–7 years) and found higher language ability linked to increased rightward POP asymmetry and bilaterally increased fractional anisotropy and decreased radial diffusivity of the arcuate fasciculus. In a more recent study of school-aged (7–10 years) boys with ASD with lower language abilities (most at least 1.5 standard deviations below the mean), we found that larger right PTR volume was associated with less decline in language over time (Knaus et al. 2016). The group with no change in language over time also had larger PT volume than the group whose language declined over time. A recent voxel-based morphometry study of high-functioning adults with ASD found that a history of language delay was associated with smaller bilateral insula, ventral basal ganglia, and right superior, middle, and polar temporal volume, and larger volume of the pons and medulla oblongata, but not in differences in volume of critical language regions (Broca’s or Wernicke’s) (Lai et al. 2015). Better current language ability was associated with larger gray matter volume of bilateral temporal pole, superior temporal regions, dorsolateral fronto-parietal and cerebellar regions, and increased white matter volume of frontal and insular regions. Another study of high-functioning adults with ASD using voxel-based morphometry found associations with a history of language delay, but no associations with current language ability (Floris et al. 2016). They found reduced leftward asymmetry of auditory cortex, including Heschl’s gyrus and the planum temporale, in adults with a history of language delay compared to adult controls. de Fossé et al. (2004), however, is the only previous study that directly compared individuals with ASD with impaired language abilities to those with average language abilities, examining school-aged boys with ASD with language impairment, boys with specific language impairment (SLI), boys with ASD without language impairment, and typically developing boys. Children with ASD with language impairment and SLI boys had rightward asymmetry of frontal language regions, while those without language impairment had leftward asymmetry. They also found a positive correlation between verbal IQ and degree of leftward asymmetry of Broca’s area (PTR + POP), suggesting that better language skill was associated with more leftward asymmetry of frontal language regions in ASD. There was also leftward PT asymmetry in the language impaired groups, but not in the language normal groups. This study’s sample size, however, was small, with only 6 individuals with ASD with average language and no children with extremely impaired language.
Most children with ASD have language delay, though by the time they are school-aged (6 years or older) many have caught up with their peers and demonstrate average or above structural language abilities assessed on standardized language measures (Pickles et al. 2014). Some individuals, however, continue to lag behind their peers, showing major impairments in expressive and receptive language abilities (Anderson et al. 2007; Pickles et al. 2014). In a longitudinal study of 2–9 year olds with ASD, Anderson and colleagues (2007) found 4 subgroups. The two most improved subgroups had language scores near or above age norms at 9 years old, although they scored below age level at the first assessment. The least improved group was mostly nonverbal, with few or no words at age 9 and the second lowest group had phrases and some sentences, but were not fluent speakers. Another study, however, distinguished 2 ASD subgroups, with ASD children with higher initial verbal skills showing increases over time in expressive language and those with low initial verbal skills having no significant gains on most language measures (Tek et al. 2014). Lombardo et al. (2015) also found two subgroups, based on pre-diagnosis (about 2 years of age) fMRI activation during speech. Toddlers with ASD with good language outcome (at 3–4 years) showed similar pre-diagnosis activation of superior temporal cortex compared to controls, while toddlers with ASD with poor language outcome had decreased pre-diagnosis superior temporal activation.
The present study addressed two main questions. First, why do some children with ASD have impaired expressive language abilities, while others have average or above abilities? Specifically, are there anatomical differences that distinguish these groups? Based on previous studies showing anatomical-behavioral relationships in ASD, we predicted that we would find differences in the anatomy of anterior language regions. Specifically, we hypothesized that the expressive language impaired group would have larger left Broca’s area (PTR + POP) and smaller right PTR compared to the average language group. Based on the findings by de Fossé and colleagues (2004), we also expected to find exaggerated leftward asymmetry of the PT in the expressive language impaired group relative to the typical language group. The second question addressed was whether there are anatomical differences that distinguish children with ASD with extremely low expressive language abilities. Most prior anatomical studies have not included individuals with very low language abilities; no studies have explored differences in language anatomy in this subgroup. We predicted that children with extremely low expressive language would show atypical anatomy of language zones including the PTR, POP, and PT.
Materials and methods
Subjects
Subjects included 34 boys with ASD, 7–11 years old, divided into an expressive language impaired group (n = 17) and an average-high language group (n = 17), based on expressive language scores. Individuals in the expressive language impaired group had expressive language scores at least 1.4 standard deviations below the mean (79 or lower) while those with average-high had expressive language scores within a standard deviation of the mean or above (88 or above). Our sample was limited to boys in order to control for potential sex-linked differences in language organization and anatomy (Blanton et al. 2004; Kansaku et al. 2000; Kulynych et al. 1994). Based on writing hand, there were 7 left-handers, 3 in the impaired expressive language group and 4 in the average language group. One of the unique aspects of our sample was that it included children with very impaired language abilities. We therefore divided our expressive language impaired sample into 2 subgroups: those with extremely low expressive language (2.93 standard deviations or more below the mean; n = 8) and those with moderately low expressive language scores (1.4–2.73 standard deviations below the mean; n = 9) for exploratory analyses.
Data was collected at Boston University School of Medicine (BUSM) (n = 16; 15 average-high language, 1 impaired expressive language) and at Louisiana State University Health Sciences Center/Children’s Hospital (LSUHSC) (n = 18; 2 average-high language, 16 impaired expressive language). All standardized tests used showed strong correlations (see below) and we did not find any scanner differences (see Scanner Effects section below). In addition, one individual oversaw all data collection and performed all anatomical measurements.
Individuals with frank neurological damage, with a known genetic disorder, who were born prematurely (less than 36 weeks), who had experienced seizures within the last 3 years, or who were on anti-seizure medication were excluded from the study. All subjects had English as their first language.
Parents and participants were informed of the procedures and parents gave written consent prior to the child’s participation in the study. All data in this manuscript were collected in compliance with the Louisiana State University Health Sciences Center and Children’s Hospital or the Boston University School of Medicine Institutional Review Boards and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Standardized tests
To assess non-verbal IQ the Leiter International Performance Scale-Revised (Leiter; Roid and Miller 1997) or Kaufman Brief Intelligence Test (KBIT-II; Kaufman and Kaufman 2004) was administered. A study showed a high correlation (r = .62) and no differences in means between these tests in children with autism and children with language impairment (Scattone et al. 2012). Receptive and expressive language abilities were assessed with the Clinical Evaluation of Language Fundamentals (CELF, 3rd ed; Semel et al. 1995) or Oral and Written Language Scales (OWLS; Carrow-Woolfolk 1995). The OWLS manual reports strong correlations with the CELF-R: receptive language = 0.80, expressive language = 0.85, and total language = 0.91. Subject characteristics are shown in Table 1.
ASD diagnoses were based on DSM-IV criteria (American Psychiatric Association 1994) using the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al. 2003) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 1999) administered by trained personnel. In addition, an expert clinician confirmed that all individuals met DSM- IV criteria for ASD (autism or PDD-NOS).
MRI acquisition
All participants were trained in a mock scanner prior to the actual MR scanning. Volumetric MR images were acquired on a Siemens 3 T Verio scanner or Philips 3 T Intera scanner. T1-weighted images were obtained as a series of 160, 1 mm gapless sagittal images. MPRage or TFE was used, with technical factors of: 256 × 256 pixel matrix, 240 or 250 mm field of view, and 8 or 9 degree flip angle. Data sets were rotated into alignment in the sagittal, axial, and coronal planes in order to eliminate any head rotation and MRI scans were maintained in real space. Each MRI scan series was assigned a blind number to assure subject confidentiality and to ensure that all measurements were performed blind to subject.
Volume measurements
Total brain volume (TBV) was measured using FSL and MRIcron (Rorden et al. 2007). The BET tool in FSL (Smith 2002) was first used to remove as much skull, dura, and non-brain tissue as possible. A mask was created in FSLView (Smith et al. 2004) and any remaining non-brain tissue was manually marked in each slice. In MRIcron, this mask was applied to the brain volume to remove the non-brain tissue and the volume was then extracted. The TBV measure included gray and white matter, the thalamus, and cerebellum, but excluded the brainstem.
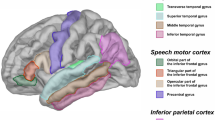
Three regions of interest (ROIs) were measured including the PT, PTR, and POP. 3D Slicer (Fedorov et al. 2012) or the MEASURE program (Barta et al. 1997) was used to manually trace the gray matter volume of each ROI in each hemisphere (Refer to Fig. 1). Given the large individual variability in the anatomy of these regions, manual measurements were performed to more accurately define these ROIs. Manual measurements also allowed the scans to be maintained in native space, which is particularly important given that our sample included children and individuals with neurodevelopmental disorders (see, Keller and Roberts 2009). All measurements were performed by a single investigator. Boundaries were the same as those used in our prior studies and inter-rater reliability for each of these measures has previously been established, with intra-class correlations (ICCs) of 0.83 or higher for each ROI (Knaus et al. 2004, 2006).
Planum temporale (PT)
The anterior boundary was defined as Heschl’s sulcus and, when present, the second Heschl’s gyrus was included in the planum measure. In the coronal plane, this image was where Heschl’s was fully visible and there was a small amount of white matter lateral to it. The posterior boundary was defined in the sagittal plane, as the point where the horizontal ramus of the Sylvian fissure turns upward into the posterior ascending ramus (PAR), so neither the PAR nor posterior descending ramus was included in the planum measurement. In cases where the Sylvian fissure gently sloped upward, the knifecut method (Witelson and Kigar 1992) was utilized. If there was no PAR, the end of the horizontal portion of the Sylvian fissure was used as the posterior boundary. This boundary was defined in the coronal plane as the most posterior slice where the Sylvian fissure was clearly visible, before it became intermixed with white matter. However, if the Sylvian fissure extended into parietal regions, the posterior boundary was defined as the image just anterior to the one where the intraparietal sulcus appeared to encircle the Sylvian fissure. The PT was measured in the coronal plane, with Heschl’s sulcus, when present, as the medial boundary. Laterally, the boundary for the planum was the edge of the Sylvian fissure, not including the lateral wall of the posterior superior temporal gyrus.
Pars triangularis (PTR)
The sagittal plane was predominantly used to measure this region because it provides the clearest view of the PTR. The anterior boundary was the anterior horizontal ramus and the posterior boundary was the anterior ascending ramus. Thus, the posterior/superior bank of the anterior horizontal ramus and the anterior/superior bank of the anterior ascending ramus were included in the PTR measurement. When extra sulci, internal notches, occurred between these two rami, the banks of these sulci were included in the measurement. The lateral boundary was defined as the most lateral sagittal image, prior to the rami being cut off, so that surface gray matter was not included. At times, only one of the sulci, either the anterior horizontal ramus or the anterior ascending ramus, was cut off, while the full extent of the other sulcus remained, in which case the bank of the remaining sulcus was still measured on images as far lateral as possible, until the sulcus was cut off. The medial boundary was defined as the most medial sagittal image in which the insula was clearly defined, prior to the white matter intruding. At this boundary, the insula appeared with clearly defined strips of white matter. The superior boundary was the inferior frontal sulcus.
Pars opercularis (POP)
The sagittal plane provides the best view of the POP, so this plane of section was primarily used to measure this region. In the sagittal plane, the anterior boundary was defined as the anterior ascending ramus and the posterior boundary was defined as the precentral sulcus. Thus, the posterior/inferior bank of the anterior ascending ramus and the anterior bank of the precentral sulcus, up to the inferior frontal sulcus, were included in the measurement. The inferior frontal sulcus, also defined in the sagittal plane, was used as the superior boundary. While on some images the precentral sulcus connects with the Sylvian fissure, it was not continuous with the Sylvian fissure throughout the full extent of the POP. In addition, in some brains, the precentral sulcus never fully connected with the Sylvian fissure. When the precentral sulcus did not connect with the Sylvian fissure, the POP was measured to the point where the Sylvian fissure would have connected with the precentral sulcus and the anterior bank of the precentral sulcus was measured from the inferior frontal sulcus, down until it disappeared. The lateral boundary was defined on sagittal images as the most lateral image prior to the rami being cut off, so that surface gray matter was not included. At times, only one of the sulci, the anterior ascending ramus or the precentral sulcus was cut off, while the other one remained, in which case the bank of the remaining sulcus was still measured on images as far lateral as possible, until the sulcus was cut off. The medial boundary was defined as one sagittal image lateral to the medial boundary of the PTR. The diagonal sulcus was measured separately and added into the POP measure. Other extra sulci that appeared between the anterior ascending ramus and the precentral sulcus were also included.
Analyses
Expressive language impaired compared to average language
To examine group differences in age and non-verbal IQ, ANOVAs were performed with group (expressive language impaired, average language) as the independent variable and age or non-verbal IQ as the dependent variable. A MANOVA was calculated to examine differences in ADOS scores, with group as the independent variable and ADOS communication and ADOS social scores as the dependent variables.
To examine differences in TBV, an ANOVA was calculated with group (expressive language impaired, average language) as the independent variable and TBV as the dependent variable. In addition, to compare the different scanners on TBV, an ANOVA was computed with scanner (BUSM, LSUHSC) as the independent variable and TBV as the dependent variable.
To examine group differences in ROI volumes, ANCOVAs were used with hemisphere as the within-subjects independent variable, group (expressive language impaired, average language) as the between-subjects independent variable, and PT, PTR, or POP gray matter volume as the dependent variable. Since there were group differences in non-verbal IQ (see below), this score was added as a covariate for all analyses. Since TBV did not differ (see below), raw volumes were examined. An asymmetry quotient (AQ) was calculated for each region, as (Left–Right)/[(Left + Right)/2], so that a positive AQ indicated leftward asymmetry and a negative quotient indicated rightward asymmetry. In order to examine group differences in degree of asymmetry, ANCOVAs were performed, with group as the independent variable, PT, PTR, or POP AQ as the dependent variable, and non-verbal IQ as the covariate. Separate ANCOVAs were run, rather than a MANCOVA because ROIs were not correlated. To examine associations between language and anatomical measures Pearson correlations were calculated across the sample, between expressive language scores and left and right PT, PTR and POP volumes and AQs.
Exploratory analyses
The expressive language impaired group was divided into an extremely low (≥ 2.93 standard deviations below the mean) and a moderately low (1.4–2.73 standard deviations below the mean) language subgroup and, due to the small sample sizes, individual data were examined. The volume of each ROI for each subject in the extremely low language subgroup was compared to the mean and standard deviation of the average language group.
Post-Hoc analyses
In order to determine if results were specific to expressive language or to a more general language problem, particularly given our findings of differences in PT anatomy, we re-classified subjects based on receptive language scores. Individuals in the receptive language impaired group had receptive language scores at least 1 standard deviation below the mean (85 or lower), while those with average-high scores had receptive language scores defined as within a standard deviation of the mean or above (89 or above). ANCOVAs were used with hemisphere as the within-subjects independent variable, group (receptive language impaired, average language) as the between-subjects independent variable, non-verbal IQ as a covariate, and PT, PTR, or POP gray matter volume as the dependent variable. To examine asymmetry, ANCOVAs were performed, with group as the independent variable, PT, PTR, or POP AQ as the dependent variable, and non-verbal IQ as the covariate.
Results
Expressive language impaired compared to average language
Refer to Table 1 for mean and range of behavioral measures for each group. There were no significant differences in age between the groups (p > .05). There was a significant group effect for non-verbal IQ (F1,32 = 20.28, p < .001), with the expressive language impaired group having lower scores than the average language group. At the multivariate level, the MANOVA examining ADOS scores was significant (F2,29 = 5.83, p = .007). At the univariate level, this was significant only for the ADOS communication score (F1,30 = 11.88, p = .002), with the impaired expressive language group having significantly higher scores (indicating more autism symptom severity) than the average language group and no differences in ADOS social scores.
There was no significant group difference in TBV (p > .05) (expressive language impaired mean = 1441.44 (144.95), average language mean = 1520.73 (97.34)). There was also no significant difference in TBV between the 2 scanners (p > .05) (BUSM mean = 1517.22 (105.45), LSUHSC mean = 1448.96 (140.37)). TBV was also not significantly correlated with any ROIs.
See Table 2 for mean volume for each group. For the PT, there was a significant language group effect (F1,31 = 3.99, p = .032), indicating overall smaller PT volume in the expressive language impaired group compared to the average language group. The hemisphere and group by hemisphere effects were not significant (p > .05). There were no significant effects for the PTR or POP (p’s > 0.05). For AQs, there were no significant group differences for any regions (p’s > 0.05) (see Table 2). While correcting for multiple comparisons may reduce Type I errors, it can increase Type II errors, allowing important findings to be missed. We performed only hypothesis-directed comparisons and given our moderate to large effect size for the PT, we did not apply corrections for multiple comparisons (see Feise 2002; Rothman 1990). Across the ASD sample, after Bonferroni correction, the right PT volume was positively correlated with expressive (r = .543, p = .001), receptive (r = .527, p = .001), and total (r = .553, p = .001) language, with larger right PT associated with better language abilities (Fig. 2).
Exploratory analyses
Subdividing the expressive language impaired group into an extremely low and a moderately low language subgroup resulted in 8 subjects in the extremely low language subgroup and 9 in the moderately low subgroup. Table 1 contains means and ranges of behavioral measures for each subgroup. There were no differences between the subgroups in age, nonverbal IQ, or ADOS scores.
Refer to Table 3 for individual volumes and AQs in each subgroup. For the right PT, most subjects (7, 88%) in the extremely low subgroup had volumes at least a standard deviation below the mean when compared to the average language group. However, the moderately low subgroup also had smaller right PT volume than the average language group, with 5 (56%) subjects having smaller volume. A Chi square comparing the extremely low and moderately low subgroups for the right PT was not significant (p > .05). For the left PT, however, most subjects in the extremely low language subgroup had volumes more than a standard deviation below the mean when compared to the average language group (4, 50%). There were no children in the moderately low language subgroup with smaller left PT compared to the average group. A Chi square comparing the extremely and moderately low subgroups for the left PT was significant (χ2 = 5.89, p = .015). See Fig. 3 for mean right and left PT volume for each group.
Interesting differences in PT asymmetry were also seen between the language subgroups. In the extremely low subgroup, 3 of 8 (38%) subjects showed an absent PT structure (1 in the left and 2 in the right hemisphere). In contrast, the subgroup with moderately low language showed a different anatomical variant. Since this subgroup had smaller right PT with similar left PT volume to the average language group, an exaggerated leftward planar asymmetry resulted, with 4 of 9 (44%) individuals having an AQ more than a standard deviation above the mean of the average language group. Only 2 (25%) in the extremely low language group had exaggerated leftward asymmetry and these were the 2 with an absent right PT.
Post-Hoc analyses
Stratifying the ASD group based on receptive language scores yielded 18 boys with impaired receptive language and 16 boys with average-high language scores. Results were the same as the prior analysis with groups stratified based on expressive language score. Specifically, there was a significant language group effect for the PT (F1,31 = 6.04, p = .020), indicating smaller PT size in the receptive language impaired group compared to the average receptive language group.
Scanner effects
Since data was collected on 2 different MRI scanners, there is a chance that results could be due to differences between the scanners. This is unlikely, given that there were regionally specific differences and if differences were due to the scanners, we would expect them to be throughout the regions measured. In addition, if the differences found in PT volume were due to differences between the scanners, it is unlikely we would find correlations between PT volume and multiple measures of language as we found. However, to verify that differences were not due to differences between the scanners, we examined the effect of the scanner in a small group of children and adolescents with impaired expressive language abilities (expressive language scores of 1.4 standard deviations or more below the mean). The sample included 9 individuals, ages 8–19 years, scanned at BUSM and 16 children, 7–10 years (included in the current study) scanned at LSUHSC. ANOVAs were used with hemisphere as the within-subjects independent variable, scanner (BUSM, LSUHSC) as the between-subjects independent variable, and PT, PTR, or POP gray matter volume as the dependent variable. For PT, there was a significant hemisphere effect (F1,23 = 11.33, p = .003), with larger left than right volume but no significant scanner or scanner by hemisphere effects (p’s > 0.05). Similarly, there were no significant scanner, hemisphere, or hemisphere by scanner effects for the PTR or POP (p’s > 0.05).
Discussion
The purpose of this study was to determine whether there were anatomical differences that distinguished children with ASD with impaired expressive language and children with ASD without expressive language deficits. Differences in the cortical anatomy of language related areas has been shown in ASD, however, it is unclear if there are specific subgroups that exist and whether differences are related to language abilities. Our study differed from most prior anatomical ASD studies in that it examined these subgroups. It differed from the one previous study which examined language based subgroups within ASD (de Fossé et al. 2004) in that we included a larger sample size, particularly of children with unimpaired language. We also used expressive language to define our groups, as opposed to more phonologically based differences, and our sample included individuals with much more impaired language. Although we were not able to include completely nonverbal children, we included children with very impaired expressive language, allowing us to determine if there were any anatomical distinctions specific to this subgroup, which has not been previously examined.
There were 3 main findings in this study. First, the PT volume was smaller in the ASD group with expressive language impairment compared to those with average or above expressive language. Second, the right PT volume was significantly correlated with language scores, with larger right volume associated with better language abilities. Third, left PT volume distinguished the extremely low expressive language subgroup from the moderately low language subgroup, with smaller left volume in the extremely low subgroup compared to both the average and moderately low groups. Each of these findings will be discussed below.
As predicted, there were differences in the anatomy of language cortex between individuals with ASD with impaired expressive language abilities relative to average abilities. We found differences, however, in the size of the PT rather than frontal language regions, which is what we had hypothesized based on several prior empiric studies. Differences in PT anatomy, however, have been shown in ASD. Rojas and colleagues found decreased left PT volume, resulting in decreased leftward asymmetry, in adults and boys with ASD relative to controls (Rojas et al. 2002, 2005). Other studies demonstrated increased leftward PT asymmetry in boys with ASD compared to typically developing controls (de Fossé et al. 2004; Herbert et al. 2002, 2005). In a recent study, we found smaller PT volume in a group of children with ASD whose language showed decline over time relative to ASD children with no change in language scores (Knaus et al. 2016). In the current study, there was reduced overall (left and right hemisphere) PT volume in the group with impaired expressive language scores relative to those with average or above scores. The PT comprises a portion of the classic Wernicke’s area, contains auditory association cortex, and is involved in higher order auditory processing important for speech and language comprehension. Since our sample was defined based on expressive language, we had anticipated finding differences predominantly in frontal language regions. The majority of the children in the expressive language impaired group, however, also had impaired receptive language abilities and expressive language relies heavily upon receptive language. Thus, differences in PT anatomy may relate to differences in overall language abilities in ASD.
Although the hemisphere by group and AQ differences were not significant, there was a tendency for the reduction to be greater in the right hemisphere (see Fig. 3) with an exaggerated leftward asymmetry in the expressive language impaired group. This is consistent with de Fossé et al.’s (2004) findings of increased leftward PT asymmetry in the impaired language ASD group compared to the unimpaired language ASD group. The right PT volume was also correlated with language measures, with larger volume associated with higher expressive, receptive, and total language scores. Given the left hemisphere’s predominant role in language in typically developing children, it was somewhat surprising that it was the right volume, rather than the left, that was related to better language abilities. Several recent studies in ASD have found associations between right language areas and language abilities. Joseph et al. (2014) reported that increased rightward POP asymmetry was correlated with increased language level in young children with ASD. Our previous study demonstrated larger right PTR volume associated with less decline in language abilities over time (Knaus et al. 2016). An fMRI study in very young children with ASD, showed increases in right language region activation during speech perception were associated with higher receptive language ability and decreased autism symptom severity (Redcay and Courchesne 2008). A larger structure in the right hemisphere may relate to the ability of some individuals to compensate and may be related to previous work which has consistently shown reduced left lateralized language functions in ASD (see, Knaus et al. 2010).
Given the extremely impaired expressive language abilities, with scores nearly 3 standard deviations below the mean in the extremely low expressive language subgroup, we had anticipated differences in language anatomy, particularly of frontal regions, in this subgroup. Although there were not differences in frontal language areas, there were some differences in the PT that distinguished this subgroup. For the right volume, there seemed to be a gradation with the extremely impaired having smaller right PT size than the average and the moderately low language subgroup falling in between. The differences in left PT between the average and expressive language impaired groups seemed to be driven by the extremely low subgroup, as this subgroup had smaller left PT volume compared to both the average and the moderately low language subgroups. Thus, individuals with moderately impaired expressive language had reduced right PT size, while those with extremely impaired expressive language had further reduced right PT size, as well as reduced left PT volume. In summary, our data provide some anatomical basis for prior behavioral findings that examined language trajectories (Anderson et al. 2007; Tek et al. 2014; Lombardo et al. 2015) and suggest three subgroups, based on PT anatomy. Although we did not examine typically developing controls in this study, the PT volume of the average-high language group was very similar to typically developing children of the same age reported in our previous study (Knaus et al. 2009). In the current study reduced right PT volume was associated with moderate expressive language impairment and was thus sufficient for some language development. Bilateral reduction in PT size, especially anomalous absence of PT, was associated with very poor expressive language abilities. This result is consistent with our prior findings where those with worse language outcomes (age equivalent scores further from chronological age) had smaller bilateral PT compared to those with no change in language scores over time (Knaus et al. 2016).
It is also interesting to note that we found 3 cases where there was no PT (2 right and 1 left hemisphere). All 3 cases were in the extremely low language subgroup. This configuration is very unusual. The lack of this structure could be a biomarker associated with language delay that results in extremely low language functions, and in some instances may be incompatible with language development. This argument is supported by the observation that this anomaly was not found in any individuals in the moderately-low language group. In addition we have not found this anatomical anomaly in any of our prior studies, including studies of higher-functioning individuals with ASD (Knaus et al. 2009), studies of adults with a diagnosis of persistent developmental stuttering (Foundas et al. 2001; Foundas 2004) or typically developing individuals (Knaus et al. 2004, 2006, 2009). In describing Sylvian fissure anatomy, Witelson and Kigar (1992) called this configuration a V-type and noted that it was rare in their sample of adult cancer patients. Leonard et al. (1997) described the V-type as one of several anomalous configurations which may be a risk factor for genetic or developmental disorders.
There are several limitations to this study. First, is the small sample size of the extremely low language subgroup. There are no prior studies that have specifically examined this group; more studies involving individuals with very impaired language with larger samples are warranted. Another limitation is that although our study involved children with extremely low expressive language, we were not able to include nonverbal children with ASD. This study also included data collected from 2 different sites, however, this allowed us to have a more diverse sample and investigate differences based on language subgroups. This study was limited to males, so it would be important for future studies to examine females with ASD to see if results are similar and to examine potential sex differences. Future studies should also include typically developing individuals to determine if their language-related anatomy is similar or different than the average language ASD group. It would also be interesting to compare ASD to other populations such as language impaired or global developmental delay. These studies could help determine the specificity of the findings for ASD. Studies including younger ages and longitudinal studies examining trajectory will also be critical for a better understanding of the anatomical basis of differing behavioral trajectories.
Overall, results from this study demonstrate anomalous PT anatomy in children with ASD with impaired expressive language compared to children with ASD with normal expressive language abilities. Although receptive and expressive language abilities are related and most subjects had deficits of receptive abilities in addition to expressive, it was somewhat surprising that differences were found only in the PT, rather than in frontal language regions. It suggests, however, that differences may be occurring earlier in the processing stream, more at the level of receptive processing, which in turn effects expressive abilities. These findings suggest that smaller PT volume may be associated with deficits in expressive language. All participants in this study had delayed language, therefore, it may be that those with larger PT volume were better able to compensate. More studies focusing on the PT are warranted, however, our results suggest PT anatomy could be a prognostic biomarker of language outcome in young children with ASD.
References
American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders (4th edn.). Washington, DC: American Psychiatric Association Press.
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th edn.). Washington, DC: American Psychiatric Association Press.
Anderson, D. K., Lord, C., Risi, S., DiLavore, P. S., Shulman, C., Thurm, A., et al. (2007). Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology, 75(4), 594–604. https://doi.org/10.1037/0022-006X.75.4.594.
Barta, P. E., Dhingra, L., Royall, R., & Schwartz, E. (1997). Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. Journal of Neuroscience Methods, 75, 111–118.
Blanton, R. E., Levitt, J. G., Peterson, J. R., Fadale, D., Sporty, M. L., Lee, M., et al. (2004). Gender differences in the left inferior frontal gyrus in normal children. NeuroImage, 22, 626–636.
Carrow-Woolfolk, E. (1995). Oral and written language scales. Circle Pines: American Guidance Services, Inc.
de Fossé, L., Hodge, S. M., Makris, N., Kennedy, D. N., Caviness, V. S. Jr., McGrath, L., et al. (2004). Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology, 56(6), 757–766.
Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J. C., Pujol, S., et al. (2012). 3D Slicer as an image computing platform for the quantitative imaging network. Magnetic Resonance Imaging, 30(9), 1323–1341. https://doi.org/10.1016/j.mri.2012.05.001.
Feise, R. J. (2002). Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology, 2, 8.
Floris, D. L., Lai, M. C., Auer, T., Lombardo, M. V., Ecker, C., Chakrabarti, B., et al. (2016). Atypically rightward cerebral asymmetry in male adults with autism stratifies individuals with and without language delay. Human Brain Mapping, 37(1), 230–253. https://doi.org/10.1002/hbm.23023.
Foundas, A. L. (2004). Brain lumps and bumps: a neural risk for autism. Annals of Neurology, 56(6), 755–756.
Foundas, A. L., Weisberg, A., Browning, C. A., & Weinberger, D. R. (2001). Morphology of the frontal operculum: a volumetric magnetic resonance imaging study of the pars triangularis. Journal of Neuroimaging, 11(2), 153–159.
Groen, W. B., Zwiers, M. P., van der Gaag, R. J., & Buitelaar, J. K. (2008). The phenotype and neural correlates of language in autism: an integrative review. Neuroscience and Biobehavioral Reviews, 32(8), 1416–1425. https://doi.org/10.1016/j.neubiorev.2008.05.008.
Herbert, M. R., Harris, G. J., Adrien, K. T., Ziegler, D. A., Makris, N., Kennedy, D. N., et al. (2002). Abnormal asymmetry in language association cortex in autism. Annals of Neurology, 52(5), 588–596.
Herbert, M. R., Ziegler, D. A., Deutsch, C. K., O’Brien, L. M., Kennedy, D. N., Filipek, P. A., et al. (2005). Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain, 128(1), 213–226.
Joseph, R. M., Fricker, Z., Fenoglio, A., Lindgren, K. A., Knaus, T. A., & Tager-Flusberg, H. (2014). Structural asymmetries of language-related gray and white matter and their relationship to language function in young children with ASD. Brain Imaging and Behavior, 8(1), 60–72.
Kansaku, K., Yamaura, A., & Kitazawa, S. (2000). Sex differences in lateralization revealed in the posterior language areas. Cerebral Cortex, 10(9), 866–872.
Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman brief intelligence test. Circle Pines: AGS Publishing.
Keller, S. S., & Roberts, N. (2009). Measurement of brain volume using MRI: software, techniques, choices and prerequisites. Journal of Anthropological Sciences, 87, 127–151.
Knaus, T. A., Bollich, A. M., Corey, D. M., Lemen, L. C., & Foundas, A. L. (2004). Sex-linked differences in the anatomy of perisylvian language cortex: a volumetric MRI study of gray matter volumes. Neuropsychology, 18(4), 738–747.
Knaus, T. A., Bollich, A. M., Corey, D. M., Lemen, L. C., & Foundas, A. L. (2006). Variability in perisylvian brain anatomy in healthy adults. Brain and Language, 97, 219–232.
Knaus, T. A., Kamps, J., & Foundas, A. L. (2016). Longitudinal language changes associated with MRI anatomy in children with autism spectrum disorder. SM Journal of Neurology and Neuroscience, 2(1), 1004.
Knaus, T. A., Silver, A. M., Dominick, K. C., Schuring, M. D., Shaffer, N., Lindgren, K. A., et al. (2009). Age-related changes in the anatomy of language regions in autism spectrum disorder. Brain Imaging and Behavior, 3(1), 51–63.
Knaus, T. A., Silver, A. M., Kennedy, M., Lindgren, K. A., Dominick, K. C., Siegel, J., et al. (2010). Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain and Language, 112, 113–120.
Kulynych, J. J., Vladar, K., Jones, D. W., & Weinberger, D. R. (1994). Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cerebral Cortex, 4(2), 107–118.
Lai, M. C., Lombardo, M. V., Ecker, C., Chakrabarti, B., Suckling, J., Bullmore, E. T., et al. (2015). Neuroanatomy of individual differences in language in adult males with autism. Cereb Cortex, 25(10), 3613–3628. https://doi.org/10.1093/cercor/bhu211.
Leonard, C. M., Krasnegor, N. A., Lyon, G. R., & Goldman-Rakic, P. S. (1997). Language and the prefrontal cortex. In Development of the prefrontal cortex: evolution, neurobiology, and behavior (pp. 141–166). Baltimore: Paul H. Brookes Publishing Co.
Lombardo, M. V., Pierce, K., Eyler, L. T., Barnes, C., Ahrens-Barbeau, C., Solso, C., S., et al (2015). Different functional neural substrates for good and poor language outcome in autism. Neuron, 86(2), 567–577. https://doi.org/10.1016/j.neuron.2015.03.023.
Lord, C., Rutter, M., DiLavore, P. C., & Risi, S. (1999). Autism diagnostic observation schedule. Los Angeles: Western Psychological Services.
Pickles, A., Anderson, D. K., & Lord, C. (2014). Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. Journal of Child Psychology and Psychiatry, 55(12), 1354–1362. https://doi.org/10.1111/jcpp.12269.
Redcay, E., & Courchesne, E. (2008). Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biological Psychiatry, 64, 589–598.
Roid, G. H., & Miller, L. J. (1997). Leiter international performance scale-revised. Torrance: Western Psychological Services.
Rojas, D. C., Bawn, S. D., Benkers, T. L., Reite, M. L., & Rogers, S. J. (2002). Smaller left hemisphere planum temporale in adults with autistic disorder. Neuroscience Letters, 328, 237–240.
Rojas, D. C., Camou, S. L., Reite, M. L., & Rogers, S. J. (2005). Planum temporale volume in children and adolescents with autism. Journal of Autism and Developmental Disorders, 35(4), 479–486.
Rorden, C., Karnath, H. O., & Bonilha, L. (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. https://doi.org/10.1162/jocn.2007.19.7.1081.
Rothman, K. J. (1990). No adjustments are needed for multiple comparisons. Epidemiology, 1(1), 43–46.
Rutter, M., Le Couteur, A., & Lord, C. (2003). Autism diagnostic interview - revised. Los Angeles: Western Psychological Services.
Scattone, D., Raggio, D. J., & May, W. (2012). Brief report: concurrent validity of the Leiter-R and KBIT-2 scales of nonverbal intelligence for children with autism and language impairments. Journal of Autism and Developmental Disorders, 42(11), 2486–2490. https://doi.org/10.1007/s10803-012-1495-y.
Semel, E., Wiig, E. H., & Secord, W. A. (1995). Clinical evaluation of language fundamentals. San Antonio: The Psychological Corporation, Harcourt Brace and Co.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, 208–219.
Tager-Flusberg, H., Paul, R., Lord, C. E., Volkmar, F., Klin, A., & Cohen, D. J. (2005). Language and communication in autism. In Handbook of autism and pervasive developmental disorder (Vol. 3, pp. 335–364). New York: Wiley.
Tek, S., Mesite, L., Fein, D., & Naigles, L. (2014). Longitudinal analyses of expressive language development reveal two distinct language profiles among young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 44(1), 75–89. https://doi.org/10.1007/s10803-013-1853-4.
Witelson, S. F., & Kigar, D. L. (1992). Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. Journal of Comparative Neurology, 323, 326–340.
Acknowledgements
This study was supported by the LSUHSC - NO Research Enhancement Fund, a program project grant from the National Institute on Deafness and Other Communication Disorders (U19 DC 03610), which was part of the NICHD/NIDCD funded Collaborative Programs on Excellence in Autism, an Autism Center of Excellence grant funded by NIDCD and NICHD (P50 DC 13027), by funding for the GCRC at Boston University School of Medicine (M01-RR0533), and by NINDS (F30 NS055511). We thank Lin Themelis for help with screening and scheduling participants and Claire Burns for assistance with screening, scheduling, and data collection. We also extend our sincere gratitude to the children and families who participated in this study.
Funding
This study was funded by the LSUHSC - NO Research Enhancement Fund, a program project grant from the National Institute on Deafness and Other Communication Disorders (U19 DC 03610), which was part of the NICHD/NIDCD funded Collaborative Programs on Excellence in Autism, an Autism Center of Excellence grant funded by NIDCD and NICHD (P50 DC 13027), the GCRC at Boston University School of Medicine (M01-RR0533), and by NINDS (F30 NS055511).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Louisiana State University Health Sciences Center and Children’s Hospital or Boston University School of Medicine and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the parent of all individual participants in the study.
Rights and permissions
About this article
Cite this article
Knaus, T.A., Kamps, J., Foundas, A.L. et al. Atypical PT anatomy in children with autism spectrum disorder with expressive language deficits. Brain Imaging and Behavior 12, 1419–1430 (2018). https://doi.org/10.1007/s11682-017-9795-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9795-7