Abstract
Transcutaneous vagal nerve stimulation (TVNS) is a promising complementary method of pain relief. However, the neural networks associated with its analgesic effects are still to be elucidated. Therefore, we conducted two functional magnetic resonance imaging (fMRI) sessions, in a randomized order, with twenty healthy subjects who were exposed to experimental heat pain stimulation applied to the right forearm using a Contact Heat-Evoked Potential Stimulator. While in one session TVNS was administered bilaterally to the concha auriculae with maximal, non-painful intensity, the stimulation device was switched off in the other session (placebo condition). Pain thresholds were measured before and after each session. Heat stimulation elicited fMRI activation in cerebral pain processing regions. Activation magnitude in the secondary somatosensory cortex, posterior insula, anterior cingulate and caudate nucleus was associated with heat stimulation without TVNS. During TVNS, this association was only seen for the right anterior insula. TVNS decreased fMRI signals in the anterior cingulate cortex in comparison with the placebo condition; however, there was no relevant pain reducing effect over the group as a whole. In contrast, TVNS compared to the placebo condition showed an increased activation in the primary motor cortex, contralateral to the site of heat stimulation, and in the right amygdala. In conclusion, in the protocol used here, TVNS specifically modulated the cerebral response to heat pain, without having a direct effect on pain thresholds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcutaneous vagal nerve stimulation (TVNS; Ventureyra 2000) was proposed as a non-invasive alternative to vagal nerve stimulation with implanted stimulators, the latter is currently used as an adjunctive therapy for epilepsy and medication-resistant major depression and is declared as a promising treatment for sleep and anxiety disorders, obesity and chronic pain (George et al. 2007; Multon and Schoenen 2005). Based on anatomic data, TVNS is usually applied to the external acoustic meatus and concha auricle that are innervated by afferent fibers of the auricular branch of the vagal nerve (Gray 1918; Peuker and Filler 2002). These afferents terminate in the brain stem nuclei of the vagal and trigeminal nerves, mainly including the ipsilateral nucleus solitarius, the principal sensory and spinal trigeminal nuclei (Nomura and Mizuno 1984). These structures are known to be involved in the transmission and processing of nociceptive stimulation, whereas analgesic effects of direct vagal stimulation seem to be mediated through the nucleus tractus solitarii (Aicher and Randich 1988). Previous fMRI investigations of TVNS in healthy volunteers confirmed an involvement of neurons in the solitary tract and the locus coeruleus in the brain stem (Dietrich et al. 2008; Kraus et al. 2013). These regions were shown to be a part of the subcortical anti-nociceptive neural network (Randich et al. 1990). The solitary tract has projections to the rostral ventromedial medulla, the periaqueductal gray, the locus coeruleus, the hypothalamus and the limbic forebrain, modulating arousal and pain perception (Rinaman 2010; Ossipov et al. 2010). When applying TVNS to the anterior wall of the acoustic meatus, a decrease of fMRI signal in limbic brain areas, including the amygdala, hippocampus, parahippocampal gyrus and the posterior cingulate cortex, was demonstrated (Dietrich et al. 2008; Kraus et al. 2007; Kraus et al. 2013). These findings suggest that TVNS targets the regulation of emotional and autonomic components in the cerebral processing of pain.
Pilot investigations found anti-nociceptive effects of TVNS on experimentally evoked pain in healthy volunteers and in patients with chronic pelvic pain (Napadow et al. 2012). Therefore, an anti-nociceptive effect of TVNS might not only be expressed in a decrease of neuronal activation in brain regions associated with the processing of pain (anterior insular cortex (AIC), secondary somatosensory cortex (S2), anterior cingulate cortex (ACC), prefrontal cortex (PFC), caudate nucleus (NC; Friebel et al. 2011), but also with areas regulating the autonomic nervous system (Becerra et al. 1999; Dietrich et al. 2008; Kraus et al. 2013).
Regarding these recent findings and aiming to further elucidate the anti-nociceptive mechanisms of non-invasive vagal nerve stimulation, we investigated the reaction of healthy volunteers to experimentally induced heat pain and their cerebral fMRI responses under TVNS in comparison with a placebo intervention. We expected TVNS to increase the heat pain threshold of study participants and to reduce the neuronal activation in brain regions involved in the processing of pain. Furthermore, we expected a modulation of areas associated with processing autonomic responses under TVNS.
Methods
Design and participants
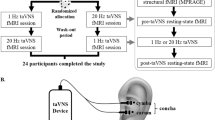
Twenty healthy right-handed volunteers (nine females), with a mean age of 28 years (range 24–44), all Caucasians, were recruited via advertisement according to eligibility criteria. None of the participants was taking medication or recreational drugs at the time of the study. The local ethics commission of the Medical Faculty of the University Medicine of Greifswald approved the protocol of this investigation and informed consent was obtained from each volunteer. Each participant underwent two fMRI sessions in a crossover manner with an inter-session-interval of at least 1 week. In both study sessions, experimental heat pain was applied and treated in randomized order with either TVNS or a placebo intervention with an inactive device. All participants were blinded in regard to the purpose of the investigation by instructing them that they would receive painful stimulation during both study sessions and would be treated with TVNS of either low or high intensity. Each study session lasted about 45 min.
Experimental pain
Experimental heat pain was elicited using a Contact Heat-Evoked Potential Stimulator (PATHWAY Model CHEPS; Medoc; Israel). A diamagnetic thermode, containing a Peltier element with 27 mm in diameter, was attached to the middle of the inner part of the right forearm (Fig. 1a). The stimulator itself was located outside the scanner room and synchronized with the MR scanner over Transmitted Through Light (TTL) signals. Individual heat pain thresholds were measured using a “Limits” test paradigm (PATHWAY Pain and Sensory Evaluation System 2008) before and after every fMRI session. During each measurement (trial), the temperature of the contact surface increased from a baseline temperature of 32 °C with a heating rate of 1.5 °C/s. The participants were instructed to stop the heating by pressing the button of the response unit as soon as they felt pain. Four trials with an inter-stimulus-interval of 2 s were performed and pain threshold was calculated as the mean value of these 4 trials, using the accompanied software PATHWAY 3.5 (Medoc Ltd., Israel). The “Ramp and Hold” paradigm (PATHWAY Pain and Sensory Evaluation System 2008) was used to induce the experimental heat pain during the fMRI sessions. The stimulation temperature, which was synchronized with MRI, was oscillating between a baseline temperature (32 °C) and the participants’ heat pain threshold, measured before the fMRI procedure, with a ramp rate of 5 °C/s. One fMRI session consisted of 8 blocks, each lasting 48 s (16 s of stimulation, 32 s of pause, Fig. 1b). After the fMRI session, participants’ heat pain threshold was measured again.
TVNS procedure
Bilateral electrical stimulation was applied to the concha of the auricle (Fig. 1a) using a self-manufactured electrode, sized 9 × 9 × 2.1 mm, described elsewhere in detail (Leutzow et al. 2013). All parts of the stimulation electrode that had been made of stainless steel were replaced by silver wire with 0.3 mm in diameter. The contact surfaces of each electrode, containing the silver wires wrapped in wool, were moistened with 0.9 % NaCl solution before each session to achieve optimal conductivity. Then the electrodes were tightly fixed inside the auricular concha, using commercially available anti-noise earplugs, made from polyurethane foam. The self-manufactured, isolated conductive silver wires were connected to a DoloBravo Dual Channel Neurostimulator (MTR GmbH, Germany) that was located outside the MR scanner. The cables were placed parallel to the body of the participant (Fig. 1a).
During the TVNS session, the device was used in the continuous mode, yielding square impulses with a frequency of 8 Hz and an impulse duration of 200 μs. At the beginning of the TVNS session, the current intensity was adjusted individually for each subject and each ear separately to a maximal level without evoking pain. For that purpose, the intensity of TVNS, perceived as a tingling sensation, was slowly increased and the participants were interviewed continuously, until the optimal, non-painful level was achieved. At the beginning of the placebo session, the stimulation was at first performed with intensity just over the sensory perception threshold. When the participants reported a weak perception of stimulation, the intensity was progressively reduced to a sub-threshold intensity within 10 s and switched off out of the subjects' view.
MRI measurement
MRI data were obtained using a 3 T MRI scanner (Siemens Verio, Erlangen, Germany) equipped with a 12-channel head coil. For each scanning session, field homogeneity was optimized by a shimming sequence. A T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence was recorded as a structural dataset (repetition time (TR): 1.69 s; echo time (TE): 2.52 ms; 176 sagittal slices; voxel size: 1 × 1 × 1 mm3; 2 times generalized autocalibrating partially parallel acquisition (GRAPPA) acceleration). In addition, a gradient echo sequence with 33 phase and magnitude images (TR1: 488 ms, TR2: 131 ms, TE1: 4.92 ms, TE2: 7.38 ms, α = 60°, FoV: 210 mm, slice spacing: 3.99 mm) was acquired to calculate a field map to correct geometric distortions in the echo planar images (EPI). EPI was acquired during the two sessions using the same field of view (FoV) as in the gradient echo sequence (TR: 2000 ms, TE: 23 ms, FoV: 210 mm, slice spacing: 3.99 mm, matrix: 104 ms, TE: 23 ms, voxel size 2 × 2 × 3 mm3). EPI and the gradient echo sequence were aligned with the anterior commissure-posterior commissure. The imaging volume covered the whole neurocranium including cerebellum and brainstem. A total of 33 slices were acquired for each of the 192 volumes recorded during one session. The pure measuring time of the protocol with the block design paradigm (Fig. 1a) took 12 min and 14 s plus the duration of the shimming sequence. Before each fMRI measurement, three pilot EPIs of the whole head during TVNS were obtained. In case of visible artifacts, electrodes and cable orientation in the head coil were corrected to avoid any visible artifacts during scanning.
fMRI data processing
Preprocessing and data analysis were performed with SPM8 (Wellcome Department of Imaging Neuroscience, London) implemented in MATLAB (MathWorks Inc., Natick, MA). Each time series was realigned after unwarping in the phase encoding direction to account for movement and susceptibility artifacts. The EPI-data were subsequently co-registered to the T1-weighted anatomical image, segmented, spatially normalized, smoothed with a Gaussian filter of 8 × 8 × 8 mm3 full width at half maximum and high-pass filtered (cut-off: 128 s). Preprocessed data were statistically evaluated for each individual using general linear modeling. A design matrix was created using a canonical hemodynamic response function. Contrast images of each participant were used for group statistics with a random effects design. Regions of interest (ROI) analysis, corrected for multiple comparisons within the ROIs with p < 0.05 (family wise error; FWE), were performed. Therefore, we selected areas known to be involved in the cerebral processing of thermal pain (for meta-analysis see Friebel et al. 2011), namely the anterior and posterior insula cortices (AIC, PIC), the somatosensory cortices (S1, S2), the ACC and the thalamus. Moreover, we added the primary motor cortex (M1c) because Brown and colleagues (2011) found classification of thermal stimuli as painful to be associated with greater fMRI-responses in the bilateral mid-insular cortices, bilateral S2, contralateral PIC, contralateral S1 and the contralateral M1c. Besides, we included the amygdala in our ROI-analysis, since it was shown to play an important role in the expectancy of pain (Ziv et al. 2010). Furthermore, areas whose activation is known to be correlated with vegetative stimulation were selected as ROIs: the ventral prefrontal cortex (PFC), the caudate nucleus (CN) (Freund et al. 2009), the dorsal brain stem and the hypothalamus (Dietrich et al. 2008; Kraus et al. 2013; Rinaman 2010; Becerra et al. 1999). For anatomic description and ROI-definition, the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al. 2002) was used. A volume of interest for the hypothalamus was created by a 5 mm centered sphere around the fMRI activation maxima at the coordinates [x = 10, y = 0, z = −4] in the Talairach space reported in a study on vegetative modulation (Kroemer et al. 2013).
To examine associations of fMRI activation of areas in the pain matrix and pain reduction by TVNS, the highest beta-values per ROI for the contrast, differentiating the verum from the placebo condition in all participants showing pain reduction under TVNS, were correlated. A linear regression analysis was performed for fMRI activation during each condition (TVNS or placebo) over all participants with changes of individual heat pain thresholds before and after the study session as a factor.
Analysis of pain thresholds
Differences in heat pain thresholds were analyzed using a repeated measures ANOVA (SPSS-Statistics; Version 22.0, IBM Corp., New York, USA) with TIME (before and after intervention) and INTERVENTION (TVNS and placebo) as within subject factors. P-values less than 0.05 were considered as statistically significant.
Results
Pain thresholds
All 20 recruited volunteers completed both study sessions successfully. The mean intensity of TVNS was 7.6 mA (range 5.0-11.5). Group (ANOVA) and post-hoc analysis did not reveal differences among the participants for the TIME and INTERVENTION factors (thresholds before: placebo 44.67 ± 0.42 °C, TVNS 44.23 ± 0.38 °C; thresholds after: placebo 44.49 ± 0.42 °C, TVNS 43.95 ± 0.49 °C). Subgroup analysis revealed that eight participants reacted with significantly increased threshold of experimental heat pain (“responder”) after TVNS (t = −3.35; P = 0.024), whereas 12 reacted with a decreased pain threshold (“non-responder”) after TVNS (t = 3.39; P = 0.012; Fig. 3a and Supplementary table). Responders and non-responders did not differ in age (p = 0.51) and gender (p = 0.66).
fMRI activation
During pain stimulation of the right forearm under placebo intervention, fMRI signal increased in the left anterior insula, the right posterior insula, the right thalamus and the anterior cingulate cortex (Table 1). During pain stimulation combined with TVNS, the left anterior insula, the posterior insula bilaterally, the right ventromedial PFC and the right caudate nucleus showed significant activation (Table 1).
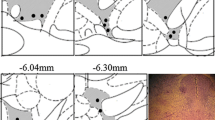
MRI-activation in the right S2, the left posterior insula as well as bilateral in the caudate nucleus and ACC was positively associated with the changes of heat pain threshold during placebo stimulation (Table 2; for insula location see Fig. 2 top). However, during TVNS, the association with changes of heat pain threshold was only seen for activation in the right anterior insula (Fig. 2 bottom; t = 5.55; MNI coordinates (x, y, z): 34, 8, 9; pFWE = 0.025).
Positive associations of insula activation with stimulation temperature. a fMRI-activation magnitude in the posterior left insula was associated with stimulation temperature during placebo stimulation. b fMRI-activation magnitude in the right anterior insula was associated with stimulation temperature during TVNS stimulation
Contrast analysis revealed decreased ACC activation during stimulation with TVNS in comparison with the placebo condition (placebo minus TVNS; t = 4.78; MNI coordinates (x, y, z):-16, 40, 3; pFWE = 0.028) and increased fMRI activation in the right amygdala (TVNS minus placebo; t = 3.88; MNI coordinates (x, y, z): 20, −6, −18; pFWE = 0.035) as well as in the M1 hand area contralateral to thermal stimulation (TVNS minus placebo; t = 3.86; MNI coordinates (x, y, z): 34, −26, 54; pFWE = 0.036).
The eight participants who reacted with an elevation of pain threshold during TVNS (Fig. 3a) showed a decreased fMRI signal compared to the placebo stimulation in the right caudate nucleus (t = 8.19; pFWE = 0.021; MNI coordinates:18, 26, 0), the vmPFC (t = 4.96, pFWE = ns, MNI coordinates: 0, 30, −12), the left anterior insula (t = 5.40; pFWE = 0.046; MNI-coordinates: −42, 14, 6), and the left hypothalamus (t = 3.94; pFWE = 0.04; MNI coordinates: −14, 0, 0; Fig. 3b–d). Participants who did not respond to TVNS with increased pain thresholds did not show any fMRI signal changes under TVNS in comparison with the placebo condition.
Results for the participants responding to TVNS with pain threshold reduction. a Difference of pain threshold temperature before and after intervention (TVNS or placebo given as mean and SEM; *p = 0.024, Bonferroni corrected. b–d In comparison to placebo TVNS yielded a reduction of fMRI activation in the left superior anterior insular cortex (Ant. IC), caudate nucleus (NC), ventromedial prefrontal cortex (vmPFC) and hypothalamus (HT)
Discussion
We used region of interest (ROI) based fMRI statistics to investigate the cerebral effects of transcutaneous vagal nerve stimulation (TVNS) applied in order to relieve experimental heat pain in healthy volunteers. During thermal pain experience without TVNS (placebo condition), we found an increase of fMRI activation in the left anterior insular and the right posterior insular cortex, the right thalamus and the anterior cingulate cortex, consistent with results of previous investigations (Apkarian et al. 2005; Freund et al. 2009; Friebel et al. 2011). Moreover, the intensity of activation in regions processing thermal pain was associated with the heat pain thresholds. This was not only observed for cortical areas processing thermal pain (S2, posterior insula, ACC) but also for the bilateral caudate nuclei. In contrast, the intensity of thermal stimulation during TVNS was only associated with right anterior insula activation.
In addition, we found decreased fMRI activation in the ACC during thermal painful stimulation under TVNS. However, we did not find an anti-nociceptive effect of TVNS for the whole group of participants.
ACC and mPFC are parts of the medial (affective-cognitive-evaluative) pain system, which includes the medial thalamic nuclei (Ingvar 1999). Investigating specific activation during experimentally induced thermal pain in comparison with pressure pain in an ALE-meta-analysis, we found activation in the left ACC as well as in other sites of representations (S2, left posterior insula, medial cingulate cortex, right inferior frontal gyrus and bilateral thalamus) to be specific for thermal pain processing in healthy participants (Friebel et al. 2011). Furthermore, the ACC has been repeatedly associated with increasing attention, especially to emotional stimuli (Bush et al. 2000) and, together with the mPFC, with skin conductance response during effortful mental processing (Naccache et al. 2005). A deactivation of the ACC during TVNS is therefore well in line with a general decreasing sympathetic effect indicated by lower attention and arousal to painful stimuli.
Although the absolute temperatures of thermal stimulation were comparable between the conditions, the association of brain activation and temperature in the presence or absence of TVNS differed considerably. During TVNS, only activation in the right anterior insula was associated with the heat intensity (temperature of aplication). Thus, the discriminative aspect of association with thermal pain may have been shifted to a more affective (Craig et al. 2000) or even anticipatory effect (Brown et al. 2008; Lickteig et al. 2013). Because of the strength of this association and specific localisation in the right anterior insula, we certainly need further studies on the combination of TVNS and thermal pain to fully understand this pehenomena.
Hypoalgesic effects of TVNS were only shown in a subgroup of the participants. This finding is in line with previous investigations, where TVNS was associated with both anti- and pro-nociceptive effects on experimentally induced pain in healthy volunteers (Johnson et al. 1991; Kraus et al. 2007; Laqua et al. 2014). The controversial results from these experimental studies are supported by findings of previous clinical investigations, revealing anti-nociceptive and pro-nociceptive reactions to experimentally induced pain in patients with an implanted vagal nerve stimulation device (Kirchner et al. 2000). Oppositional responses to TVNS could be explained by differing individual susceptibility to TVNS (Laqua et al. 2014). However, the underlying mechanisms are still to be elucidated.
When focusing our analysis on participants who reacted with an increased pain threshold to TVNS we found a reduction of fMRI activation in the right caudate nucleus, the ventromedial PFC, left AIC and the left hypothalamus. The caudate nucleus is interconnected with prefrontal areas that are functionally related to various cognitive processes (Alexander et al. 1986; Lehéricy et al. 2004). Together with the PFC, the anterior cingulate as well as the insular cortices, the caudate nucleus was shown to be involved in the neural processing of experimentally induced heat pain (Freund et al. 2009).
The reduced neuronal activity of the hypothalamus in TVNS responders is in line with the neurophysiological hypothesis of non-invasive vagal nerve stimulation. Connectivity analysis might help to understand an interaction of this area with the anterior insula, the caudate nucleus and the PFC during TVNS in future studies. A decrease of vmPFC activation during TVNS is well in accordance with a broad line of literature demonstrating the association of the vmPFC with psychophysiological responses associated with emotional processing. For example, increased vagal impact decreasing stress and conflict is known to be associated with activation in the vmPFC (Anders et al. 2004). In addition, Seifert et al. (2009) reported reduced vmPFC activity during central sensitization under systemic sodium channel blockade by lidocaine.
Although our investigation has the strength of a crossover design, allowing comparisons of intra-individual psychophysical responses to TVNS in contrast to placebo during thermal pain stimulation, our study lacks statistical power for demonstrating a more detailed cerebral network associated with the TVNS effects on thresholds of heat pain. Moreover, the extent of genuine stimulation of vagal nerve afferents is unknown. Other researchers suggested stimulation of the anterior wall of the outer auditory canal to be most effective for TVNS (Kraus et al. 2007; Kraus et al. 2013).
Since the effect sizes were expected to be small, we used an ROI-approach. Future studies with larger samples sizes and an improved design might be able to analyze the whole brain instead of ROI.
The method of “Limits”, used to measure the thresholds of experimental heat pain, seems to be rather insensitive to discriminate effects of TVNS.
Another limitation of our methodology is that it excluded the possibility to measure the perception of pain under noxious heat stimulation during fMRI session, thus, leaving the question of habituation to pain unanswered. Using the same temperature for noxious heat stimulation during fMRI sessions, rather than randomizing other painful or non-painful temperatures, we could not exclude the expectancy and habituation effects either. All this should be avoided in future studies, according to a recent systematic review (Duerden and Albanese 2013).
In order to measure anti-nociceptive effects of TVNS, more sensitive measurement methods (e.g., using so-called dynamic methods of experimental pain measurement) should be used for that purpose (Grosen et al. 2013). Therefore, it might be advantageous to apply a sensitization model for allodynia (Jürgens et al. 2014). Another strategy would be to pre-select participants who react with measurable autonomic response to TVNS outside of the MRI scanner.
Moreover, the pathophysiological considerations and clinical data suggest that patients with chronic pain would benefit from TVNS more than healthy volunteers within the experimental pain model (Eichhammer 2011; Napadow et al. 2012). Therefore, combining the crossover design with an improved sensitive measurement of experimental pain and neuroimaging focused on the limbic system will have certain advantage in future studies involving patients with chronic pain.
Conclusion
In summary, thermal stimulation elicited changes of fMRI signal in cerebral regions that are involved in the processing of pain. Transcutaneous vagal nerve stimulation led to decreased neuronal activation in the medial pain system, whereas the anti-nociceptive effect of TVNS was also associated with decreased activation of areas involved in emotional and cognitive pain regulation (superior anterior insula, vmPFC and the caudate nucleus) as well as the hypothalamus as a brain region releasing stress hormones.
References
Aicher, S. A., & Randich, A. (1988). Effects of intrathecal antagonists on the antinociception, hypotension, and bradycardia produced by intravenous administration of [D-Ala2]-methionine enkephalinamide (DALA) in the rat. Pharmacology Biochemistry and Behavior, 30(1), 65–72.
Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381.
Anders, S., Lotze, M., Erb, M., Grodd, W., & Birbaumer, N. (2004). Brain activity underlying emotional valence and arousal: A response-related fMRI study. Human Brain Mapping, 23(4), 200–209.
Apkarian, A. V., Bushnell, M. C., Treede, R. D., & Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463–484.
Becerra, L. R., Breiter, H. C., Stojanovic, M., Fishman, S., Edwards, A., Comite, A. R., et al. (1999). Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magnetic Resonance in Medicine, 41(5), 1044–1057.
Brown, C. A., Seymour, B., El-Deredy, W., & Jones, A. K. (2008). Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain, 139(3), 324–332.
Brown, J. E., Chatterjee, N., Younger, J., & Mackey, S. (2011). Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PloS One, 6(9), e24124.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science, 4(6), 215–222.
Craig, A. D., Chen, K., Bandy, D., & Reiman, E. M. (2000). Thermosensory activation of insular cortex. Nature Neuroscience, 3(2), 184–190.
Dietrich, S., Smith, J., Scherzinger, C., Hofmann-Preiss, K., Freitag, T., Eisenkolb, A., et al. (2008). A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech, 53(3), 104–111.
Duerden, E. G., & Albanese, M. C. (2013). Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Human Brain Mapping, 34(1), 109–149.
Eichhammer, P. (2011). (2011). Potential for transcutaneous vagus nerve stimulation in pain management. Pain Manag, 1(4), 287–289.
Freund, W., Klug, R., Weber, F., Stuber, G., Schmitz, B., & Wunderlich, A. P. (2009). Perception and suppression of thermally induced pain: a fMRI study. Somatosensory and Motor Research, 26(1), 1–10.
Friebel, U., Eickhoff, S. B., & Lotze, M. (2011). Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. NeuroImage, 58(4), 1070–1080.
George, M. S., Nahas, Z., Borckardt, J. J., Anderson, B., Burns, C., Kose, S., & Short, E. B. (2007). Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders. Expert Review of Neurotherapeutics, 7(1), 63–74.
Gray H. (1918). Anatomy of the human body. X. The organs of the senses and the common integument. The external ear. Lea & Febiger 146.
Grosen, K., Fischer, I. W., Olesen, A. E., & Drewes, A. M. (2013). Can quantitative sensory testing predict responses to analgesic treatment? European Journal of Pain, 17(9), 1267–1280.
Ingvar, M. (1999). Pain and functional imaging. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 354(1387), 1347–1358.
Johnson, M. I., Hajela, V. K., Ashton, C. H., & Thompson, J. W. (1991). The effects of auricular transcutaneous electrical nerve stimulation (TENS) on experimental pain threshold and autonomic function in healthy subjects. Pain, 46(3), 337–342.
Jürgens, T. P., Sawatzki, A., Henrich, F., Magerl, W., & May, A. (2014). An improved model of heat-induced hyperalgesia--repetitive phasic heat pain causing primary hyperalgesia to heat and secondary hyperalgesia to pinprick and light touch. PLoS One, 9(6), e99507.
Kirchner, A., Birklein, F., Stefan, H., & Handwerker, H. O. (2000). Left vagus nerve stimulation suppresses experimentally induced pain. Neurology, 55(1), 1167–1171.
Kraus, T., Hösl, K., Kiess, O., Schanze, A., Kornhuber, J., & Forster, C. (2007). BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. Journal of Neural Transmission, 114(11), 1485–1493.
Kraus, T., Kiess, O., Hösl, K., Terekhin, P., Kornhuber, J., & Forster, C. (2013). CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimulation, 6(5), 798–804.
Kroemer, N. B., Guevara, A., Vollstädt-Klein, S., & Smolka, M. N. (2013). Nicotine alters food-cue reactivity via networks extending from the hypothalamus. Neuropsychopharmacology, 38(11), 2307–2314.
Laqua, R., Leutzow, B., Wendt, M., & Usichenko, T. (2014). Transcutaneous vagal nerve stimulation may elicit anti- and pro-nociceptive effects under experimentally-induced pain - a crossover placebo-controlled investigation. Autonomic Neuroscience, 185(10), 120–122.
Lehéricy, S., Ducros, M., Van de Moortele, P. F., Francois, C., Thivard, L., Poupon, C., et al. (2004). Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology, 55(4), 522–529.
Leutzow, B., Lange, J., Gibb, A., Schroeder, H., Nowak, A., Wendt, M., & Usichenko, T. I. (2013). Vagal sensory evoked potentials disappear under the neuromuscular block – an experimental study. Brain Stimulation, 6(5), 812–816.
Lickteig, R., Lotze, M., & Kordass, B. (2013). Successful therapy for temporomandibular pain alters anterior insula and cerebellar representations of occlusion. Cephalgia, 33(15), 1248–1257.
Multon, S., & Schoenen, J. (2005). Pain control by vagus nerve stimulation: from animal to man…and back. Acta Neurologica Belgica, 105(2), 62–67.
Naccache, L., Dehaene, S., Cohen, L., Habert, M. O., Guichart-Gomez, E., Galanaud, D., & Willer, J. C. (2005). Effortless control: executive attention and conscious feeling of mental effort are dissociable. Neuropsychologia, 43(9), 1318–1328.
Napadow, V., Edwards, R. R., Cahalan, C. M., Mensing, G., Greenbaum, S., Valovska, A., et al. (2012). Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Medicine, 13(6), 777–789.
Nomura, S., & Mizuno, N. (1984). Central distribution of primary afferent fibers in the Arnold’s nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Research, 292(2), 199–205.
Ossipov, M. H., Dussor, G. O., & Porreca, F. (2010). Central modulation of pain. The Journal of Clinical Investigation, 120(11), 3779–3787.
PATHWAY Pain & Sensory Evaluation System. (2008). System Overview (8th ed., p. 17). Israel: Medoc Ltd.
Peuker, E. T., & Filler, T. J. (2002). Nerve supply of the human auricle. Clinical Anatomy, 15(1), 35–37.
Randich, A., Ren, K., & Gebhart, G. F. (1990). Electrical stimulation of cervical vagal afferents. II. Central relays for behavioral antinociception and arterial blood pressure decreases. Journal of Neurophysiology, 64(4), 1115–1124.
Rinaman, L. (2010). Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Research, 1350(9), 18–34.
Romoli, M., Allais, G., Airola, G., Benedetto, C., Mana, O., Giacobbe, M., et al. (2014). Ear acupuncture and fMRI: a pilot study for assessing the specificity of auricular points. Neurological Sciences, 35(S1), 189–193.
Seifert, F., Bschorer, K., De Col, R., Filitz, J., Peltz, E., Koppert, W., & Maihöfner, C. (2009). Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. The Journal of Neuroscience, 29(19), 6167–6175.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject. NeuroImage, 15(1), 273–289.
Ventureyra, E. C. (2000). Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Child Nerv Syst, 16(2), 101–102.
Ziv, M., Tomer, R., Defrin, R., & Hendler, T. (2010). Individual sensitivity to pain expectancy is related to differential activation of the hippocampus and amygdala. Human Brain Mapping, 31(2), 326–338.
Acknowledgments
The authors thank Vasyl Gizhko from the Department of Experimental Physics, University of Kiev, Ukraine, for his design and tests of the stimulation electrode; MTR GmbH, Germany for providing the TENS device; Dr. Konrad Meissner for providing the CHEPS thermode and the volunteers, who participated in this investigation. We would like to thank Henriette Hacker and Dr. Mike Cummings for carefully rechecking the manuscript for style and spelling mistakes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study
Conflict of interest
Taras Usichenko, René Laqua, Bianca Leutzow and Martin Lotze declare that he/she has no conflict of interest.
Additional information
Dr. Usichenko and Mr. Laqua made equal contributions in conducting the investigation and writing the manuscript
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Usichenko, T., Laqua, R., Leutzow, B. et al. Preliminary findings of cerebral responses on transcutaneous vagal nerve stimulation on experimental heat pain. Brain Imaging and Behavior 11, 30–37 (2017). https://doi.org/10.1007/s11682-015-9502-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9502-5