Abstract
Adaptive social behavior appears to require flexible interaction between multiple large-scale brain networks, including the executive control network (ECN), the default mode network (DMN), and the salience network (SN), as well as interactions with the perceptual processing systems these networks function to modulate. Highly connected cortical “hub” regions are also thought to facilitate interactions between these networks, including the dorsolateral prefrontal cortex (DLPFC), dorsomedial prefrontal cortex (DMPFC), anterior cingulate cortex (ACC), and anterior insula (AI). However, less is presently known about the relationship between these network functions and individual differences in social-cognitive abilities. In the present study, 23 healthy adults (12 female) underwent functional magnetic resonance imaging (fMRI) while performing a visually based social judgment task (requiring the evaluation of social dominance in faces). Participants also completed both self-report and performance-based measures of emotional intelligence (EI), as well as measures of personality and facial perception ability. During scanning, social judgment, relative to a control condition involving simple perceptual judgment of facial features in the same stimuli, activated hub regions associated with each of the networks mentioned above (observed clusters included: bilateral DLPFC, DMPFC/ACC, AI, and ventral visual cortex). Interestingly, self-reported and performance-based measures of social-cognitive ability showed opposing associations with these patterns of activation. Specifically, lower self-reported EI and lower openness in personality both independently predicted greater activation within hub regions of the SN, DMN, and ECN (i.e., the DLPFC, DMPFC/ACC, and AI clusters); in contrast, in the same analyses greater scores on performance-based EI measures and on facial perception tasks independently predicted greater activation within hub regions of the SN and ECN (the DLPFC and AI clusters), and also in the ventral visual cortex. These findings suggest that lower confidence in one’s own social-cognitive abilities may promote the allocation of greater cognitive resources to, and improve the performance of, social-cognitive functions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The neuro-cognitive basis of social functioning has become a topic of considerable research interest (Adolphs 2009; Van Overwalle 2009). In particular, a large body of recent literature has emerged within the field of cognitive neuroscience that has highlighted specific neural networks engaged by tasks requiring social perception, theory of mind (ToM; also often referred to as “mentalizing”) abilities, and adaptive decision-making in social situations (e.g., Amodio and Frith, 2006; Barrett and Satpute, 2013; Decety and Sommerville, 2003; Frith and Frith, 2006; Heatherton, 2006; Keysers et al., 2010; Li et al., 2014; Lombardo et al., 2010; Meyer et al., 2015; Seidel et al., 2010; Zaki and Ochsner, 2012). At present, this body of literature suggests that the brain is organized into several functionally distinct—yet highly interacting—neural networks, and that adaptive social cognition is the result of complex, context-specific interactions between these networks. Three networks of particular interest are the “default mode network” (DMN; also sometimes called the “mentalizing” network), the frontoparietal “executive control” network, and the “salience” network (Barrett and Satpute 2013; Lindquist and Barrett 2012; Yeo et al. 2011). According to Barrett and Satpute (2013), the DMN’s function can be understood to involve the high-level conceptualization of sensory input based on past experience; it includes dorsal and ventral regions of the medial prefrontal cortex (MPFC), posterior/retrosplenial cingulate cortex, the temporoparietal junction (TPJ), and both medial and lateral anterior temporal regions. The executive control network, which includes dorsolateral prefrontal (DLPFC) and posterior parietal (PPC) cortical regions, is implicated in the top-down modulation (both excitatory and inhibitory) of sensory processing associated with selectively attending to, and holding in mind (i.e. working memory), perceptual information that is relevant to one’s current goals (Power and Petersen 2013). Finally, the salience network includes anterior insula (AI) and anterior cingulate cortex (ACC) regions, and may function to direct attention (and related cognitive processing resources) to homeostatically/emotionally relevant stimuli (Taylor et al. 2009).
In a social cognition task, therefore, appropriate conceptualization of the mental states of others (i.e., ToM) will typically recruit DMN regions (Frith and Frith 2006). However, executive control network regions will be needed to selectively modulate sensory processing in a goal-appropriate manner (Sripada et al. 2014), and the selective deployment of cognitive processing resources (such as those proximally controlled by the executive control network) will also need to be guided by salience network regions, such that their allocation remains based on current needs/goals, as well as estimations of the uncertainty of current perceptual estimates (Barrett and Simmons 2015; Brown et al. 2011; Feldman and Friston 2010; Gu et al. 2013). Due to the observation that the brain exhibits a “small-world” network architecture in which networks interact through “rich club hubs” (van den Heuvel et al. 2008; Zippo et al. 2013), some regions would also be expected to make more important contributions to network interactions than others. Specifically, the AI, dorsal MPFC/ACC (DMPFC/dACC), and DLPFC appear to represent important hub regions that allow for the network interactions described above (van den Heuvel and Sporns 2011, 2013).
Although, this broad account of the neural basis of social cognition has considerable support, many details remain to be explored. One particular area that deserves considerable further attention pertains to the potential factors (e.g., intelligence, personality factors, emotion recognition ability) that may explain individual differences in social cognition ability. Researchers interested in the concept of emotional intelligence (EI), for example, have designed multiple measures for assessing, and have observed, individual differences in the cognitive abilities needed to function adaptively in social situations (Davis and Humphrey 2012; Fernández-Berrocal et al. 2006; Hertel et al. 2009; Lopes et al. 2005; Mayer et al. 2004; O’Boyle et al. 2011; Pérez et al. 2005; Schutte et al. 2010). However, studies of the neural basis of EI have thus far met with mixed results; this is in part due to a fairly limited number of studies performed that have attempted to directly relate EI measures to differences in neural structure/function (Bar-On et al. 2003; Barbey et al. 2014; Killgore et al. 2012; Takeuchi et al. 2011; Takeuchi et al. 2013a, b; Takeuchi et al., 2013a, b; Webb et al. 2013) and in part due to disagreement over the most valid and useful way to conceptualize EI (e.g., Conte, 2005; Locke, 2005; Petrides, 2011). Specifically, different proposed measures conceptualize EI in different ways, and it is unclear at present how distinct the construct of EI is from standard intelligence (i.e., IQ) on the one hand, and how distinct it is from standard personality measures on the other (Harms and Crede 2010; Locke 2005; Mayer et al. 2001; Webb et al. 2013). Measures of self-perceived vs. actual performance on EI-related tasks also often disagree with one another (Brackett et al. 2006; Goldenberg et al. 2006), and this further highlights the question of what measures of EI are appropriate to use in neuroimaging studies. In general, therefore, it remains unclear what cognitive and personality factors explain differences in social cognition abilities, and how these relate to the neural networks supporting these abilities.
In the present study, we were interested in exploring the neural correlates of social cognitive ability, with a specific focus on examining what cognitive- and/or personality-related factors may explain individual differences in these abilities. In particular, based on the tension between self-reported and performance-based measures of social-cognitive abilities described above (Brackett et al. 2006; Goldenberg et al. 2006), we were interested in exploring the possibility that self-reported beliefs about one’s own social characteristics/abilities might show distinct relationships with neural network activations from performance-based social ability measures. To do so, we first designed a visually based social judgment task, which we predicted would engage regions of the DMN, executive control network, salience network, and visual cortex that were previously observed in the neuroimaging studies on social cognition discussed above. Second, we had participants complete multiple self-report and performance-based measures of social and cognitive abilities. Self-report measures included widely used personality (Costa and McCrae 1992) and EI questionnaires (Bar-On 2006; Brackett et al. 2006), whereas performance measures included a standard cognitive intelligence (IQ) test (Wechsler 1999), a widely used performance-based EI measure (Mayer et al. 2002), and independent measures of the ability to perceive state- and trait-related psychological attributes in others (Ekman and Friesen 1976). Finally, we ran step-wise multiple regression analyses, using regional brain activation differences as outcome variables and our self-report and performance-based measures as predictor variables. The brain regions we focused on were the task-activated “hub” regions (AI, DMPFC/dACC, and DLPFC) believed to serve large-scale network interactions, as well as task-related regions of visual cortex. The regression analyses described above were done in order to explore whether differences in large-scale brain network activation during a social cognition task can be explained by these other widely used individual differences measures of both cognitive/emotional abilities and self-reported beliefs regarding one’s own social characteristics/abilities. We first predicted that self-report measures would explain significant portions of the variance in activation levels within DMN and salience network hub regions (i.e., AI and DMPFC/dACC), due to the fact that such measures plausibly reflect the consciously accessible beliefs/values contributing to conceptualization and the evaluation of current needs/goals (i.e., the functions most associated with those networks). We further predicted that performance-based measures would instead explain significant portions of the variance in activation levels within executive control network hub regions (i.e., left and right DLPFC) and in visual system activation. This second hypothesis was based on the idea that actual performance should depend on the degree to which executive control systems modulate visual processing in a goal−/task-appropriate manner.
Materials and methods
Participants
23 healthy adults (12 female) participated in the present study. These participants ranged in age from 21 to 43 years (M = 30.78, SD = 6.76). Participants did not have any history of psychiatric, neurological or substance use disorders, and all provided written informed consent prior to participating. Although a subset of behavioral data from some of these participants has been reported elsewhere (Killgore et al. 2012, 2013; Webb et al. 2013), the primary data from the fMRI task and its correlations with behavioral variables are novel and have never been published in any forum. The research protocol of the present study was also reviewed and approved by the US Army Human Research Protections Office, as well as by the Institutional Review Board of McLean Hospital.
Procedure
Social judgment task
While undergoing fMRI scanning, participants were asked to complete a social judgment task. In this task, participants were simultaneously presented with pictures of three male faces per trial (See Fig. 1a), and asked to make a particular condition-specific judgment. In the two “social judgment” conditions, participants were asked to press the button (i.e., out of buttons 1, 2, or 3) corresponding to which of the faces appeared to be the “strongest” or “weakest” of the three. In these conditions, each face set consisted of one strong-dominance, one weak-dominance, and one average-dominance face. Aside from these conditions, there was also a “different” condition in which two of the three faces presented on each trial were identical, whereas the third face was different. Participants were asked to press the button that corresponded to the face that was different from the other two. All faces used in this task were taken from a validated database of computer-generated faces manipulated in shape and reflectance for perceived dominance from low-dominance (−3 SD) to high-dominance (+3 SD) (freely available at: http://tlab.princeton.edu/databases/dominancefaces/) using the FaceGen Modeller program (http://facegen.com) Version 3.1 (Todorov and Oosterhof 2011; Todorov et al. 2013); the use of high-dominance (+3 SD), low-dominance (−3 SD), and average-dominance (0 SD) faces, and their location of visual presentation (i.e., left, right, middle) was counterbalanced across conditions. Twenty-five facial identities were used and counterbalanced in similar fashion. During the task run (inside the scanner), each face set was shown for 4.75 s (followed by a 0.25 s black screen) in a condition-specific block-design format. There were 4 face sets shown in each “different” block (D), whereas there were 6 face sets shown in each “strong” (S) and “weak” (W) block. The block order was: D, W, D, S, D, W, D, S, D, W, D, S. Task stimuli were presented using ePrime2 presentation software (http://www.pstnet.com/eprime.cfm).
a Examples of average-dominance (left), high-dominance (middle), and low-dominance (right) faces used in the Social Judgment Task. b Examples of high-trustworthiness (left) and low-trustworthiness (right) faces used in the Trustworthiness Judgment Task. c FMRI results of Social > Perceptual Judgment. Visible clusters are present within the DMPFC/dACC, left and right DLPFC, left and right AI, left and right ventral visual cortex, left PPC, right thalamus, posterior midbrain, and cerebellum
In addition to the fMRI task described above, participants were also asked to complete a series of other self-report and performance-based measures of both cognitive/emotional abilities and self-reported personality traits.
Cognitive measures
Emotional Intelligence (EI)
In order to assess the role of emotional intelligence in social judgment, participants completed previously validated, commercially available tests of distinct models of EI. One test – the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) – is based on the “Ability model,” which defines emotional intelligence in terms of the cognitive capacities that allow one to reason about and solve emotion-related problems, and assesses EI based on participants’ performance on a range of different tasks/assessments (Mayer et al. 2002). It therefore treats EI as similar to traditional notions of intelligence (Mayer et al. 2001). It uses computer-administered items that are designed to measure abilities such as identifying emotions, understanding the causes of emotions, and utilizing emotions to guide behavior and accomplish goals. The MSCEIT provides a total emotional intelligence score (as well as several subscale scores). For this study, raw scores were converted to scaled scores on the basis of the general normative group, without adjustment for sex. There is also a validated self-report inventory designed to tap into similar EI capacities as the MSCEIT, called the Self-Rated Emotional Intelligence Scale (SREIS) (Brackett et al. 2006), which we used to assess the agreement between actual performance on the abilities measured by the MSCEIT and self-perceived performance with regard to those same abilities. The SREIS is a 19-item self-report questionnaire with items such as “By looking at people’s facial expressions, I recognize the emotions they are experiencing” that are rated on a 5-point Likert scale ranging from 1 (“very inaccurate”) to 5 (“very accurate”).
In contrast, the other major test of EI we used – the Bar-On Emotional Quotient Inventory (EQ-i) – is based on the “Trait model,” which uses self-report inventories (as opposed to problem solving tests), and views EI as set of personal competencies reflecting an individual’s potential to cope with environmental demands (Bar-On 2006). It contains 125 items, and provides a total EI score (as well as several subscale scores). Items consist of statements such as ‘I am aware of the way I feel’ and ‘I do not hold up well under stress’, which must be answered on a five-point Likert scale ranging from ‘Very Seldom or Not True of Me’ to ‘Very Often True of Me or True of Me.’
Standard intelligence
Intelligence quotient (IQ) was assessed with the Wechsler Abbreviated Scale of Intelligence (WASI; Pearson Assessment, Inc., San Antonio, TX) (Wechsler 1999) in order to assess the role of standard intelligence in social judgment. This test provides scores for Full Scale IQ (and also subscale measures of Verbal IQ and Performance IQ). The WASI is a widely used intelligence scale with reported reliability of .98 for Full Scale IQ, with extremely high test-retest reliability; it also correlates .92 with the more comprehensive Wechsler Adult Intelligence Scale-III (WAIS; Pearson Assessment, Inc., San Antonio, TX). A trained and experienced bachelor’s level research assistant (blind to study hypotheses) administered the WASI under the supervision of a licensed doctoral level neuropsychologist.
Emotional facial recognition
As an independent means of assessing the ability to perceptually detect state-related information in others’ faces, participants were asked to complete the Ekman 60 faces test (Ekman and Friesen 1976). This is a computerized emotional recognition task during which participants are presented with photographs of 10 different actors (6 photographs of each), all displaying each of the basic emotions one time (happiness, anger, disgust, fear, surprise, and sadness). Participants are asked to choose which emotion best describes the facial expression shown (from a presented list). Photographs were presented in a pseudorandom order, and a total score (0–60) of correct responses for each participant was calculated.
Facial trustworthiness judgment task
As an independent means of assessing the ability to perceptually detect trait-related information in others’ faces, participants were asked to complete a computerized task, using ePrime2 presentation software (http://www.pstnet.com/eprime.cfm), in which they were shown pairs of faces and asked to decide which of the two presented faces looked most trustworthy (See Fig. 1B). The instructions were also clarified by saying “in other words, if you were in danger or needed help, which person would you be more likely to trust?” Answers were provided by pressing “1″ or “2″ on a laptop, and participants were asked to answer as quickly and accurately as possible (no time limit was enforced). Faces were taken from a validated database of computer-generated faces manipulated in shape and reflectance for perceived trustworthiness from low-trustworthiness (−3 SD below average) to high-trustworthiness (+3 SD above average) (freely available at: http://tlab.princeton.edu/databases/trustworthinessfaces/) using the FaceGen Modeller program (http://facegen.com) Version 3.1 (Todorov and Oosterhof 2011; Todorov et al. 2013). A total of 100 face pairs were presented, 25 at each of 4 levels of difficulty based on the difference in SD level of the faces. Difficulty level 1 used face pairs with values of −3 and +3 SD, level 2 used face pairs with values of −2 and +2 SD, level 3 used face pairs with values of −1 and +1 SD, and level 4 used face pairs with values of −1 and 0 SD. The database includes 25 facial identities, and all identities were used and counterbalanced for location (left/right) and difficulty level.
The motivation for including the two independent of measures of social perception ability described immediately above is that the perception subtests within the MSCEIT have received considerable criticism. In particular, studies have found that scores on these MSCEIT subtests do not correlate with other validated measures of facial emotion perception (Roberts et al. 2006). This leads to the concern that they may not represent sufficiently valid measures of such social perception abilities. Therefore, we were interested in using these independent measures to ensure that we acquired reliable performance-based data regarding individual differences in the ability to perceptually detect socially relevant information.
Personality inventory
To assess the possible role of personality differences in social judgment, participants were asked to complete a computerized version of the NEO Personality Inventory – Revised (NEO-PI-R) (Costa and McCrae 1992). The NEO-PI-R contains 240 items rated on a five-point Likert scale from “strongly disagree” to “strongly agree.” This test has been found to have excellent internal consistency and also shows convergent validity with Eysenck’s personality dimensions (Costa and McCrae 1995; Costa 1996). It provides scores for five domains of personality (Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness), and several “facets” within each domain.
Neuroimaging methods
Neuroimaging was performed using a 3 T (Siemens Tim Trio, Erlangen, Germany) scanner with a 12-channel head coil. T1-weighted structural 3D MPRAGE images were acquired (TR/TE/flip angle =2.1 s/2.25 ms/12 degree) covering 128 sagittal slices (256 × 256) with a slice thickness of 1.33 mm (voxel size =1.33 × 1 × 1). Functional T2*-weighted scans were acquired over 42 transverse slices (3.5 mm thickness). An interleaved sequence was used (TR/TE/flip angle =2.5 s/30 ms/90 degree), and the voxel size of the T2* sequence was 3.5 × 3.5 × 3.5 mm. The field of view (FOV) was 22.4 cm, with a 64 × 64 acquisition matrix.
Image processing
Preprocessing steps on all MRI scans, as well as subsequent statistical analyses, were performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Raw functional images were realigned, unwarped, and coregistered to each subject’s MPRAGE image in accordance with standard algorithms. Images were then normalized to Montreal Neurological Institute (MNI) coordinate space, spatially smoothed (6 mm full-width at half maximum), and resliced to 2 × 2 × 2 mm voxels. The standard canonical hemodynamic response function in SPM was used, low-frequency confounds were minimized with a 128-s high-pass filter, and serial autocorrelation was corrected using the AR(1) function. The Artifact Detection Tool (http://www.nitrc.org/projects/artifact_detect/) was also used to regress out scans as nuisance covariates in the first-level analysis exceeding 3 SD in mean global intensity and scan-to-scan head motion that exceeded 1.0 mm.
Statistical analysis
For each participant, a general linear model was specified to contrast activation within the social judgment task between trials when social judgments were made (i.e., strong/weak) and trials when perceptual judgments were made (i.e., different). Motion regressors (generated by ART – see image processing above) were also added to each of these 1st-level designs. These contrast images were then entered into a second-level SPM analysis (one-sample T-test) to assess the main effect of our contrast of interest (i.e., Social Judgment > Perceptual Judgment). Analyses were thresholded using a height threshold of p < .001 (uncorrected) and family-wise error (FWE) corrected cluster extent threshold of p < .05.
Contrast estimates (i.e., the first eigenvariates) were also extracted from select visual system and “network hub region” clusters that were found to activate within these analyses (see results section). This was done by first individually selecting the activation clusters of interest within SPM8 and then using SPM8’s built-in volume-of-interest (VOI) time-series extraction tool. For each contrast estimate we extracted, the whole activation cluster defined the volume of interest. These contrast estimates were then regressed against our self-report and performance-based measures, to determine the relative contribution of these variables in explaining patterns of brain activation. Specifically, the following categories of explanatory variables were considered: (i) Performance-based cognitive-emotional abilities measures: WASI total IQ score, MSCEIT total score, Ekman 60 total score, facial trustworthiness judgment total accuracy score, and (ii) Self-reported cognitive-emotional trait/ability measures: Bar-On EQi total score, NEO personality inventory 5-factor T-scores (Neuroticism, Extroversion, Openness, Agreeableness, Conscientiousness), and SREIS total scores. Within SPSS 20, these measures were entered into stepwise linear regression models with the eigenvariate of each of our neural activation clusters of interest as the outcome variable. Results were considered significant at p < 0.05. Multiple collinearity checks were also run in SPSS (described further in the results section) to ensure that correlations between predictor variables in these regression analyses were not above acceptable limits.
Results
fMRI activation contrasts
Social judgment > perceptual judgment
As predicted, this contrast revealed significant activation within clusters spanning the left DLPFC, right and left ventral visual cortex, right DLPFC/AI, DMPFC/dACC (bilaterally), left AI, and left PPC. Other activation clusters were also observed within the medial cerebellum and right thalamus (see Table 1 and Fig. 1c). One further cluster within the posterior midbrain was noted because it survived FWE-correction at the peak-level. It did not survive FWE-correction at the cluster level, but this is likely due to the small size of the gray matter nuclei within this subcortical region (e.g., the superior colliculus and periacqueductal gray).
Social judgment task: performance
When required to identify the “different” face from among the sets of three, accuracy scores had a mean of 96 % (+/− 9.8 %), suggesting that this perceptual judgment condition was fairly easy for all participants. Accuracy scores when determining the “strongest/weakest” member of each set of three faces had a numerically lower mean of 82 % (+/− 13 %), suggesting, as expected, that this social judgment condition was more difficult than the perceptual judgment condition. Similarly, average median reaction times across the group were also numerically shorter for the “different” condition (1669.89 +/− 408.83 ms) than for the “strongest” or “weakest” conditions (strong: 2311.52 +/− 332.51 ms; weak: 2320.37 +/− 277.88 ms). The median reaction time was used instead of the mean in order to avoid the possibility that occasional attentional lapses during the task would inappropriately skew the mean toward longer reaction time estimates.
Performance-based and self-report measures
The mean and standard deviation for scores on each of our performance-based and self-report measures is listed in Table 2. Significant predictors of the activation observed within each of our regions of interest (i.e., DMPFC, right DLPFC/AI, left and right ventral visual cortex, left DLPFC, and left AI) are detailed below. These cortical clusters were specifically chosen due to their known participation as hub regions within the salience, DMN, and executive control networks, or, in the case of the visual cortex clusters, because these regions plausibly reflect the targets of top-down modulation from these anterior cognitive control networks (Barrett and Satpute 2013; Barrett and Simmons 2015; Lindquist and Barrett 2012; Sripada et al. 2014; Yeo et al. 2011).
Greater DMPFC/dACC activation was best predicted by lower EQi total scores (β = −0.478, p = 0.008) and lower NEO openness scores (β = −0.418, p = 0.018). EQi scores accounted for 34 % of the variance (R2 = 0.369, p = 0.002), and NEO openness scores accounted for an additional 16 % of the variance (R2 change =0.158, p = 0.018) in DMPFC activation.
In terms of greater right DLPFC/AI activation, the model that best fit the data included higher facial trustworthiness judgment scores (β = 0.592, p = 0.001) and lower SREIS scores (β = −0.592, p = 0.001). Facial trustworthiness judgment scores accounted for 24 % of the variance (R2 = 0.237, p = 0.019), and SREIS scores accounted for an additional 34 % of the variance (R2 change =0.339, p = 0.001) in right DLPFC/AI activation. Although MSCEIT scores were excluded by this stepwise analysis, there was a trend-level positive relationship between MSCEIT total scores and right DLPFC/AI activation (β = 0.266, p = 0.076). We therefore ran a post-hoc correlation, and observed that right DLPFC/AI activation was significantly positively correlated with MSCEIT total scores (r = 0.429, p = 0.041).
Greater left ventral visual cortex activation was best predicted by higher Ekman 60 total scores (β = 0.717, p < 0.001) and higher facial trustworthiness judgment total accuracy scores (β = 0.415, p = 0.002). Ekman 60 total scores accounted for 57 % of the variance (R2 = 0.568, p < 0.001), and facial trustworthiness judgment total scores accounted for an additional 17 % of the variance (R2 change =0.171, p = 0.002) in left ventral visual cortex activation.
For right ventral visual cortex activation, higher Ekman 60 total scores (β = 0.745, p < 0.001), and higher facial trustworthiness judgment scores (β = 0.343, p = 0.004), were also significant independent predictors, as well as lower NEO openness scores (β = −0.305, p = 0.01). Ekman 60 total scores accounted for 54 % of the variance (R2 = 0.542, p < 0.001), facial trustworthiness judgment total scores accounted for an additional 18 % of the variance (R2 change =0.178, p = 0.002), and NEO openness scores accounted for an additional 9 % of the variance (R2 change =0.085, p = 0.01) in right ventral visual cortex activation.
In the model predicting greater left DLPFC activation that best fit the data, lower NEO openness scores (β = −0.417, p = 0.048) were found to be the only significant predictor accounting for 17 % of the variance (R2 = 0.174, p = 0.048).
Lower NEO openness scores (β = −0.457, p = 0.028) were also found to be the only significant predictor of greater left AI activation, accounting for 21 % of the variance (R2 = 0.209, p = 0.028).
For each of the regression analyses described above, we also took multiple measures to ensure that potential collinearity issues did not threaten the validity of our results. First, as suggested by Field (2013), we ran correlation analyses between all predictor variables and confirmed that no predictor variables were correlated above a value of r = 0.8 (average correlation value within this correlation matrix was r = 0.04, SD = 0.26). Second, using SPSS collinearity diagnostics, we confirmed that the highest variance inflation factor (VIF) in these analyses was not greater than 10, and that the average VIF was not substantially greater than 1 (Bowerman and O’Connell 1990).
Discussion
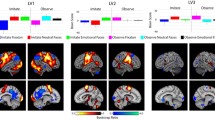
As predicted, we first observed that social judgment, relative to perceptual judgment, activated regions associated with the executive control network (left and right DLPFC, left PPC), salience network (left and right AI and DMPFC/dACC), the DMN (anterior portion of the DMPFC/dACC cluster), and left and right regions of ventral visual cortex known to be involved in facial processing (e.g., Druzgal and D’Esposito, 2001a, 2001b; O’Craven and Kanwisher, 2000; Vuilleumier et al., 2001). The DMPFC/dACC, in particular, has been associated previously with visual evaluations of social dominance (Freeman et al. 2009). Although not predicted in advance, social judgment also activated regions of the cerebellum, right thalamus, and posterior midbrain. These further results are, nevertheless, consistent with current models in which cortical cognitive control networks interact, via subcortical nuclei, with a cerebellar “error” network that functions to optimize cortical network performance through error minimization processes (Dosenbach et al. 2008; Power and Petersen 2013). The posterior midbrain also includes the superior colliculi, which are implicated in the control of eye movements and spatial attention during visual tasks (Krauzlis et al. 2013). Thus, the present findings supported the hypothesized role of the executive control, salience, and default mode networks in social cognition; they also provided the predicted clusters of activation within network hub regions (believed to serve network interactions) that allowed us to explore the relationship between individual difference measures and associated neural responses. Consistent with our a priori hypotheses regarding these individual difference measures, we subsequently observed that distinct performance-based and self-report measures were able to explain a significant proportion of the variance in activation within distinct network regions (Fig. 2). As we describe in more detail below, a broad trend was observed across our results, converging on the overarching theme that lower self-reported confidence in one’s social abilities may promote the allocation of greater cognitive resources to, and therefore improve performance on, actual social-cognitive tasks.
Graphical illustration of the overarching theme observed within our individual difference-based step-wise multiple regression analyses. These findings suggest that stronger responses within large-scale neural networks (and the perceptual systems they modulate) are associated with both better performance (higher MSCEIT, Ekman 60, and Facial Trustworthiness Judgment scores) and reduced self-reported openness/confidence in one’s own social cognitive abilities (lower NEO openness, SREIS, and Bar-On EQi scores). We propose that this may reflect the fact that salience network and DMN regions (AI and DMPFC/dACC) predict the need for increased effort to succeed in individuals with low openness/confidence, and that this promotes increased engagement of DLPFC executive control network functions (which improves performance by promoting additional visual processing). SREIS Self-Rated Emotional Intelligence Scale, MSCEIT Mayer–Salovey–Caruso Emotional Intelligence Test, EQi Bar-On Emotional Quotient Inventory
Individual differences and large-scale network function
First, self-report measures of personality and EI – NEO openness and total EQi scores – were each negatively associated with, and explained significant independent portions of the variance in, activation within the DMPFC/dACC cluster, which overlaps with regions of both the salience network and the DMN and may serve as an important hub for interactions between these networks (van den Heuvel and Sporns 2011, 2013). This is consistent with our a priori hypothesis that self-reportable beliefs/values would be most closely associated with networks that function to evaluate one’s homeostatic/emotional needs and conceptualize sensory input based on expectations/beliefs derived from background knowledge and past experience. Overall, these findings are in line with earlier work showing that self-reported EI was inversely correlated with activation of the medial prefrontal cortex and insular regions (Killgore and Yurgelun-Todd 2007). SREIS scores (another self-report EI measure) were also negatively associated with individual differences in the activation of the right DLPFC/AI cluster; since the right AI is part of the salience network, this is also broadly consistent with what we predicted. However, since this single cluster also encompassed the right DLPFC (an executive control network region), these results are not able to differentiate between the influence of SREIS scores on the salience vs. executive control network regions. However, as the DLPFC and AI are both important hub regions serving interactions between these networks (van den Heuvel and Sporns 2011, 2013), and given that they were both activated together in our task, this suggests that SREIS scores could also relate to important interactions between salience and executive control networks. The inverse relationship between self-perceived competencies and brain activation appears explicable in terms of how self-reported personality traits and self-related beliefs would be expected to interact with perceived salience. That is, it seems plausible that, when trying to evaluate the relative dominance of another person (as in our fMRI task), this would invoke greater concern/perceived salience in a person who was less open to new experiences (i.e., lower NEO openness scores), or less confident in their ability to adaptively assess and respond to emotionally charged social situations (i.e., lower EQi and SREIS scores). This increased salience might also provoke greater use of background knowledge from past experience to construct internal models for use in making these judgments (i.e., greater DMN activation). In general then, we suggest that these findings could be interpreted to indicate that reduced self-reported confidence/openness may lead to increased concern (and the predicted need for greater effort/resources to succeed at a social-cognitive task), and therefore increased salience of task stimuli and motivation to engage greater cognitive control to perform successfully.
We also observed that facial trustworthiness judgment accuracy scores (a performance measure) explained a significant independent portion of the variance in the right DLPFC/AI cluster; this is consistent with our hypotheses regarding the relationship between performance and executive control regions, but it was not a prediction we made regarding the right AI. In contrast to the SREIS, trustworthiness accuracy scores were positively associated with right DLPFC/AI activation. Interestingly, our post-hoc correlation analysis of MSCEIT total score also demonstrated a significant positive relationship between MSCEIT scores and right DLPFC/AI activation; thus, both of these performance measures increased with increasing activation. In contrast, we observed a negative relationship between both the left DLPFC and left AI clusters and NEO openness scores, but no other measure came out significantly associated with these clusters within our stepwise regression analyses. Thus, self-report measures were consistently negatively associated with, and performance measures were consistently positively associated with, the activation of these brain regions believed to serve network interactions. Similar to our suggestion above regarding the DMPFC/dACC and right DLPFC/AI, the observation that lower NEO openness scores were associated with greater left AI and left DLPFC activation could also reflect the fact that making social judgments may be perceived as more concerning/salient (greater left AI activation), and also promote allocation of greater processing resources to the task (greater left DLPFC activation), among those who are less open to new experiences. This account could also explain why greater right DLPFC/AI activation is associated with greater performance (i.e., higher MSCEIT scores and facial trustworthiness judgment accuracy), and also with lower self-reported abilities (i.e. lower SREIS scores). That is, greater cognitive/attentional resources may be allocated to task performance in those who are less confident in their abilities.
Individual differences and sensory cortex function
In further support of the overarching theme we propose, our analyses regarding the left and right ventral visual cortex clusters demonstrated that facial emotion recognition accuracy (as measured by the Ekman 60 test), and facial trustworthiness judgment accuracy, were each positively associated with, and explained significant independent portions of the variance in, visual system activation associated with facial processing. The fact that these areas were more activated in social vs. perceptual judgment, and that greater activation increases predicted better scores on performance-based measures, both suggest that the allocation of additional visual processing resources was required to infer ToM-related information from facial perception. In addition, however, our results suggest that independent portions of the variance in this allocation of additional processing resources are associated with the ability to accurately infer emotions (a state-related psychological attribute) and trustworthiness (a trait-related psychological attribute). The ability to infer these two types of psychological attributes plausibly allows for distinctly useful influences on decision-making. For example, the ability to infer a person’s emotional state has important implications for deciding how to interact with them in the present moment (e.g., if they are angry vs. afraid, different strategies would likely be optimal for helping them feel better), whereas the ability to perceptually infer a psychological trait plausibly has more long-term decision-making implications (e.g., if they are not trustworthy, it would be a better idea to not keep them as a friend). These results therefore highlight the possibility that different internal control “decisions” regarding top-down allocation of visual processing resources may underlie each of these abilities; future research should examine what further factors might contribute to an individual’s ability to learn to infer each of these useful types of information accurately.
Relation to previous literature
To our knowledge, few studies to date have examined the relationship between large-scale network interactions and individual difference measures of social-cognitive abilities, such as EI. However, our results do appear consistent with, and able to build upon, several previous findings. For example, earlier studies have found disagreement between self-report vs. performance-based measures of EI (Brackett et al. 2006; Goldenberg et al. 2006); relatedly, previous studies have found that self-reported EI measures are related to personality variables, whereas performance-based EI measures are related to IQ scores (KV Petrides et al. 2007; Webb et al. 2013). Our findings appear consistent with this work, but also suggest a way in which the beliefs/values reflected in self-reported EI and personality measures may promote cognitive resource allocation in a way that can improve performance. This may therefore clarify the nature of the disagreements previously observed between self-reported and performance-based measures, and highlight how they may influence the neural networks that implement social-cognitive functions. Our results also build on previous neuroimaging work that has explicitly examined correlates of social-cognitive abilities. For example, previous studies have found that differences in gray matter and white matter within the insula are related to differences in self-reported EI (Takeuchi et al. 2011; Takeuchi et al., 2013a, b), as are resting state functional connectivity estimates associated with the medial frontal cortex, DLPFC, and visual cortical regions (Takeuchi et al., 2013a, b), as well as cerebellum (Pan et al. 2014). Measures of ability EI have also been shown to correlate with gray matter volume of the insular cortex (Killgore et al. 2012; Tan et al. 2014) and greater functional responsiveness of the insular cortex to dynamically changing expressions communicating trustworthiness (Killgore et al. 2013). The visual cortex regions we observed also overlap with the same regions found in meta-analyses of emotion face processing (Fusar-Poli et al., 2009a, b; Fusar-Poli et al., 2009a, b). Our results appear to offer additional insights, however, regarding the way activity in these regions relates to individual differences in multiple conceptually distinct performance measures.
Limitations and conclusion
The present study has several limitations. First, the sample size is only moderate, and therefore future research should attempt to replicate the relationships we observed between performance-based/self-report measures and activation of particular large-scale neural network regions. Given that our fMRI task only presented male faces, a replication study (using a larger sample size) might also examine the possibility that neural responses in some brain regions could show gender interactions. Second, although our study was guided by some broad a priori hypotheses, the stepwise multiple regression approach we used, and the different measures we gathered, served an exploratory function. These findings should therefore be seen as mainly hypothesis-generating, which further highlights the importance for future studies to replicate the particular directional relationships we observed. In particular, it was somewhat surprising that WASI total IQ scores did not account for a significant proportion of the variance in the activation of any of the brain regions examined. This could perhaps suggest that IQ is less relevant to the type of social-cognitive task we used, or alternatively, it is possible that this was due to some partially shared variance between IQ scores and other stronger predictors in our analyses. Therefore, future studies should employ additional statistical analyses to further examine such issues. Third, it is notable that none of the cluster activations we observed were associated with accuracy on the social judgment task itself. This is surprising, as judgments of dominance appear to reflect a similar trait-related inference as that of facial trustworthiness, which were in fact, related to brain activation. We suggest that this is probably best explained by the fact that the dominance judgments were designed to be considerably easier than facial trustworthiness judgments, particularly in the difficult conditions of the facial trustworthiness task in which the faces presented were only separated by one standard deviation (in terms of the previously rated trait features). Therefore, the greater difficulty, reflected in the lower mean accuracy (68 % vs. 82 %), in trustworthiness vs. dominance judgment may have helped to bring out more meaningful individual differences. It is also possible, however, that accuracy differences may be explained by activations in the midbrain/cerebellar clusters which we chose not to further examine.
Fourth, although most of our chosen performance-based and self-report measures are previously validated, it bears highlighting that the facial trustworthiness task and the social judgment task were of our own creation. Thus, despite the fact that the trustworthiness/dominance face sets we used have been validated in previous work, less is known about the exact psychometric properties of the particular ways in which we chose to use them. Fifth, as we also highlighted above, the activation clusters we observed did not always permit distinguishing the contributions of neighboring cortical areas. Specifically, a single cluster spanned the right DLPFC and AI, and another spanned the left and right DMPFC/dACC, which created difficulty in discerning the distinct functional contributions of these regions. Therefore, future studies should perhaps take specific steps (such as using a smaller smoothing kernel) to facilitate the ability to observe and analyze more localized activation clusters in these regions. Finally, as in previous work (e.g., Oosterwijk et al. 2012), we chose to investigate network activation through examining the responses of brain regions known to participate in those networks. However, this strategy is limited by the fact that it does not allow inferences about interactions between regions of activation. Therefore, future work should also perform independent component analyses (ICA) to further examine the relation between individual difference measures and the distributed patterns of correlated neural activity that are detectable with such methods.
In conclusion, using a social judgment task to activate the multiple neural networks known to be engaged by, and interact during, successful social cognition, we were able to identify particular contributions of different individual difference factors to the functioning of hub regions thought to subserve interactions between these distinct contributing networks. First, we found that lower self-reported traits related to social and emotional competence were associated with greater activation of network regions that are involved in assessing the salience of, conceptualizing, and directing cognitive resources to task-relevant perception. Second, we found that higher scores on measures of better objective social/emotional performance were associated with greater activation within executive control regions as well as the visual processing regions modulated by them. Together these findings suggest that better performance is associated with greater allocation of cognitive processing resources, and that the decision to allocate resources in this way may be promoted by lower subjective estimates of performance ability (i.e., those who think they are the least capable may put forth the greatest cognitive effort). Finally, we found evidence that distinct portions of variance in activation within visual processing regions may account for the ability to correctly infer state-related vs. trait-related psychological information about others. If replicated in future work, these results may have important implications for the interaction between self-reported beliefs, objective performance, and interacting large-scale neural networks within the context of adaptive social functioning.
References
Adolphs, R. (2009). The social brain: neural basis of social knowledge. Annual Review of Psychology, 60, 693–716. doi:10.1146/annurev.psych.60.110707.163514.
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–277.
Bar-On, R. (2006). BarOn Emotional Quotient Inventory: a measure of emotional intelligence – technical manual. North Tonawanda, NY: Multi-Health Systems.
Bar-On, R., Tranel, D., Denburg, N., & Bechara, A. (2003). Exploring the neurological substrate of emotional and social intelligence. Brain, 126(Pt 8), 1790–1800. doi:10.1093/brain/awg177.
Barbey, A., Colom, R., & Grafman, J. (2014). Distributed neural system for emotional intelligence revealed by lesion mapping. Social Cognitive and Affective Neuroscience, 9(3), 265–272. doi:10.1093/scan/nss124.
Barrett, L., & Satpute, A. (2013). Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Current Opinion in Neurobiology, 23(3), 361–372. doi:10.1016/j.conb.2012.12.012.
Barrett, L., & Simmons, W. (2015). Interoceptive predictions in the brain. Nature reviews. Neuroscience, 16(7), 419–429. doi:10.1038/nrn3950.
Bowerman, B., & O’Connell, R. (1990). Linear statistical models: An applied approach (2nd ed.). Belmont, CA: Duxbury.
Brackett, M., Rivers, S., Shiffman, S., Lerner, N., & Salovey, P. (2006). Relating emotional abilities to social functioning: a comparison of self-report and performance measures of emotional intelligence. Journal of Personality and Social Psychology, 91(4), 780–795. doi:10.1037/0022-3514.91.4.780.
Brown, H., Friston, K., & Bestmann, S. (2011). Active inference, attention, and motor preparation. Frontiers in Psychology, 2, 218. doi:10.3389/fpsyg.2011.00218.
Conte, J. (2005). A review and critique of emotional intelligence measures. Journal of Organizational Behavior, 26(4), 433–440. doi:10.1002/job.319.
Costa, P. (1996). Work and personality: use of the NEO-PI-R in industrial/organisational psychology. Applied Psychology, 45, 225–241.
Costa, P., & McCrae, R. (1992). Revised NEO Personality Inventory (NEOPI- R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources, Inc..
Costa, P., & McCrae, R. (1995). Primary traits of Eysenck’s P-E-N system: three- and five-factor solutions. Journal of Personality and Social Psychology, 69, 308–317.
Davis, S., & Humphrey, N. (2012). Emotional intelligence predicts adolescent mental health beyond personality and cognitive ability. Personality and Individual Differences, 52(2), 144–149. doi:10.1016/j.paid.2011.09.016.
Decety, J., & Sommerville, J. (2003). Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences, 7, 527–533.
Dosenbach, N., Fair, D., Cohen, A., Schlaggar, B., & Petersen, S. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. doi:10.1016/j.tics.2008.01.001.
Druzgal, T., & D’Esposito, M. (2001a). Activity in fusiform face area modulated as a function of working memory load. Cognitive Brain Research, 10(3), 355–364. doi:10.1016/S0926-6410(00)00056-2.
Druzgal, T., & D’Esposito, M. (2001b). A neural network reflecting decisions about human faces. Neuron, 32(5), 947–955. doi:10.1016/S0896-6273(01)00519-0.
Ekman, P., & Friesen, W. (1976). Pictures of facial Affect. Palo Alto, CA: Consulting Psychologists Press.
Feldman, H., & Friston, K. (2010). Attention, uncertainty, and free-energy. Frontiers in Human Neuroscience, 4, 215. doi:10.3389/fnhum.2010.00215.
Fernández-Berrocal, P., Alcaide, R., Extremera, N., & Pizarro, D. (2006). The role of emotional intelligence in anxiety and depression among adolescents. Individual Differences Research, 4(1), 16–27.
Field, A. (2013). Discovering statistics using IBM SPSS statistics (4th ed.). SAGE Publications.
Freeman, J., Rule, N., Adams, R., & Ambady, N. (2009). Culture shapes a mesolimbic response to signals of dominance and subordination that associates with behavior. NeuroImage, 47(1), 353–359. doi:10.1016/j.neuroimage.2009.04.038.
Frith, C., & Frith, U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–534.
Fusar-Poli, P., Placentino, A., Carletti, F., Allen, P., Landi, P., Abbamonte, M., et al. (2009b). Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters, 452(3), 262–267. doi:10.1016/j.neulet.2009.01.065.
Fusar-Poli, P., Placentino, A., Carletti, F., Landi, P., Allen, P., Surguladze, S., et al. (2009a). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34(6), 418–432.
Goldenberg, I., Matheson, K., & Mantler, J. (2006). The assessment of emotional intelligence: a comparison of performance-based and self-report methodologies. Journal of Personality Assessment, 86(1), 33–45. doi:10.1207/s15327752jpa8601_05.
Gu, X., Hof, P., Friston, K., & Fan, J. (2013). Anterior insular cortex and emotional awareness. The Journal of Comparative Neurology, 521(15), 3371–3388. doi:10.1002/cne.23368.
Harms, P., & Crede, M. (2010). Remaining issues in emotional intelligence research: construct overlap, method artifacts, and lack of incremental validity. Industrial and Organizational Psychology, 3(2), 154–158. doi:10.1111/j.1754-9434.2010.01217.x.
Heatherton, T. (2006). Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience, 1, 18–25.
Hertel, J., Schütz, A., & Lammers, C.-H. (2009). Emotional intelligence and mental disorder. Journal of Clinical Psychology, 65(9), 942–954. doi:10.1002/jclp.20597.
Keysers, C., Kaas, J. H., & Gazzola, V. (2010). Somatosensation in social perception. Nature reviews. Neuroscience, 11(6), 417–428. doi:10.1038/nrn2833.
Killgore, W., Schwab, Z., Tkachenko, O., Webb, C., DelDonno, S., Kipman, M., et al. (2013). Emotional intelligence correlates with functional responses to dynamic changes in facial trustworthiness. Social Neuroscience, 8(4), 334–346. doi:10.1080/17470919.2013.807300.
Killgore, W., Weber, M., Schwab, Z., DelDonno, S., Kipman, M., Weiner, M., & Rauch, S. (2012). Gray matter correlates of trait and ability models of emotional intelligence. Neuroreport, 23(9), 551–555. doi:10.1097/WNR.0b013e32835446f7.
Killgore, W., & Yurgelun-Todd, D. (2007). Neural correlates of emotional intelligence in adolescent children. Cognitive, Affective, & Behavioral Neuroscience, 7(2), 140–151. doi:10.3758/CABN.7.2.140.
Krauzlis, R., Lovejoy, L., & Zénon, A. (2013). Superior colliculus and visual spatial attention. Annual Review of Neuroscience, 36, 165–182. doi:10.1146/annurev-neuro-062012-170249.
Li, W., Mai, X., & Liu, C. (2014). The default mode network and social understanding of others: what do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8, 74. doi:10.3389/fnhum.2014.00074.
Lindquist, K., & Barrett, L. (2012). A functional architecture of the human brain: emerging insights from the science of emotion. Trends in Cognitive Sciences, 16(11), 533–540. doi:10.1016/j.tics.2012.09.005.
Locke, E. (2005). Why emotional intelligence is an invalid concept. Journal of Organizational Behavior, 26(4), 425–431. doi:10.1002/job.318.
Lombardo, M. V., Chakrabarti, B., Bullmore, E. T., Wheelwright, S. J., Sadek, S. A., Suckling, J., & Baron-Cohen, S. (2010). Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience, 22(7), 1623–1635. doi:10.1162/jocn.2009.21287.
Lopes, P., Salovey, P., Coté, S., & Beers, M. (2005). Emotion regulation abilities and the quality of social interaction. Emotion, 5(1), 113–118. doi:10.1037/1528-3542.5.1.113.
Mayer, J., Salovey, P., & Caruso, D. (2002). Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) – User’s Manual. North Tonawanda, NY: Multi-Health Systems.
Mayer, J., Salovey, P., & Caruso, D. (2004). Emotional intelligence: theory, findings, and implications. Psychological Inquiry, 15(3), 197–215.
Mayer, J., Salovey, P., Caruso, D., & Sitarenios, G. (2001). Emotional intelligence as a standard intelligence. Emotion, 1(3), 232–242.
Meyer, M., Taylor, S., & Lieberman, M. (2015). Social working memory and its distinctive link to social cognitive ability: an fMRI study. Social cognitive and affective neuroscience, nsv065–. doi:10.1093/scan/nsv065
O’Boyle, E., Humphrey, R., Pollack, J., Hawver, T., & Story, P. (2011). The relation between emotional intelligence and job performance: A meta-analysis. Journal of Organizational Behavior, 32(5), 788–818. doi:10.1002/job.714.
O’Craven, K. M., & Kanwisher, N. (2000). Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience, 12(6), 1013–1023. doi:10.1162/08989290051137549.
Oosterwijk, S., Lindquist, K., Anderson, E., Dautoff, R., Moriguchi, Y., & Barrett, L. (2012). States of mind: emotions, body feelings, and thoughts share distributed neural networks. NeuroImage, 62(3), 2110–2128. doi:10.1016/j.neuroimage.2012.05.079.
Pan, W., Wang, T., Wang, X., Hitchman, G., Wang, L., & Chen, A. (2014). Identifying the Core Components of Emotional Intelligence : Evidence from Amplitude of Low-Frequency Fluctuations during Resting State, 9(10), 1–8. doi:10.1371/journal.pone.0111435
Pérez, J., Petrides, K., & Furnham, A. (2005). Measuring trait emotional intelligence. In R. Schulze & R. Roberts (Eds.), Emotional intelligence: An International Handbook (pp. 181–202). Hogrefe.
Petrides, K., Pita, R., & Kokkinaki, F. (2007). The location of trait emotional intelligence in personality factor space. British journal of psychology (London, England : 1953), 98(Pt 2), 273–289. doi:10.1348/000712606X120618.
Petrides, K. V. (2011). Ability and trait emotional intelligence. The Wiley-Blackwell Handbook of Individual Differences, 656–678. doi:10.1002/9781444343120.ch25.
Power, J., & Petersen, S. (2013). Control-related systems in the human brain. Current Opinion in Neurobiology, 23(2), 223–228. doi:10.1016/j.conb.2012.12.009.
Roberts, R., Schulze, R., O’Brien, K., MacCann, C., Reid, J., & Maul, A. (2006). Exploring the validity of the Mayer-Salovey-Caruso emotional intelligence test (MSCEIT) with established emotions measures. Emotion, 6(4), 663–669. doi:10.1037/1528-3542.6.4.663.
Schutte, N., Malouff, J., Simunek, M., McKenley, J., & Hollander, S. (2010). Characteristic emotional intelligence and emotional well-being. Cognition and Emotion, 16(6), 769–785. doi:10.1080/02699930143000482.
Seidel, E., Eickhoff, S., Kellermann, T., Schneider, F., Gur, R. C., Habel, U., & Derntl, B. (2010). Who is to blame? neural correlates of causal attribution in social situations. Social Neuroscience, 5(4), 335–350. doi:10.1080/17470911003615997.
Sripada, C., Angstadt, M., Kessler, D., Phan, K., Liberzon, I., Evans, G., et al. (2014). Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. NeuroImage, 89, 110–121. doi:10.1016/j.neuroimage.2013.11.006.
Takeuchi, H., Taki, Y., Nouchi, R., Sekiguchi, A., Hashizume, H., Sassa, Y., et al. (2013a). Resting state functional connectivity associated with trait emotional intelligence. NeuroImage, 83, 318–328. doi:10.1016/j.neuroimage.2013.06.044.
Takeuchi, H., Taki, Y., Sassa, Y., Hashizume, H., Sekiguchi, A., Fukushima, A., & Kawashima, R. (2011). Regional gray matter density associated with emotional intelligence: evidence from voxel-based morphometry. Human Brain Mapping, 32(9), 1497–1510. doi:10.1002/hbm.21122.
Takeuchi, H., Taki, Y., Sassa, Y., Hashizume, H., Sekiguchi, A., Nagase, T., et al. (2013b). White matter structures associated with emotional intelligence: evidence from diffusion tensor imaging. Human Brain Mapping, 34(5), 1025–1034. doi:10.1002/hbm.21492.
Tan, Y., Zhang, Q., Li, W., Wei, D., Qiao, L., Qiu, J., et al. (2014). The correlation between emotional intelligence and gray matter volume in university students. Brain and Cognition, 91, 100–107. doi:10.1016/j.bandc.2014.08.007.
Taylor, K., Seminowicz, D., & Davis, K. (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping, 30(9), 2731–2745. doi:10.1002/hbm.20705.
Todorov, A., Dotsch, R., Porter, J., Oosterhof, N., & Falvello, V. (2013). Validation of data-driven computational models of social perception of faces. Emotion, 13(4), 724–738. doi:10.1037/a0032335.
Todorov, A., & Oosterhof, N. (2011). Modeling social perception of faces. Signal Processing Magazine, IEEE, 28, 117–122.
van den Heuvel, M., & Sporns, O. (2011). Rich-club organization of the human connectome. The Journal of Neuroscience, 31(44), 15775–15786. doi:10.1523/JNEUROSCI.3539-11.2011.
van den Heuvel, M., & Sporns, O. (2013). An anatomical substrate for integration among functional networks in human cortex. The Journal of Neuroscience, 33(36), 14489–14500. doi:10.1523/JNEUROSCI.2128-13.2013.
van den Heuvel, M., Stam, C., Boersma, M., & Hulshoff Pol, H. (2008). Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage, 43(3), 528–539. doi:10.1016/j.neuroimage.2008.08.010.
Van Overwalle, F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30(3), 829–858. doi:10.1002/hbm.20547.
Vuilleumier, P., Armony, J. L., Driver, J., & Dolan, R. J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron, 30(3), 829–841.
Webb, C., Schwab, Z., Weber, M., DelDonno, S., Kipman, M., Weiner, M., & Killgore, W. (2013). Convergent and divergent validity of integrative versus mixed model measures of emotional intelligence. Intelligence, 41(3), 149–156. doi:10.1016/j.intell.2013.01.004.
Wechsler, D. (1999). Wechsler abbreviated scale of intelligence. Bloomington, MN: Psychological Corporation.
Yeo, B., Krienen, F., Sepulcre, J., Sabuncu, M., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi:10.1152/jn.00338.2011.
Zaki, J., & Ochsner, K. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–680. doi:10.1038/nn.3085.
Zippo, A., Gelsomino, G., Van Duin, P., Nencini, S., Caramenti, G., Valente, M., & Biella, G. (2013). Small-world networks in neuronal populations: a computational perspective. Neural Networks, 44, 143–156. doi:10.1016/j.neunet.2013.04.003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was funded by a USAMRAA grant to WDSK (grant number W81XWH-09-1-0730).
Conflict of interest
Ryan Smith declares that he has no conflict of Interest. Anna Alkozei declares that she has no conflict of interest. W.D. “Scott” Killgore declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Smith, R., Alkozei, A. & Killgore, W.D.S. Contributions of self-report and performance-based individual differences measures of social cognitive ability to large-scale neural network functioning. Brain Imaging and Behavior 11, 685–697 (2017). https://doi.org/10.1007/s11682-016-9545-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9545-2