Abstract
Eating behaviors are closely related to body weight, and eating traits are depicted in three dimensions: dietary restraint, disinhibition, and hunger. The current study aims to explore whether these aspects of eating behaviors are related to intrinsic brain activation, and to further investigate the relationship between the brain activation relating to these eating traits and body weight, as well as the link between function connectivity (FC) of the correlative brain regions and body weight. Our results demonstrated positive associations between dietary restraint and baseline activation of the frontal and the temporal regions (i.e., food reward encoding) and the limbic regions (i.e., homeostatic control, including the hypothalamus). Disinhibition was positively associated with the activation of the frontal motivational system (i.e., OFC) and the premotor cortex. Hunger was positively related to extensive activations in the prefrontal, temporal, and limbic, as well as in the cerebellum. Within the brain regions relating to dietary restraint, weight status was negatively correlated with FC of the left middle temporal gyrus and left inferior temporal gyrus, and was positively associated with the FC of regions in the anterior temporal gyrus and fusiform visual cortex. Weight status was positively associated with the FC within regions in the prefrontal motor cortex and the right ACC serving inhibition, and was negatively related with the FC of regions in the frontal cortical-basal ganglia-thalamic circuits responding to hunger control. Our data depicted an association between intrinsic brain activation and dietary restraint, disinhibition, and hunger, and presented the links of their activations and FCs with weight status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Eating behavior, in particular abnormal eating, is one of the major contributions to being overweight and obese. A number of psychological scales, such as the Three Factor Eating Questionnaire (TFEQ) (Stunkard and Messick 1985). were developed to measure eating behaviors from multiple perspectives. The TFEQ assesses eating behaviors under three subscales: (1) dietary restraint (cognitive restraint on eating), (2) disinhibition (of control) and (3) (susceptibility to) hunger (Stunkard and Messick 1985). Previous studies have validated the reliability of the TFEQ (Boschi et al. 2001; Bond et al. 2001). and have also demonstrated that TFEQ scores are correlated with a variety of physical measures (neurotransmitter, weight, body composition, and energy expenditure) (Langlois et al. 2011) and psychological traits (mood and binge eating) (Elfhag 2005). The TFEQ is employed in clinical diagnosis (Khazaal et al. 2010) and investigations on weight loss effect (Wing and Phelan 2005; Lemoine et al. 2007). as well as in the treatment of bulimia nervosa and anorexia nervosa (Walsh et al. 1997). Compared to the Dutch Eating Behavior Questionnaire, the Revised Restraint Scale and the Current Dieting Questionnaire, the TFEQ restraint scale is a more valid measure of the intent to diet and actual caloric restriction (Williamson et al. 2007). Collectively, the TFEQ has become an effective instrument widely used in research on obesity and eating disorders.
Recently, functional magnetic resonance imaging (fMRI) technology has been used to investigate the association between neural activations and dietary restraint, disinhibition, and hunger, respectively (Santel et al. 2006; Kullmann et al. 2013; Martin et al. 2010; DelParigi et al. 2005; Lee et al. 2013; Maayan et al. 2011; Jurriaan M Born et al. 2011; DelParigi et al. 2007). Dietary restraint is linked with executive, motivation, and reward circuits responding to food intake. Dietary restraint is positively associated with neural activity in the dorsal prefrontal cortex, and is negatively correlated with neural changes in the orbitofrontal cortex (OFC) (DelParigi et al. 2007). It is also negatively associated with activation in the temporal visual processing network when exposed to food stimuli (Kullmann et al. 2013). Relative to healthy control groups, dietary restraint is negatively correlated with activation of the left inferior parietal lobe (IPL) and right lingual gyrus in anorexia nervosa patients (Santel et al. 2006). suggesting a decreased somatosensory-gustatory responsiveness to food. In addition, dietary restraint is inversely related to the neural activities in the left thalamus, cingulate cortex, amygdala, and most of the striatum for hedonic processing after meals (Jurriaan M Born et al. 2011). It is positively correlated with food reward value in the left nucleus accumbens before eating breakfast; while, during the meal, dietary restraint can lead to a decreased rewarding value of food in the nucleus accumbens, indicating that dietary restraint is also associated with the activation of the brain basal reward system (Jurriaan M Born et al. 2011). Disinhibition is demonstrated to be tightly associated with the activation of regions related to food sensory and reward; it is positively associated with activity in the left IPL and right lingual gyrus (Santel et al. 2006). and negatively correlated with pre-meal activities of the anterior cingulate cortex (ACC) (Martin et al. 2010). One study has reported that higher disinhibition scores are accompanied with increased blood flow in the insula in obese subjects relative to lean individuals after consuming a liquid meal (DelParigi et al. 2005). Lee and colleagues have revealed that low-dietary disinhibition participants show lowered ventral medial prefrontal cortex (VMPFC) activations for food evaluation after being fed (Lee et al. 2013). In addition, Maayan, et al. find that disinhibition scores present a strong association with OFC gray matter volume in the lean group individuals (Maayan et al. 2011). As for hunger, previous studies have indicated that feeling hungry was associated with augmented activations in regions for sensory, memory, motivation, and reward processing of food, such as the insula, parahippocampal gyrus, hippocampus, anterior cingulate cortex, left striate and extrastriate cortex, and right anterior lateral orbitofrontal cortex (Haase et al. 2009; Siep et al. 2009; Tataranni et al. 1999). To our knowledge, only one study has examined the relationship between the hunger subscale of the TFEQ and brain activation, in which hunger is positively correlated with pre-meal activities of the medial prefrontal cortex (MPFC) (Martin et al. 2010). The aforementioned studies shed light on the neural mechanisms underlying eating behaviors traits. However, those findings were obtained during exposure to food cues (Santel et al. 2006; Kullmann et al. 2013; Martin et al. 2010). or in fasting or satiety (Lee et al. 2013; Kullmann et al. 2013; DelParigi et al. 2005). or were compared across different weight groups (Santel et al. 2006; Kullmann et al. 2013; Martin et al. 2010; DelParigi et al. 2005; Maayan et al. 2011). Resting fMRI studies have indicated that basal brain activation could predict behavior performance (Gu et al. 2010; Zou et al. 2013; García-García et al. 2013). Thus far, the use of resting fMRI now offers the opportunity to avoid the influence of external tasks and describe the intrinsic patterns of brain activition associated with a particular eating behavior, namely dietary restraint, disinhibition, and hunger. A systematic investigation of intrinsic brain activations related to eating behavior traits is able to provide more evidence to understand food intake and design a better weight control strategy. Thus, the first objective of the current study is to explore the brain activations related to dietary restraint, disinhibition, and hunger during the resting state.
The relationship between eating behaviors examined by looking at the TFEQ and weight status have also been reported by a variety of behavioral studies (Provencher et al. 2003; Lindroos et al. 1997; de Castro and Lilenfeld 2005; Nicholas P Hays et al. 2002; Westenhoefer et al. 1999; Castellanos et al. 2009; Riou et al. 2011; Foster et al. 1998). Until now, a complex behavioral relationship between dietary restraint and weight status has been demonstrated. Some investigations find that the overweight/obese group has a lower score for dietary restraint than the lean group (Castellanos et al. 2009; Kullmann et al. 2013) and that the dietary restraint score is inversely related to BMI (Riou et al. 2011; Boschi et al. 2001; Foster et al. 1998). The relationship between increased dietary restraint scores and decreased BMI has also been reported in obesity treatment studies (Laurenius et al. 2012; Foster et al. 1998). Some other studies have demonstrated that there is no significant difference in dietary restraint among various body mass index (BMI) groups (Provencher et al. 2003; Lindroos et al. 1997) and that dietary restraint is not significantly correlated with BMI (Nicholas P Hays et al. 2002). However, two studies have found that obese adolescents score higher in dietary restraint than lean individuals (Maayan et al. 2011). and dietary restraint is positively correlated with BMI (Fayet et al. 2012). A recent study further indicates an inverted U-shaped relationship between dietary restraint and BMI, and implies this relationship is also moderated by disinhibited eating (Dietrich et al. 2014).
Findings about the relationship between disinhibition and weight status as well as between hunger and weight status are relatively consistent. A variety of studies have reported higher ratings of self-reported disinhibition and hunger scores in obese subjects than in lean individuals (Maayan et al. 2011; Kullmann et al. 2013; Castellanos et al. 2009; Lindroos et al. 1997; Harden et al. 2009). Weight loss is associated with significant decreases in disinhibition and hunger (Foster et al. 1998; Lemoine et al. 2007). Disinhibition can strongly predict weight gain and current BMI (Nicholas P Hays et al. 2002). and the mean hunger score has been shown to forecast weight change per year (Nicholas P. Hays et al. 2006). Parental disinhibition scores are positively correlated with the offspring’s BMI (Gallant et al. 2013).
In addition, a few neuroimaging publications have reported that eating behavior traits measured with the TFEQ and weight status are associated with common brain activations. Activations of the IPL are negatively correlated with dietary restraint and positively associated with BMI (Santel et al. 2006). The percentage of body fat and disinhibition scores are positively correlated with regional cerebral blood flow in the middle-dorsal insular region (DelParigi et al. 2005). In addition, there is no association between disinhibition and OFC volume for obese individuals, whereas there is a strong association for the lean group (Maayan et al. 2011). These studies extend the link between eating behavior and weight status from the perspective of neuroimaging and are ultimately helpful in understanding the neural mechanisms of obesity. Currently, the relationships between weight status and the intrinsic brain subsystems associated to eating behavior traits have not been fully investigated. The second objective of the current study is therefore to investigate the connection between weight status and regional activations of the intrinsic brain subsystem relating to each eating behavior traits, and the link of weight status and the interregional functional connectivity of the intrinsic brain subsystem.
In the current study, we first calculated the amplitude of low frequency fluctuations (ALFF) for each individual, and built group-level regression models to explore brain activations related to dietary restraint, disinhibition, and hunger. The influences of age, gender, and handedness were regressed out. The ALFF values of all voxels in an activated region were averaged to get the mean ALFF value of the region. Then, Pearson correlations on the mean ALFF value of each region and BMI were conducted. Ultimately, the functional connectivity of the paired brain regions relating to each TFEQ score was examined. A Pearson correlation analysis was performed to investigate the association between functional connectivity and BMI.
Methods
Participants and data acquisition
A total of 99 healthy adults with the TFEQ test were included in the present analysis (female: 37, male: 62; range: 20–57; age, mean ± SE: 35.1 ± 1.14 years). The data were downloaded from the International Neuroimaging Data-sharing Initiative (INDI) online database NKI/Rockland Sample (http://fcon 1000.projects.nitrc.org/indi/pro/nki.html)provided by the Nathan Kline Institute (Nooner et al. 2012). The data consisted of 2 lean subjects (BMI < 18), 43 normal weight subjects (18 < BMI < 25), 26 overweight subjects (25 < BMI < 30) and 28 obese subjects (BMI > 30). Participants were asked to fast prior to the MRI scan, which began at 9:00 am local time and lasted one hour. Then, TFEQ measurement started at 10:45 am.
Structural MRI scans were acquired with a Siemens MAGNETOM Tim Trio 3.0 T Scanner (TR 2500 ms; TE 3.5 ms; TI 1200 ms; FOV:256 × 256; slice thickness 1 mm; Flip angle 8°; matrix size 256 × 256; 200 Transverse slices). Functional MRI images were scanned with a gradient echo echo-planar sequence (TR = 2500 ms; TE = 35 ms; flip angle =80°; FOV: 256 × 256; in-plane resolution =3 × 3 mm2, slice thickness 3 mm). Demographic information and scan details are available at http://fcon_1000.projects.nitrc.org/indi/pro/nki.html. Written informed consent was obtained from all participants and the NKI institutional review board approved all procedures for collection and sharing of data (Nooner et al. 2012).
Functional image preprocessing
Before processing, the first five functional scans were discarded. Data were analyzed by using a DPARSF toolbox (Chao-Gan and Yu-Feng 2010). Preprocessing included slice timing, realignment, detrending, and a temporal band-pass filter (0.01–0.08 Hz). A multiple regression was performed to remove nuisance signals from the BOLD data. Nuisance variables included the mean whole brain signal of whole brain voxels, mean ventricular signal, mean white matter signal from white matter regions of interest, and six head realignment parameters and their derivatives. Images were normalized to the MNI template with a 2 × 2 × 2 mm3 voxel size and smoothed with a Gaussian kernel of 4-mm3 full width at half maximum. Then, the amplitude of low frequency fluctuations (ALFF) was calculated for each subject (http://restfmri.net/forum/index.php). ALFF was defined as the total power within the frequency range between 0.01 and 0.08 Hz, which represented the strength or intensity of low frequency oscillations (Yu-Feng et al. 2007). After activated brain mappings were obtained, the Pearson correlations of the mean ALFF value of each region and BMI were calculated. Functional connectivity was calculated between each pair of activated brain mappings. The mean time series was calculated for each activated brain area, and Pearson correlation coefficients were calculated for each pair of activated brain regions. Then, group level Pearson correlations were conducted on BMI and functional connectivity of each pair of activated brain areas. It should be noted that we did not explore all brain circuits related to BMI with our functional connectivity analysis in pre-determined region pairs. As the current study aimed to examine the link of body weight status with the functional connectivity of brain areas associated with dietary restraint, disinhibition, and hunger, our analyses are appropriate to fulfil the aim, but excluded some brain circuits previously shown to be correlated with BMI.
Statistical analysis
One-way ANOVAs were performed on the scores of dietary restraint, disinhibition, and hunger. Subjects were divided into three groups (two lean subjects were excluded): normal weight (18 < BMI < 25), overweight (25 < BMI < 30), and obese (BMI > 30). Three group-level multiple linear regressions of ALFF mappings and scores of each TFEQ factor were conducted respectively. In the model, the score of each TFEQ factor had a variable of interest, and age, gender, and handedness were entered as covariates. The resulting statistical maps with a significance level of P < 0.001 and continuous cluster size >30 voxels (Goldstone et al. 2009; Frank et al. 2012) were identified as activations.
Results
Demographics
In the current study, the mean score of dietary restraint was: 6.86 (SE = 0.44) (Table 1). Dietary restraint scores of the three groups were (mean ± SE): 6.58 ± 0.68 (normal weight), 7.46 ± 0.64 (overweight) and 6.71 ± 0.96 (obese). Mean disinhibition score was 4.11 (SE = 0.33). Disinhibition scores of the three groups were (mean ± SE): 4.02 ± 0.51 (normal weight), 4.04 ± 0.61 (overweight) and 4.32 ± 0.61 (obese). Mean hunger score was 4.52 (SE = 0.32). Hunger scores of the three groups were (mean ± SE): 3.49 ± 0.42 (normal weight), 4.96 ± 0.67 (overweight) and 5.68 ± 0.34 (obese). The one-way ANOVA detected a significant group effect in the hunger scores (F(2, 96) = 4.394, p = 0.015) due to a significantly lower hunger score for the normal weight group than that of the obese group (p = 0.005). No significant group effect was found for the scores of dietary restraint (ps. > 0.420) and disinhibition respectively (ps. > 0.707). Mean BMI was 27.18 (SE = 0.58). BMIs of the three groups were: 22.21 ± 0.27 (normal weight), 27.75 ± 0.28 (overweight) and 34.28 ± 0.75 (obese) (Table 1). Association analysis found no significant correlation between dietary restraint score and BMI (r = 0.01, p = 0.95), as well as the disinhibition score and BMI (r = −0.01, p = 0.89). Meanwhile, the hunger score and BMI were significantly correlated (r = 0.22, p = 0.03).
Intrinsic brain activation related to dietary restraint, disinhibition, and hunger
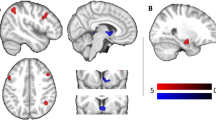
The results of the regression analysis are shown in Table 2. The dietary restraint score was positively correlated with ALFF in the right middle temporal gyrus and middle occipital gyrus (rMTGb), left anterior cingulate (rACC), left middle temporal gyrus (lMTG), left rectal gyrus, left medial frontal gyrus (lMedFG), right fusiform gyrus (rFFG), right lentiform nucleus and hypothalamus (rHPT), left inferior temporal gyrus (ITG), right middle temporal gyrus (rMTGa), right inferior temporal gyrus, and left Cerebellar Tonsil (lCERT)(Fig. 1).
Brain regions with baseline activity significantly correlated with dietary restraint (P < 0.001, cluster size >30 voxels). Abbreviation: rMTGa and rMTGb, right middle temporal gyrus; lACC, left anterior cingulate cortex; lMTG, left middle temporal gyrus; lMedFG, left medial frontal gyrus; rFFG, right fusiform gyrus; rHPT, right hypothalamus; lITG, left inferior temporal gyrus; lCERT, left cerebellar tonsil
The disinhibition score was positively correlated with ALFF in several brain regions within the frontal cortex, cingulate, and cerebellum, including the left middle frontal gyrus, precentral gyrus, and cingulate gyrus (lMidFG), left postcentral gyrus and precentral gyrus (lPoCG), right anterior cingulate gyrus (rACC), left cingulate gyrus (lCG), right medial frontal gyrus (rMedFG), right declive and culmen (rCULM). (Fig. 2).
Brain regions with baseline activity significantly correlated with disinhibition (P < 0.001, cluster size >30 voxels). Abbreviation: lMidFG, left middle frontal gyrus; lPoCG, left postcentral gyrus; lPoCG, left postcentral gyrus; lCG, left cingulate gyrus; lMedFG, left medial frontal gyrus; rCULM, right culmen
The hunger score was positively correlated with ALFF of a number of brain regions in the frontal, limbic, temporal cortices, and cerebellum, including the left fusiform gyrus, sub-gyral and middle temporal gyrus (lFFG), left medial frontal gyrus and cingulate gyrus (lCG), right thalamus (rTHAL), left caudate (lCAUD), right culmen, declive and right dentate (rCULM), left middle frontal gyrus (lMidFG), right superior frontal gyrus (rSFG), left inferior frontal gyrus (lIFG), left postcentral gyrus (lPoCG), left dentate and declive (lDEC) Fig. 3.
Brain regions with baseline activity significantly correlated with Hunger (P < 0.001, cluster size >30 voxels). Abbreviation: lFFG, left fusiform gyrus; lMedFG, left medial frontal gyrus; rTHAL, right thalamus; lCAUD, left caudate; rCULM, right culmen; lMidFG, left middle frontal gyrus; rSFG, right superior frontal gyrus; lIFG, left inferior frontal gyrus; lPoCG, left postcentral gyrus; lDENT, left dentate
Relationship between weight status and intrinsic brain activations relating to dietary restraint, disinhibition and hunger
Among the brain regions related to dietary restraint, BMI was positively associated with the ALFF of the lITG (r = 0.28, p = 0.005), the rMTGb (r = 0.28, p = 0.006) and the lACC (r = 0.20, p = 0.042) respectively. Among the brain regions associated with disinhibition, the BMI was positively associated with the ALFF of the lPoCG (r = 0.21, p = 0.036). Among the brain regions being linked to hunger, the BMI was positively associated with the ALFF of the lPoCG (r = 0.25, p = 0.014) and the lDEC (r = 0.21, p = 0.036). The BMI was marginally associated and the ALFF of the rTHAL (r = 0.19, p = 0.055).
Relationship between weight status and intrinsic functional connectivity
Within the brain subsystem related to dietary restraint, BMI was negatively correlated with the FC of lMTG and lCERT, as well as the FC of lITG and lMTG. On the other hand, BMI was positively correlated with the FC of the paired brain regions: rFFG and lITG, as well as rFFG and lMTG (Table 3).
Within the brain subsystem involved in disinhibition, BMI was negatively correlated with FC of lCG and rCULM. BMI was positively correlated with FC of the lMidFG and rACC (Table 3).
Within the brain networks related to hunger, our data showed negative correlations between BMI and the FC of paired brain regions: lMedFG and lDEC, lMidFG and lDEC, lPoCG and lDEC, lPoCG and rCULM, lMedFG and rCULM, lMidFG and rTHAL, and rSFG and rTHAL (Table 3).
Discussion
The current study investigated the intrinsic brain subsystem related to dietary restraint, disinhibition (of control), and (susceptibility to) hunger. Our results demonstrated positive associations between dietary restraint and baseline activation of the frontal and temporal regions (i.e., food reward encoding) and the limbic regions (i.e., homeostatic control, including the hypothalamus). Disinhibition was positively associated with brain activation in the frontal motivational system (i.e., OFC) and premotor cortex. Hunger was positively related to the prefrontal, temporal, and limbic and cerebellum regions. Within the brain regions related to dietary restraint, weight status was negatively correlated with FC of the regions in the anterior temporal gyrus and was positively associated with the FC of regions in the anterior temporal gyrus and fusiform visual cortex. Meanwhile, Weight status was positively associated with the FC within regions in the prefrontal motor cortex and the right ACC serving inhibition, and was negatively related to the FC of regions in the frontal cortical-basal ganglia-thalamic circuits responding to hunger control. Our data depicted an association between eating behavior traits and intrinsic brain activation, and presented the links between their activations and FCs with weight status.
Eating behavior results
Collectively, the mean TFEQ scores in the current sample were close to those in previous studies (DelParigi et al. 2005; Cornier et al. 2007; J. M. Born et al. 2010; Provencher et al. 2003). 19 participants had a high dietary restraint trait (dietary restraint score > = 11), 19 participants presented a high disinhibition trait (disinhibition score > = 8), and 22 participants showed a high hunger trait (disinhibition score > = 7). In addition, the mean dietary restraint score was lower than that in anorexia patients, and the mean disinhibition score was a bit higher than that in anorexia patients (Santel et al. 2006). With regard to TFEQ scores in each group, the overweight group exhibited numerically larger dietary restraint scores than the normal weight and obese groups. Dietary restraint was defined as a tendency to consciously restrict energy intake and types of foods eaten with concerns about body shape and weight gain (Stunkard and Messick 1985). Our data were consistent with the findings that the obese subjects might attempt to curb the intake of high-calorie foods because they were aware of the weight gain effect (Nicholas P Hays et al. 2002; Drapeau et al. 2003; Lindroos et al. 1997). It also indicated that the overweight group could be more sensitive to body shape and weight gain (Stunkard and Messick 1985). Obese individuals presented higher hunger scores than the normal groups. It was in line with the previous studies that obese individuals had more behavioral susceptibility in the perception of body symptoms for food intake compared with the normal weight subjects. As for disinhibition score, there was little variation among the three groups, as we discussed in the section on limitations.
Neuroimaging results
In the current study, dietary restraint was associated with baseline activations of the frontal and temporal regions (i.e., food reward encoding) and the limbic regions (i.e., homeostatic control, including the hypothalamus). We discovered that dietary restraint was related to activation in the ACC. The ACC is associated with reward-related attentional control, decision-making, and motivation monitoring (Bush et al. 2002; Tang et al. 2012; Ashley N. Gearhardt et al. 2013). The ACC is also believed to be involved in cognitive control and reward expectation during food intake (Hon et al. 2006; Hinkle et al. 2013). Obese individuals have been found to have less activation in the ACC than normal-weight participants (Tang et al. 2012; Hare et al. 2009). Positive correlations between dietary restraint and baseline activation in the ACC indicate that enough food reward encoding is important for dietary restraint, consistent with reward-deficiency theory (Jurriaan M Born et al. 2011; Fineberg et al. 2009). Dietary restraint was associated with activation in the right hypothalamus and left OFC, both important parts for appetite regulation (Blundell et al. 2012; De Silva et al. 2012; Piech et al. 2009; Scharmüller et al. 2012). The hypothalamus has been found to act as a central gateway modulating homeostatic drives to eat (De Silva et al. 2012). Its lateral parts are believed to respond to hunger, thirst, and emotions, and the medial parts are indicated to eliminate the feeling of hunger (De Silva et al. 2012; Scharmüller et al. 2012). Both of them serve as a counterbalance for one another. In the current study, the voxel coordinate with maximum significance was close to the medial hypothalamus, indicating that strong dietary restraint was also related to basic limbic regions involved with homeostatic balance. However, due to limited spatial resolution of the data, we could not differentiate hypothalamic sub-regions; these results should be checked with higher spatial resolution fMRI data. In the current study, activations of the left orbitofrontal gyrus lie in its medial part. This area has been found to respond to low-calorie foods but not to high-calorie foods, and to be involved in food evaluation with the aim of energy balance (W. D. Killgore and Yurgelun-Todd 2006). The positive coupling of dietary restraint with the ALFF of the orbitofrontal gyrus may indicate that people with stronger dietary restraint are more concerned about the homeostatic aspect of food. These above results may indicate that dietary restraint is significantly related to frontal, limbic, and paralimbic cortices for homeostatic balance and food reward.
Among the regions with intrinsic brain related to dietary restraint, BMI was positively associated with the intrinsic activation in lACC, lITG, and rMTG. In addition, we found that BMI was negatively correlated with functional connectivity of the rMTG and lITG. The middle temporal gyrus and inferior temporal gyrus are mostly related to the objects’ semantic representation and working memory retrieval. Temporal cortices have been observed to respond greater in obesity groups than in normal weight groups for food stimuli (Rothemund et al. 2007; W. D. S. Killgore et al. 2003; Ashley N. Gearhardt et al. 2013; De Silva et al. 2012; Cornier et al. 2007). and for food commercials versus non-food commercials (Ashley N. Gearhardt et al. 2013). A tight connectivity between rMTG and lITG indicates a powerful semantic retrieval brain network for food, thus contributing to the prevention of weight gain.
Disinhibition is defined as a tendency to overeat in the presence of palatable foods or other stimuli such as boredom or sadness (Stunkard and Messick 1985). The current study found that disinhibition was positively associated with intrinsic activation of rMedFG. The rMedFG lies in the lateral part of the orbitofrontal cortex, and was associated with visual, taste, olfactory, and somatosensory information and then encodes this with the reward of food (Edmund T. Rolls 2004; Siep et al. 2009; Volkow and Fowler 2000; De Silva et al. 2012). The lateral OFC is involved in food motivation, reward sensitivity, and impulsivity (Beaver et al. 2006). The positive correlation of the activities of the lateral OFC and disinhibition to eating is consistent with the idea that hyper-activation of the motivation system contributed to food anticipation and intake (De Silva et al. 2012).
We discovered that BMI was negatively associated with functional connectivity between the cingulate cortex and the right declive and culmen. The cingulate gyrus (BAs 24 and 32) is anatomically connected with the autonomic nervous system, including the hypothalamus, caudate, putamen, and cerebellum (Matthews et al. 2004). and functionally contributes to the representation of food intake requirements (Weston 2012). The culmen is also anatomically connected with the hypothalamus and is demonstrated to be related to food anticipation (Allen et al. 2005). Participants also exhibit greater neural responses in the right culmen to food commercials compared to television shows (Ashley N. Gearhardt et al. 2013). The right culmen shows reduced activation in response to high-calorie relative to low-calorie food cues after a Roux-en-Y gastric bypass operation (Ochner et al. 2012). On the other hand, individuals who display less activation in the culmen for a difficult delay-discounting task gain more weight than those who display greater activation (Kishinevsky et al. 2012). A strong functional connectivity between the cingulate gyrus and culmen may indicate more sensitivity to homeostasis balance, which is beneficial to controlling weight.
Hunger refers to the susceptibility to perceive body symptoms that signal the need for food (Stunkard and Messick 1985). In the current study, the hunger score was associated with regions in the frontal cortical-basal ganglia-thalamic circuits. These results are consistent with Tataranni’s findings that hunger is associated with increased regional cerebral blood flow in the ACC, thalamus, caudate, dentate, and cerebellum (Tataranni et al. 1999). The ACC is involved in homeostasis-related behaviors, such as thirst and hunger (Fuhrer et al. 2008). It has a greater response during hunger than satiety states (Hinton et al. 2004; Weston 2012). The thalamus has been demonstrated to be a hub of sensory information during food intake (Edmund T Rolls 2005). Visual information and taste of food, somatosensory, gustatory, and visceroceptive information pass through the thalamus to the orbitofrontal cortex (Berthoud and Morrison 2008). Augmented thalamus activity has been suggested to lead to an increase in food-seeking behavior, which may in turn cause over-consumption (Kelley et al. 2005). The caudate is more related to promoting habitual learning and the direct consummatory response to rewards (Kenny 2011). All of these regions are widely believed to play an important role in the processing of nutrition-related stimuli, and also tend to be affected by weight status and food energy (Kelley et al. 2005). The left IFG is a key region for selecting information among competing sources and generating action meanings, which is involved in motor planning (Swick et al. 2008; Molnar-Szakacs et al. 2005). The left IFG, together with the premotor cortices, form the motor planning and controlling system of eating. In summary, these results show that the sensation of hunger is not only related to the cortices for homeostasis maintenance, but is also associated with frontal and temporal regions for food encoding. BMI was negatively related to the functional connectivity of several frontal cortices and limbic regions and the cerebellum. It has been demonstrated that dopamine signals from the nuclear accumbens pass through the dorsal pallidum and ventral pallidum, and then reach the dorsal and ventral thalamus, and finally motivate sensorimotor and premotor cortex to execute food intake (Berthoud and Morrison 2008). Thus, the right thalamus and prefrontal motor cortex make up a key circuit for motor planning. A strong functional synchronization of them may have the benefit of improving motor planning of food intake. On the other hand, the culmen and declive have been implicated as involving in motor function, and there is also a body of research considering them as playing a key role in food intake and energy homeostasis (Ashley N Gearhardt et al. 2014; Kishinevsky et al. 2012). Increased BMI was linearly associated with higher bilateral activation in the declive (Tomasi et al. 2009). Compared to healthy controls, there is less activation of the left culmen and the left declive in the group with metabolic syndrome, indicating an autonomic function of the left culmen and declive (Green et al. 2015). The strong functional connectivity between the frontal and premotor regions, the premotor regions and the thalamus, and the premotor regions and the left culmen and declive may indicate a powerful ability of motor planning during food intake, which in turn has a benefit for maintaining weight. These findings are in line with the reward-deficiency theory.
Limitations
The current study has a number of limitations that need to be acknowledged. First, the dietary restraint, disinhibition, and hunger scores were measured with a self-report questionnaire, the responses to which may vary from person to person. In addition, the resting-state fMRI is also low in signal-contrast ratio. Although there are a number of studies that have demonstrated that phenotypic differences are associated with variability in the intrinsic functional architecture of the brain (Vaidya and Gordon 2013). it should be noted that there is considerable variability that may limit the statistical power of the regression model. Thus, it is difficult to fully explore the intrinsic functional subsystem associated with dietary restraint, disinhibition, and hunger scores in small number of subjects. We therefore selected a comparably loose threshold on cluster size to acquire the final brain activation mappings according to previous studies (Goldstone et al. 2009; Frank et al. 2012). Further studies should employ a larger number of participants to effectively explore the brain activations that are associated with eating behavior and weight status. Second, there is limited variation among the three groups in disinhibition scores, which may limit the statistical power of the regression model. Brain activation associated with disinhibition should be examined in a more extensive range of subjects.
Conclusion
In the current study, we found that dietary restraint, disinhibition, and hunger were related with distributed intrinsic subsystems in frontal, temporal and limbic cortices for homeostasis balance and reward encoding of food. The functional connectivity analysis on these intrinsic brain subsystems further indicates that BMI was also related to the regional functional synchronization. Compared to the single use of regional frequency analysis and whole brain functional connectivity analysis (seed-based connectivity and independent component analysis), our findings demonstrate the association between eating behaviors and weight status in multi-dimensional neural activations.
References
Allen, G., McColl, R., Barnard, H., Ringe, W. K., Fleckenstein, J., & Cullum, C. M. (2005). Magnetic resonance imaging of cerebellar–prefrontal and cerebellar–parietal functional connectivity. NeuroImage, 28(1), 39–48.
Beaver, J. D., Lawrence, A. D., van Ditzhuijzen, J., Davis, M. H., Woods, A., & Calder, A. J. (2006). Individual differences in reward drive predict neural responses to images of food. The Journal of Neuroscience, 26(19), 5160–5166.
Berthoud, H.-R., & Morrison, C. (2008). The brain, appetite, and obesity. Annual Review of Psychology, 59, 55–92.
Blundell, J. E., Caudwell, P., Gibbons, C., Hopkins, M., Naslund, E., King, N., et al. (2012). Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Disease Models & Mechanisms, 5(5), 608–613. doi:10.1242/dmm.009837.
Bond, M. J., McDowell, A. J., & Wilkinson, J. Y. (2001). The measurement of dietary restraint, disinhibition and hunger: an examination of the factor structure of the Three Factor Eating Questionnaire (TFEQ). International Journal of Obesity and Related Metabolic Disorders, 25(6), 900–906. doi:10.1038/sj.ijo.0801611.
Born, J. M., Lemmens, S. G., Rutters, F., Nieuwenhuizen, A. G., Formisano, E., Goebel, R., et al. (2010). Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. International Journal of Obesity, 34(1), 172–181. doi:10.1038/ijo.2009.221.
Born, J. M., Lemmens, S. G., Martens, M. J., Formisano, E., Goebel, R., & Westerterp-Plantenga, M. S. (2011). Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. The American Journal of Clinical Nutrition, 94(2), 392–403.
Boschi, V., Iorio, D., Margiotta, N., D'Orsi, P., & Falconi, C. (2001). The three-factor eating questionnaire in the evaluation of eating behaviour in subjects seeking participation in a dietotherapy programme. Annals of Nutrition & Metabolism, 45(2), 72–77.
Bush, G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, D., Jenike, M. A., et al. (2002). Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences, 99(1), 523–528. doi:10.1073/pnas.012470999.
Castellanos, E. H., Charboneau, E., Dietrich, M. S., Park, S., Bradley, B. P., Mogg, K., et al. (2009). Obese adults have visual attention bias for food cue images: evidence for altered reward system function. International Journal of Obesity, 33(9), 1063–1073. doi:10.1038/ijo.2009.138.
Chao-Gan, Y., & Yu-Feng, Z. (2010). DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Frontiers in Systems Neuroscience, 4, 13. doi:10.3389/fnsys.2010.00013.
Cornier, M.-A., Von Kaenel, S. S., Bessesen, D. H., & Tregellas, J. R. (2007). Effects of overfeeding on the neuronal response to visual food cues. The American Journal of Clinical Nutrition, 86(4), 965–971.
de Castro, J. M., & Lilenfeld, L. R. R. (2005). Influence of heredity on dietary restraint, disinhibition, and perceived hunger in humans. Nutrition, 21(4), 446–455. doi:10.1016/j.nut.2004.07.010.
De Silva, A., Salem, V., Matthews, P. M., & Dhillo, W. S. (2012). The Use of Functional MRI to Study Appetite Control in the CNS. Experimental Diabetes Research, 2012, 13. doi:10.1155/2012/764017.
DelParigi, A., Chen, K., Salbe, A. D., Reiman, E. M., & Tataranni, P. A. (2005). Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. NeuroImage, 24(2), 436–443. doi:10.1016/j.neuroimage.2004.08.035.
DelParigi, A., Chen, K., Salbe, A., Hill, J., Wing, R., Reiman, E., et al. (2007). Successful dieters have increased neural activity in cortical areas involved in the control of behavior. International Journal of Obesity, 31(3), 440–448.
Dietrich, A., Federbusch, M., Grellmann, C., Villringer, A., & Horstmann, A. (2014). Body weight status, eating behavior, sensitivity to reward/punishment, and gender: relationships and interdependencies. Frontiers in Psychology, 5, 1073.
Drapeau, V., Provencher, V., Lemieux, S., Despres, J. P., Bouchard, C., & Tremblay, A. (2003). Do 6-y changes in eating behaviors predict changes in body weight? Results from the Quebec Family Study. International Journal of Obesity and Related Metabolic Disorders, 27(7), 808–814.
Elfhag, K. (2005). Personality correlates of obese eating behaviour: Swedish universities Scales of Personality and the Three Factor Eating Questionnaire. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity, 10(4), 210–215.
Fayet, F., Petocz, P., & Samman, S. (2012). Prevalence and correlates of dieting in college women: a cross sectional study. Int J Womens Health, 4, 405–411. doi:10.2147/ijwh.s33920.
Fineberg, N. A., Potenza, M. N., Chamberlain, S. R., Berlin, H. A., Menzies, L., Bechara, A., et al. (2009). Probing Compulsive and Impulsive Behaviors, from Animal Models to Endophenotypes: A Narrative Review. Neuropsychopharmacology, 35(3), 591–604.
Foster, G. D., Wadden, T. A., Swain, R. M., Stunkard, A. J., Platte, P., & Vogt, R. A. (1998). The Eating Inventory in obese women: clinical correlates and relationship to weight loss. International Journal of Obesity and Related Metabolic Disorders, 22(8), 778–785.
Frank, G. K., Reynolds, J. R., Shott, M. E., Jappe, L., Yang, T. T., Tregellas, J. R., et al. (2012). Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology, 37(9), 2031–2046.
Fuhrer, D., Zysset, S., & Stumvoll, M. (2008). Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring), 16(5), 945–950. doi:10.1038/oby.2008.33.
Gallant, A. R., Tremblay, A., Perusse, L., Despres, J. P., Bouchard, C., & Drapeau, V. (2013). Parental eating behavior traits are related to offspring BMI in the Quebec Family Study. International Journal of Obesity, 37(11), 1422–1426. doi:10.1038/ijo.2013.14.
García-García, I., Jurado, M. Á., Garolera, M., Segura, B., Sala-Llonch, R., Marqués-Iturria, I., et al. (2013). Alterations of the salience network in obesity: A resting-state fMRI study. Human Brain Mapping, 34(11), 2786–2797. doi:10.1002/hbm.22104.
Gearhardt, A. N., Yokum, S., Stice, E., Harris, J. L., & Brownell, K. D. (2013). Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nst059.
Gearhardt, A. N., Yokum, S., Stice, E., Harris, J. L., & Brownell, K. D. (2014). Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience, 9(7), 932–938.
Goldstone, A. P., Prechtl de Hernandez, C. G., Beaver, J. D., Muhammed, K., Croese, C., Bell, G., et al. (2009). Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience, 30(8), 1625–1635.
Green, E., Jacobson, A., Haase, L., & Murphy, C. (2015). Neural correlates of taste and pleasantness evaluation in the metabolic syndrome. Brain Research, 1620, 57–71.
Gu, H., Salmeron, B. J., Ross, T. J., Geng, X., Zhan, W., Stein, E. A., et al. (2010). Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage, 53(2), 593–601. doi:10.1016/j.neuroimage.2010.06.066.
Haase, L., Cerf-Ducastel, B., & Murphy, C. (2009). Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage, 44(3), 1008–1021.
Harden, C. J., Corfe, B. M., Richardson, J. C., Dettmar, P. W., & Paxman, J. R. (2009). Body mass index and age affect Three-Factor Eating Questionnaire scores in male subjects. Nutrition Research, 29(6), 379–382. doi:10.1016/j.nutres.2009.04.001.
Hare, T. A., Camerer, C. F., & Rangel, A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–648. doi:10.1126/science.1168450.
Hays, N. P., Bathalon, G. P., McCrory, M. A., Roubenoff, R., Lipman, R., & Roberts, S. B. (2002). Eating behavior correlates of adult weight gain and obesity in healthy women aged 55–65 y. The American Journal of Clinical Nutrition, 75(3), 476–483.
Hays, N. P., Bathalon, G. P., Roubenoff, R., McCrory, M. A., & Roberts, S. B. (2006). Eating Behavior and Weight Change in Healthy Postmenopausal Women: Results of a 4-Year Longitudinal Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(6), 608–615.
Hinkle, W., Cordell, M., Leibel, R., Rosenbaum, M., & Hirsch, J. (2013). Effects of Reduced Weight Maintenance and Leptin Repletion on Functional Connectivity of the Hypothalamus in Obese Humans. PloS One, 8(3), e59114. doi:10.1371/journal.pone.0059114.
Hinton, E. C., Parkinson, J. A., Holland, A. J., Arana, F. S., C. Roberts, A., & Owen, A. M. (2004). Neural contributions to the motivational control of appetite in humans. European Journal of Neuroscience, 20(5), 1411–1418, doi:10.1111/j.1460-9568.2004.03589.x.
Hon, N., Epstein, R. A., Owen, A. M., & Duncan, J. (2006). Frontoparietal activity with minimal decision and control. The Journal of Neuroscience, 26(38), 9805–9809, doi:10.1523/jneurosci.3165-06.2006.
Kelley, A. E., Baldo, B. A., & Pratt, W. E. (2005). A proposed hypothalamic–thalamic–striatal axis for the integration of energy balance, arousal, and food reward. The Journal of Comparative Neurology, 493(1), 72–85. doi:10.1002/cne.20769.
Kenny, P. J. (2011). Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron, 69(4), 664–679.
Khazaal, Y., Billieux, J., Fresard, E., Huguelet, P., Van der Linden, M., & Zullino, D. (2010). A measure of dysfunctional eating-related cognitions in people with psychotic disorders. Psychiatric Quarterly, 81(1), 49–56.
Killgore, W. D., & Yurgelun-Todd, D. A. (2006). Affect modulates appetite-related brain activity to images of food. International Journal of Eating Disorders, 39(5), 357–363.
Killgore, W. D. S., Young, A. D., Femia, L. A., Bogorodzki, P., Rogowska, J., & Yurgelun-Todd, D. A. (2003). Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage, 19(4), 1381–1394, doi:10.1016/S1053-8119(03)00191-5.
Kishinevsky, F. I., Cox, J. E., Murdaugh, D. L., Stoeckel, L. E., Cook Iii, E. W., & Weller, R. E. (2012). fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite, 58(2), 582–592. doi:10.1016/j.appet.2011.11.029.
Kullmann, S., Pape, A. A., Heni, M., Ketterer, C., Schick, F., Haring, H. U., et al. (2013). Functional network connectivity underlying food processing: disturbed salience and visual processing in overweight and obese adults. Cerebral Cortex, 23(5), 1247–1256. doi:10.1093/cercor/bhs124.
Langlois, F., Langlois, M.-F., Carpentier, A. C., Brown, C., Lemieux, S., & Hivert, M.-F. (2011). Ghrelin levels are associated with hunger as measured by the Three-Factor Eating Questionnaire in healthy young adults. Physiology & Behavior, 104(3), 373–377.
Laurenius, A., Larsson, I., Bueter, M., Melanson, K. J., Bosaeus, I., Forslund, H. B., et al. (2012). ing Roux-en-Y gastric bypass. International Journal of Obesity, 36(3), 348–355. doi:10.1038/ijo.2011.217.
Lee, Y., Chong, M. F., Liu, J. C., Libedinsky, C., Gooley, J. J., Chen, S., et al. (2013). Dietary disinhibition modulates neural valuation of food in the fed and fasted states. The American Journal of Clinical Nutrition, 97(5), 919–925. doi:10.3945/ajcn.112.053801.
Lemoine, S., Rossell, N., Drapeau, V., Poulain, M., Garnier, S., Sanguignol, F., et al. (2007). Effect of weight reduction on quality of life and eating behaviors in obese women. Menopause, 14(3 Pt 1), 432–440. doi:10.1097/gme.0b013e31802e46c2.
Lindroos, A. K., Lissner, L., Mathiassen, M. E., Karlsson, J., Sullivan, M., Bengtsson, C., et al. (1997). Dietary intake in relation to restrained eating, disinhibition, and hunger in obese and nonobese Swedish women. Obesity Research, 5(3), 175–182.
Maayan, L., Hoogendoorn, C., Sweat, V., & Convit, A. (2011). Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring), 19(7), 1382–1387. doi:10.1038/oby.2011.15.
Martin, L. E., Holsen, L. M., Chambers, R. J., Bruce, A. S., Brooks, W. M., Zarcone, J. R., et al. (2010). Neural Mechanisms Associated With Food Motivation in Obese and Healthy Weight Adults. Obesity, 18(2), 254–260. doi:10.1038/oby.2009.220.
Matthews, S. C., Paulus, M. P., Simmons, A. N., Nelesen, R. A., & Dimsdale, J. E. (2004). Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage, 22(3), 1151–1156. doi:10.1016/j.neuroimage.2004.03.005.
Molnar-Szakacs, I., Iacoboni, M., Koski, L., & Mazziotta, J. C. (2005). Functional Segregation within Pars Opercularis of the Inferior Frontal Gyrus: Evidence from fMRI Studies of Imitation and Action Observation. Cerebral Cortex, 15(7), 986–994. doi:10.1093/cercor/bhh199.
Nooner, K. B., Colcombe, S., Tobe, R., Mennes, M., Benedict, M., Moreno, A., et al. (2012). The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. [Review]. Frontiers in Neuroscience, 6, doi:10.3389/fnins.2012.00152.
Ochner, C. N., Laferrère, B., Afifi, L., Atalayer, D., Geliebter, A., & Teixeira, J. (2012). Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neuroscience Research, 74(2), 138–143. doi:10.1016/j.neures.2012.08.002.
Piech, R. M., Lewis, J., Parkinson, C. H., Owen, A. M., Roberts, A. C., Downing, P. E., et al. (2009). Neural correlates of appetite and hunger-related evaluative judgments. PloS One, 4(8), e6581. doi:10.1371/journal.pone.0006581.
Provencher, V., Drapeau, V., Tremblay, A., Despres, J. P., & Lemieux, S. (2003). Eating behaviors and indexes of body composition in men and women from the Quebec family study. Obesity Research, 11(6), 783–792. doi:10.1038/oby.2003.109.
Riou, M.-È., Doucet, É., Provencher, V., Weisnagel, S. J., Piché, M.-È., Dubé, M.-C., et al. (2011). Influence of physical activity participation on the associations between eating behaviour traits and body mass index in healthy postmenopausal women. Journal of Obesity, 2011, 9. doi:10.1155/2011/465710.
Rolls, E. T. (2004). The functions of the orbitofrontal cortex. Brain and Cognition, 55(1), 11–29. doi:10.1016/S0278-2626(03)00277-X.
Rolls, E. T. (2005). Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiology & Behavior, 85(1), 45–56.
Rothemund, Y., Preuschhof, C., Bohner, G., Bauknecht, H.-C., Klingebiel, R., Flor, H., et al. (2007). Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage, 37(2), 410–421. doi:10.1016/j.neuroimage.2007.05.008.
Santel, S., Baving, L., Krauel, K., Münte, T. F., & Rotte, M. (2006). Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Research, 1114(1), 138–148. doi:10.1016/j.brainres.2006.07.045.
Scharmüller, W., Übel, S., Ebner, F., & Schienle, A. (2012). Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neuroscience Letters, 518(2), 106–110. doi:10.1016/j.neulet.2012.04.063.
Siep, N., Roefs, A., Roebroeck, A., Havermans, R., Bonte, M. L., & Jansen, A. (2009). Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural Brain Research, 198(1), 149–158. doi:10.1016/j.bbr.2008.10.035.
Stunkard, A. J., & Messick, S. (1985). The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research, 29(1), 71–83.
Swick, D., Ashley, V., & Turken, A. U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9, 102. doi:10.1186/1471-2202-9-102.
Tang, D. W., Fellows, L. K., Small, D. M., & Dagher, A. (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology & Behavior, 106(3), 317–324. doi:10.1016/j.physbeh.2012.03.009.
Tataranni, P. A., Gautier, J.-F., Chen, K., Uecker, A., Bandy, D., Salbe, A. D., et al. (1999). Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences, 96(8), 4569–4574. doi:10.1073/pnas.96.8.4569.
Tomasi, D., Wang, G.-J., Wang, R., Backus, W., Geliebter, A., Telang, F., et al. (2009). Association of body mass and brain activation during gastric distention: implications for obesity. PloS One, 4(8), e6847. doi:10.1371/journal.pone.0006847.
Vaidya, C. J., & Gordon, E. M. (2013). Phenotypic variability in resting-state functional connectivity: current status. Brain Connectivity, 3(2), 99–120. doi:10.1089/brain.2012.0110.
Volkow, N. D., & Fowler, J. S. (2000). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex, 10(3), 318–325. doi:10.1093/cercor/10.3.318.
Walsh, B. T., Wilson, G. T., Loeb, K. L., Devlin, M. J., Pike, K. M., Roose, S. P., et al. (1997). Medication and psychotherapy in the treatment of bulimia nervosa. American Journal of Psychiatry, 154(4), 523–531.
Westenhoefer, J., Stunkard, A. J., & Pudel, V. (1999). Validation of the flexible and rigid control dimensions of dietary restraint. International Journal of Eating Disorders, 26(1), 53–64. doi:10.1002/(SICI)1098-108X(199907)26:1<53::AID-EAT7>3.0.CO;2-N.
Weston, C. S. (2012). Another major function of the anterior cingulate cortex: the representation of requirements. Neuroscience and Biobehavioral Reviews, 36(1), 90–110. doi:10.1016/j.neubiorev.2011.04.014.
Williamson, D. A., Martin, C. K., York-Crowe, E., Anton, S. D., Redman, L. M., Han, H., et al. (2007). Measurement of dietary restraint: validity tests of four questionnaires. Appetite, 48(2), 183–192. doi:10.1016/j.appet.2006.08.066.
Wing, R. R., & Phelan, S. (2005). Long-term weight loss maintenance. The American Journal of Clinical Nutrition, 82(1), 222S–225S.
Yu-Feng, Z., Yong, H., Chao-Zhe, Z., Qing-Jiu, C., Man-Qiu, S., Meng, L., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development, 29(2), 83–91. doi:10.1016/j.braindev.2006.07.002.
Zou, Q., Ross, T. J., Gu, H., Geng, X., Zuo, X.-N., Hong, L. E., et al. (2013). Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Human Brain Mapping, 34(12), 3204–3215. doi:10.1002/hbm.22136.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Funding
This paper is supported by National Natural Science Foundation of China under Grant Nos. 61473235, 81271549, 61131003, 81470816, 61431013, 81201081, 81227901, 81120108005; the Fundamental Research Funds for the Central Universities No. QN2012031; the Shaanxi Provincial Natural Science Foundation under Grant No. 2015JM3117.
Data collection and sharing project was funded in part by the New York State Office of Mental Health and Research Foundation for Mental Hygiene. Additional project support provided by the NKI Center for Advanced Brain Imaging (CABI), the Brain Research Foundation (Chicago, IL), the Stavros Niarchos Foundation, and NIH grant P50 MH086385-S1. The project Directors were F. Xavier Castellanos, Bennett Leventhal, and Michael Milham. The Project Coordinator was Kate Nooner. David Gutman and Maarten Mennes finished Computational Infrastructure and Data Analytic Development/Support. The NKI/Rockland Sample Team included Melissa Benedict, Bharat Biswal, Barbara Coffey, Stan Colcombe, David Guilfoyle, David Gutman, Harold S. Koplewicz, Matthew Hoptman, Dan Javitt, Larry Maayan, Maarten Mennes, Kate Nooner, Nunzio Pomara. I would like to express my gratitude to Heather S. Pixley for the English editing of this paper.
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained when study participants joined the International Neuroimaging Data-sharing Initiative (INDI) database.
Additional information
NKI/Rockland data Sample used in preparation of this article was obtained from the International Neuroimaging Data-sharing Initiative (INDI) online database NKI/Rockland Sample (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). The investigators within the INDI project contributed to the design and implementation of INDI and provided data but did not take part in the data analysis or the writing of this paper. A complete listing of INDI investigators can be found at: http://fcon_1000.projects.nitrc.org/indi/pro/nki.html
Rights and permissions
About this article
Cite this article
Zhao, J., Li, M., Zhang, Y. et al. Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: an fMRI study. Brain Imaging and Behavior 11, 264–277 (2017). https://doi.org/10.1007/s11682-015-9491-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9491-4