Abstract
Beech stands are considered part of the ancient forest ecosystems in the northern hemisphere. In mixed stands in beach forest ecosystems, the type of associated tree species can significantly affect soil functions, but their influence on microbial activity, nutrient cycling and belowground properties is unknown. Here, we considered forest patches in northern Iran that are dominated by different tree species: Fagus orientalis Lipsky, Quercus castaneifolia C. A. Mey., Pterocarya fraxinifolia (Lam.), Tilia begonifolia Stev., Zelkova carpinifolia Dippe, Acer cappadocicum Gled, Acer velutinum Boiss., Fraxinus excelsior L., Carpinus betulus L., and Alnus subcordata C. A. Mey. For each forest patch–tree species, litter and soil samples (25× 25 × 10 cm, 100 of each) were analyzed for determine soil and litter properties and their relationship with tree species. The litter decomposition rate during a 1-year experiment was also determined. A PCA showed a clear difference between selected litter and soil characteristics among tree species. F. orientalis, Q. castaneifolia, P. fraxinifolia, T. begonifolia, Z. carpinifolia, A. cappadocicum, and A. velutinum enhanced soil microbial biomass of carbon, whereas patches with F. excelsior, C. betulus and A. subcordata had faster litter decomposition and enhanced biotic activities and C and N dynamics. Thus, soil function indicators were species-specific in the mixed beech forest. A. subcordata (a N-fixing species), C. betulus and F. excelsior were main drivers of microbial activities related to nutrient cycling in the old-growth beech forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyrcanian forests are unique forests that have maintained their remnants from the last ice age (Sagheb-Talebi et al. 2014). The Caspian vegetation region on the shores of the Caspian Sea, form a green belt, 110 km wide and 800 km long, of temperate deciduous trees. This ecoregion receives 600 to 2000 mm of rainfall per year. Beech forests are part of the ancient, valuable forest ecosystems in the northern hemisphere because the beech trees have been created by natural regeneration and belong to the third geological period. Based on published statistics, this species alone comprises 23.63% of the number of forests in northern Iran and 29.96% of the volume (Sefidi et al. 2016; Azaryan et al. 2021). In mixed forests, the composition of a mature stand is determined by various dynamic processes that influence the establishing and developing of a stand (Levula et al. 2003). The overstorey composition of trees also significantly affects litter quality and topsoil properties (Kemner et al. 2021; Kim et al. 2021; Wang et al. 2021; Qin and Wang 2022).
Soil directly contributes to various ecosystem functions and services including net primary production, climate regulation, nutrient cycle and carbon sequestration (Singh et al. 2018; Parhizkar et al. 2021). Soil is a finite resource because it develops over very long periods of time. On the human timescale, soil can be considered a non-renewable natural resource. Plant community composition at the ground level alters soil processes and functions through a variety of factors including microclimate change, bedding and root secretions, and habitat or resource provisions for soil microbial communities (Lin et al. 2021). Therefore, understanding the impact of plant communities on multiple soil functions is of great value.
The fertility of forest soils is seen in its ability to provide nutrients for forest trees (Nguemezi et al. 2020). The forest canopy cover can affect soil fertility as an important part of forest sites. Amount and quality of litter (Majasalmi and Rautiainen 2020), through-fall and stem flow, rooting patterns, microbial activities and forest floor processes are typically affected by soil fertility, directly or indirectly (de Vries et al. 2021), and result in different levels of forest productivity (Majasalmi and Rautiainen 2020).
Soil quality is promoted through ongoing organic material input and decomposability via microbial activities that generate soil fertility hotspots. These processes lead to an increase in soil microbial diversity and respiration under the tree canopy (Wang et al. 2022) and create a microhabitat for increasing biodiversity (Catenazzi and Donnelly 2007; Rathore et al. 2022). These hotspots of fertility have been hypothesized to form through both biotic and abiotic processes (i.e., litterfall and decomposition) (Charley and West 1977), atmospheric deposition (Fenn et al. 2003) and microbial activity (Žifčáková et al. 2016; Wang et al. 2022) that make feedback loops and fortify high nutrient accumulation (Schlesinger et al. 1996). The attributes of the fertility hotspots will also differ based on factors such leaf quality, species composition and the ecosystem (Alameda et al. 2012). For instance, the canopy cover of trees provides a suitable environment for microbial colonization (Ortiz et al. 2022) and activity by reducing solar radiation, temperature and soil water evaporation (Berry et al. 2013). Changes in forest tree composition due to global climate change also obviously affect ecosystem performance (Mueller et al. 2012). Hence, the impact of trees on biogeochemical cycles has widely been studied, but the results have been inconsistent (Wang et al. 2018, 2021; De Andres 2019).
Beech species can accelerate soil acidification and thus leaching of nutrients, decreasing topsoil fertility. Therefore, the presence of other tree species in beech forest stands may help improve soil characteristics and nutrient cycling (Kooch 2012). In the forests of northern Iran, the contributions of the other tree species that grow with the old-growth beech trees to microbial activity, nutrient cycles, and belowground properties have not been considered yet. Here, we thus assessed the effect of mixed forest stands in composition with different tree species on soil functional indicators in organic and mineral layers. We hypothesized that the soil fertility and microbial hotspots would predominantly correlate with the tree species and litter properties. Our findings on soil functions will serve as a base to optimize forest structure and improve ecosystem services.
Materials and methods
Study area
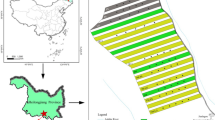
The Golband region (study site), consisting of 36,855 ha in northern Iran (Fig. 1A), is characterized by uneven-aged stands (i.e., tree diameters between 10–150 cm). The Golband watershed lies between 51°17′ E to 51°46′ E, 36°27' N to 36°35 N. The mean altitude is 2000 m above sea level, mean total rainfall is 900 mm, and mean annual temperature is 11 °C. The slope of the region varies between 5 and 70%. According to the American USDA Soil Taxonomy classification (Hughes et al. 2017), the soil of the region is Alfisols. The area consists of mixed beech forest dominated by oriental beech (Fagus orientalis Lipsky), wingnut (Pterocarya fraxinifolia Lam.), oak (Quercus castaneifolia C. A. Mey.), lime tree (Tilia begonifolia Stev.), maple tree (Acer velutinum Boiss.), Caucasian zelkova (Zelkova carpinifolia Dippe), ash (Fraxinus excelsior L.), hornbeam (Carpinus betulus L.), Cappadocian maple (Acer cappadocicum Gled), and Caucasian alder (Alnus subcordata C.A. Mey.). Less-frequent species (< 10%) are elm (Ulmus glabra Huds.), wild cherry (Prunus avium L.), and wild service tree (Sorbus torminalis Crantz). Herbaceous species such as Asperula odorata L., Hypericum androsaemum L., Euphorbia amygdaloides L., and Polystichum sp. cover more than 85% of the forest floors (Anonymous 2018).
Location of the study area (Golband Forest) in the Mazandaran Province, northern Iran, with 100 studied patches (plots) of dominated tree species (A, B). Soil samples (0–10 cm depth) were taken in a 25 × 25 cm area under each canopy cover (C). Note: Fagus: Fagus orientalis Lipsky, Quercus: Quercus castaneifolia C. A. Mey., Pterocarya: Pterocarya fraxinifolia Lam., Tilia: Tilia begonifolia Stev., Zelkova: Zelkova carpinifolia Dippe, Acer C.: Acer cappadocicum Gled, Acer V.: Acer velutinum Boiss., Fraxinus: Fraxinus excelsior L., Carpinus: Carpinus betulus L., and Alnus: Alnus subcordata C.A. Mey
Sampling design and laboratory measurements
Patches (hereafter plots) of dominant tree species were identified in the study area. In total, 100 plots (10 replications for each tree species) were considered (see Fig. 1A, B). Each plot includes an individual tree (DBH about 50 cm) of the dominant tree species that are always surrounded by similar tree species. All plots were located between 1000 and 1100 m a.s.l., had a similar aspect (north), slope class (12%–16%), forest management (preserved areas and intact without harvesting) and were at least 1000 m from each other. In September, a litterbag experiment was set up to assess litter decomposition in the field (Wieder and Lang 1982). Four litter traps (1 m × 1 m) were placed in each plot at the four sides of the studied tree species at a distance of one-third of the crown radius from the stem, with 40 bags of laboratory-dried litter from each species within each plot. In August, soil samples (25 cm × 25 cm × 10 cm; see Fig. 1C) were taken at the site of each litter trap. In total, 400 (i.e., 10 tree species × 10 replicated plots × 4 samples) litter and soil samples were gathered, the litter and soil samples within each plot (n = 4) were bulked separately to yield one composite litter and one composite soil sample for each plot (total: 100 litter and 100 soil samples) for analysis. In addition, litter (forest floor or O-horizon) thickness was measured at the litter trap sites (Dechoum et al. 2015).
An elemental analyzer (Fisons EA1108, Milan, Italy) was used to measure total C in samples and nutrient components in litter (Parsapour et al. 2018). Bulk and particle densities (BD and PD, respectively) of soils were determined with clod (Plaster 1985) and pycnometer (Blake and Hartge 1986) methods. Soil porosity was computed as 1 − (BD / PD) (Pires et al. 2014). The distribution of aggregate size (0.053 and 0.25 mm for microaggregates and 0.25 and 0.50 mm for macroaggregates) was determined as described by Cambardella and Elliott (1992). Aggregate stability and texture of soils were determined using the Yoder and Bouyoucos methods (Bouyoucos 1962; Kemper and Rosenau 1986). Soil pH was determined using an Orion Ionalyzer Model 901 pH meter in a 1:2.5, soil: water solution. EC (Electrical Conductivity) was determined using an Orion Ionalyzer Model 901 EC meter in a 1:2.5 soil: water solution. Soil organic C and total N were determined using the Walkley–Black and Kjeldahl methods (see Allison 1975; Bremner and Mulvaney 1982). C and N sequestrations in this study were computed as C or N sequestration = C or N content × Soil depth × BD × 0.1, where 0.1 is a conversion factor. Particulate organic C and particulate organic N (POC and PON) were measured using physical sundering (Cambardella and Elliot 1992). Dissolved organic C and dissolved organic N (DOC and DON) was analyzed using the procedure of Jones and Willett (2006). Soil extraction solutions were used for the colorimetric determination of NH4+ (at 645 nm) and NO3− (at 420 nm) concentrations (Li et al. 2014). Soil available K, Ca, and Mg were measured using atomic absorption spectrophotometry (Bower et al. 1952) and available P using spectrophotometry (Homer and Pratt 1961). The separated fine roots (i.e., diameter < 2 mm) were dried at 70 °C, then weighed (Neatrour et al. 2005).
Soil water content and temperature (as soil climate variables) and biota were measured in summer (15 August) and fall (15 November). Soil water content was measured by drying soil samples at 105 °C for 24 h. Soil temperature was measured using a digital probe-thermometer sensor (TFA Dostmann, Model 30.1048, Ottersberg, Germany) in the field (Zancan et al. 2006). Before drying earthworms were picked from the soil samples and categorized into ecological classes based on exterior specifications (Kooch et al. 2014). Extraction and Acari and Collembola counts were obtained using the Berless-Tulgreen funnel method; to obtain mesofauna counts, a certain amount of soil was weighed and placed in the Berless-Tulgreen funnel; after 4 days, mesofauna were collected in 0.5% v/v formalin and counted) (Page 1986). Nematodes were extracted using tray (Whitehead and Hemming 1965) and centrifugation (Niknam 1991) methods, then fixed and transferred to glycerin. Densities of soil protozoa were determined using an extraction method and light microscopy (Mayzlish and Steinberger 2004). Bacterial and fungal counts on nutrient agar and potato dextrose agar were obtained by serial dilution and plate count methods (Kooch et al. 2020). Soil basal respiration (BR) was measured using CO2 emission method, and CO2 absorption was measured in an alkaline solution (Alef 1995). Substrate-induced respiration (SIR) (based on CO2 production; Anderson and Domsch 1990) and microbial biomass C, N and P (i.e., MBC, MBN and MBP) were measured using a fumigation-extraction method (Brookes et al. 1985). The metabolic quotient (qCO2), microbial ratio or entropy and C availability index were calculated based on the stoichiometry of soil organic C, respiration and microbial biomass C (Insam and Domsch 1988; Anderson and Domsch 1990; Cheng et al. 1996). Soil enzyme activities were calculated using the Schinner and von Mersi (1990) protocol. Soil C and net N mineralization (Cmin and Nmin) were measured as described by Raiesi (2012a, b a, b) controlled laboratory conditions.
Statistical analyses
Normality of data was assessed using a Kolmogorov–Smirnov test and homogeneity of variance was checked using Levene’s test. One-way analysis of variance (ANOVA) was used to compare litter and soil properties among different tree species; means that differed significantly were analyzed using Duncan’s test (P ≤ 0.05). All analyses were done using SPSS (version 20; IBM, Armonk, NY, USA). In addition, principal component analysis (PCA using PC-Ord version 5.0; Mc Cune and Mefford 1999) was performed to identify any patterns in the changes in litter and soil characteristics between different tree species.
Results
Litter properties
Litter chemistry differed significantly among the various tree species. The contents of NPK, Mg and Ca in the A. subcordata litter was highest. Litter thickness was greatest under F. orientalis (≈ Q. castaneifolia). Litter C was significantly higher under F. orientalis, Q. castaneifolia and P. fraxinifolia, which had the same functional traits. In addition, F. orientalis had the highest litter C/N ratio (Table 1). The highest litter decomposition rates after 360 days were found under Alnus, but the trend in litter decomposition was the same for all tree species over the experiment. The ANOVA results revealed that significant differences in litter decomposition were due to litter types of the various tree species. At all sites, the litter lost half of its initial mass during the incubation period (Fig. 2 and Table S1).
Litter mass remaining (kg ha−1) under different tree species. Data in details are presented in Appendix 1. Note: Fagus: Fagus orientalis Lipsky, Quercus: Quercus castaneifolia C. A. Mey., Pterocarya: Pterocarya fraxinifolia Lam., Tilia: Tilia begonifolia Stev., Zelkova: Zelkova carpinifolia Dippe, Acer C.: Acer cappadocicum Gled, Acer V.: Acer velutinum Boiss., Fraxinus: Fraxinus excelsior L., Carpinus: Carpinus betulus L., and Alnus: Alnus subcordata C.A. Mey
Soil properties
All soil properties, except soil density, the amount of sand and C sequestration, were affected by the tree species. The most available P and K was found under Alnus, whereas litter under A. subcordata ≈ C. betulus species had the most available Ca and Mg. Soil organic C, POC and DOC were significantly higher at sites having F. orientalis and Q. castaneifolia than at the other sites, but soil C/N ratio was highest under F. orientalis, Q. castaneifolia and P. fraxinifolia. Fine root biomass was highest in the topsoil of A. subcordata ≈ C. betulus and the lowest under F. orientalis (Table 2). In fact, these two species had similar values for some soil characteristics. Overall, the various tree species affected the soil microclimate and biotic characteristics (Table 3). In addition, soil biota population differed significantly among the tree species, and seasonal changes in all traits evaluated were similar among the tree species. The soil water content was higher under F. orientalis ≈ Q. castaneifolia, with maximum values during the autumn. In the summer, soil temperatures were highest in A. subcordata ≈ C. betulus ≈ F. excelsior stands (Table 3), which have the same function as other species. Except for soil bacteria and fungi, which were more abundant in the summer, the activities of other soil biotas were higher in the autumn when an ideal soil climate (i.e., higher soil water content and lower soil temperature) prevailed for most of the studied soil organisms (Table 3, Fig. 3).
The different tree species exerted significant effects due to the habitat types on the activity of ecological groups of earthworms. Maximum activity was observed under A. subcordata trees. The densities of soil acarina were highest under A. subcordata ≈ C. betulus ≈ F. excelsior, whereas greater densities of collembola, nematode and bacteria were observed under A. subcordata species. Moreover, higher densities of protozoa were detected under A. subcordata and C. betulus and more fungi under A. subcordata ≈ C. betulus ≈ F. excelsior ≈ A. velutinum. (Table 3). The effects of trees on changes in soil microbial and enzymatic activities were significant (Table 4). Soil BR, SIR, MBN, MBP, qCO2, urease and acid phosphatase activities were highest under A. subcordata. Arylsulfatase activity was highest under A. subcordata ≈ C. betulus and invertase activity highest under A. subcordata ≈ C. betulus ≈ F. excelsior. The soil under F. orientalis had a higher MBC, while the highest MBC/MBN ratio was measured for F. orientalis or Q. castaneifolia stands (Table 4). After 17 weeks of incubation, the soil C mineralization in the litter was differentially affected by the tree species in rank order of A. subcordata ≈ C. betulus ≈ F. excelsior > A. velutinum. > A. cappadocicum. > Z. carpinifolia ≈ T. begonifolia > P. fraxinifolia ≈ Q. castaneifolia ≈ F. orientalis. However, soil N mineralization after 35 days, was differentially affected by tree species in rank order of A. subcordata > C. betulus > F. excelsior ≈ A. velutinum. > A. cappadocicum. ≈ Z. carpinifolia > T. begonifolia > P. fraxinifolia ≈ Q. castaneifolia ≈ F. orientalis (Fig. 4; Table S2).
Mean (± SE; n = 10) of soil C and N mineralisation (A, B) under different tree species. Data in details are presented in Appendix 2. Note: Fagus: Fagus orientalis Lipsky, Quercus: Quercus castaneifolia C. A. Mey., Pterocarya: Pterocarya fraxinifolia Lam., Tilia: Tilia begonifolia Stev., Zelkova: Zelkova carpinifolia Dippe, Acer C.: Acer cappadocicum Gled, Acer V.: Acer velutinum Boiss., Fraxinus: Fraxinus excelsior L., Carpinus: Carpinus betulus L., and Alnus: Alnus subcordata C.A. Mey
PCA based on the correlation matrix of the tree species (A), litter and soil properties (B, C). Note: Fagus: Fagus orientalis Lipsky, Quercus: Quercus castaneifolia C. A. Mey., Pterocarya: Pterocarya fraxinifolia Lam., Tilia: Tilia begonifolia Stev., Zelkova: Zelkova carpinifolia Dippe, Acer C.: Acer cappadocicum Gled, Acer V.: Acer velutinum Boiss., Fraxinus: Fraxinus excelsior L., Carpinus: Carpinus betulus L., and Alnus: Alnus subcordata C.A. Mey
Relationship among trees with litter and soil properties
In the PCA analysis of the 70 variables evaluated for litter and soil samples, two principal components (PC1 and PC2) explained over 55% (PC1 = 50.13%, PC2 = 6.90%) of the total variance. The PCA outcomes showed a clear discrimination in the litter and soil properties among tree species due to functional traits or habitat types of trees. Two categories of drivers for soil fertility and microbial activities were revealed Fig. 5. Group 1 (F. orientalis, Q. castaneifolia, P. fraxinifolia, T. begonifolia, Z. carpinifolia, A. cappadocicum. and A. velutinum.) enhanced soil microbial biomass of carbon and had a positive effect on soil properties, whereas group 2 (F. excelsior, C. betulus and A. subcordata) showed litter nutrients and enhanced biota activities, C and N cycles (Fig. 3A − C).
Discussion
Litter properties
Our findings clearly indicate litter chemistry and decomposition were differentially affected by the various tree species. In addition to the quality of litter, the availability and content of nutrients that are returned to the soil environment are fundamental for soil fertility and optimum tree growth (Cao et al. 2020). Houle et al. (2015) believed that the type of tree species and the quality of their litter determine the amount of available nutrients and the mechanism of litter decomposition. Chen et al. (2020) pointed to the importance and role of forest species in restoring nutrients to the soil and stated that the litter quality (thickness and litter elements like C, N, K, P, Ca, Mg and C/N ratio) strongly affects decomposition rate and soil fertility. In this study, we showed that A. subcordata provides more P, K, Ca, Mg, and total N in the litter than the other species. In addition to the nutrients, the C/N ratio is an important indicator in decomposition dynamics (Goh and Totua 2004; Vesterdal et al. 2012; Kooch and Bayranvand 2019). The litter of A. subcordata species had a higher N content than that of the other species. As a consequence, the C/N ratio was lower. We therefore hypothesize that A. subcordata releases more C into the soil than the other species do. A. subcordata is a pioneer species in Hyrcanian forests that adds new organic substances to the forest soil layers every year (Koupar et al. 2011). It also forms a symbiosis with N-fixing actinomycetes and fixes atmospheric N into the soils, increasing soil fertility and providing good quality litter (i.e., lower C/N ratio and higher values of N, P, K, Ca and Mg) (Taleshi et al. 2009; Parsapour et al. 2018). The high N content of A. subcordata litter also increases the populations of soil organisms and the mineralization rate of elements (Glaser et al. 2018). The higher C/N ratio in F. orientalis litter, compared to the other species, may cause a higher recalcitrance and lower decomposition rate than for litter of the other tree types.
Soil properties
As mentioned earlier, soil properties differ significantly among tree species (Wang et al. 2021). The trees drive biogeochemical regulation in ecosystems via the stabilisation of organic C among other things. The lower amount of organic C under A. subcordata and C. betulus is the result of a rapid mineralisation (Kooch 2012; Błońska et al. 2018), which is related to the occurrence of fertility hotspots. In comparison to contents in other trees, the higher contents of N of A. subcordata and C. betulus improve the soil N (Sayyad 2009). The content of soil macro elements is also associated to the release of nutrients by trees and nutrient cycling in the forest floor (Dijkstra and Smits 2002; Osborne et al. 2020). Humus formation and nutrients cycling can also be affected by the canopy of trees (Majasalmi and Rautiainen 2020). A. subcordata and C. betulus can lead to an increase in soil pH and fertility (Zeng et al. 2014; Majasalmi and Rautiainen 2020), whereas Q. castaneifolia and F. orientalis species have greater acidifying capabilities (Augusto et al. 2002) than other deciduous trees do.
Soil biological activities were generally lower under F. orientalis. At A. subcordata stands, the higher quality of the forest floor enhanced soil biota populations (Knops et al. 2002; Osborne et al. 2020). Our data indicated a temporal change in earthworms, acarina, nematodes, protozoa, collembola and densities of fungal and bacterial populations due to seasonal environmental changes (Chaudhuri and Paliwal 2008; Suthar 2012). Soil moisture as an important factor affects the physiology of microorganisms directly, affecting access to water and regulating access to organic matter, which in turn affects macroorganismal and microbial soil populations (Andrade et al. 2017). Soil temperature and water content are major drivers of variations in soil biota under different trees (Lozano-Parra et al. 2015). Higher soil temperature and less topsoil water under various tree species are unsuitable for epigeic activity in the summer. The faster reaction of epigeic earthworms than the other ecological groups is due to the higher sensitivity of epigeic earthworms to the soil microclimate compared to anecic and endogeic forms (Lagerlof et al. 2002), which anecic and endogeic groups can move to various soil layers when conditions are favourable (Nuutinen and Butt 2009). Higher soil fauna activities in the fall have also been confirmed (Crumsey et al. 2013; Ren et al. 2018). In the fall season, maximum earthworm activity was recorded among tree species having favourable soil water. The lower earthworm population in the summer is a result of the drier and warmer conditions (see Fig. 2). Crumsey et al. (2013) reported significant effects of soil water on the comparative frequency of earthworm species comparative frequency of earthworm species and that earthworm species richness was mainly regulated by soil water content rather than pH or soil organic C. Similar results were reported by Suthar (2012); low physiological activity and high temperatures in summer limit the activity of soil organisms.
Differences in soil fertility can also be the result of differences in the earthworm, acarina, collembola, nematode and protozoa populations and preferences (Sackett et al. 2013; Sigurdsson and Gudleifsson 2014). Rich stands with more nutrients and litter having a low C/N ratio (Rehschuh et al. 2021) are preferred by earthworms. Several studies (Drouin et al. 2016; Tucker Serniak 2017; Zhang et al. 2020) revealed a crucial influence of the chemical composition of plants (such as N, lignin, and phenols) on soil properties and fauna. According to our results, the lowest earthworm density and biomass was found at F. orientalis plots having a higher C/N ratio. Desirable conditions for earthworms were provided by A. subcordata, which had the lowest C/N ratio. Thus, soil bacteria activity was higher in A. subcordata plots and more fungi were present in plots with A. subcordata, C. betulus, F. excelsior and A. velutinum. Variations in physical properties (e.g., lower aggregate stability) and soil fertility can also alter bacterial and fungal populations in F. orientalis and Q. castaneifolia plots (Kim et al. 2021). Previous studies showed provisional schemas of soil fungi and bacteria population in various trees and indicated that soil bacteria were more abundant during the summer than the fall (Kuffner et al. 2012; Preusser et al. 2019; Li et al. 2021). Generally, the soil organismal activities in A. subcordata plots might be attributable to higher litter quality and more favourable topsoil temperatures (Glaser et al. 2018). Bacteria are more sensitive to a low pH. Therefore, the highest biomass can normally be related to neutral to slightly alkaline conditions of the soil at A. subcordata stands (pH > 7). Hence, there is a positive correlation between bacterial biomass and pH. Differences in quantity and quality of the litter influences microbial and enzymatic activities, nutrient accessibility and, thus, all biogeochemical cycles (Zheng et al. 2018). Compared to other species, the highest value of BR, SIR, MBN, MBP, and qCO2 was recorded in soils at A. subcordata stands. Tardy et al. (2014) showed a negative effect of low soil fertility on BR and SIR, similar to our findings. The results of Sasongko et al. (2019) revealed that an increase in forest soil nutrients can lead to higher microbial activities, BR, and SIR in the soil.
Our results showed significant differences in enzymatic activities among the tree species. Soil enzymatic activities are affected by soil management strategies and trees (Silva et al. 2012) and can be used to predict changes in soil quality (Guo and Han 2008; Yao et al. 2020). Higher enzymatic activity causes faster decomposition and higher availability of organic nutrients (Wang et al. 2013). The clay fraction seems to contribute to the accumulation of soil enzymes via their stabilisation and protection (Zhong et al. 2015) under A. subcordata. In soils at the A. subcordata sites, the activity of urease increases with increasing pH, EC, total N, and nutrients (Cheng et al. 2013) and decreases with higher C/N ratios. Acid phosphatase activities also can significantly differ depending on the tree species (Chodak et al. 2021) and are strongly affected by pH, soil water, total N and organic C content (Wang et al. 2021). The activity of sulfatase is correlated with soil particle size and highest in the clay fraction (Ling et al. 2014) under A. subcordata. Greater soil water can reduce the revival oxidation potential anaerobic conditions of the soil that constrain enzyme activities (Brockett et al. 2012) under F. orientalis. Low sulfatase activities at the F. orientalis stands can also be a result of low soil pH (Wang et al. 2016). Invertase plays a key role in the N and C cycles by hydrolyzing sucrose into glucose and fructose (Zhong et al. 2015). A. subcordata gives rise to a higher decomposition rate of litter and accelerates N cycling and invertase activity compared to F. orientalis (Zhong et al. 2015). In addition, higher pH (Li et al. 2009) and better fertility (Zhong et al. 2015) at A. subcordata stands improves the invertase enzyme activity. According to our findings, the soil at A. subcordata, C. betulus and F. excelsior stands had the highest mineralisation rate of C and N in comparison with other species. Parallel with our data, previous researches (Eickenscheidt et al. 2014; Uri et al. 2014; Tarighat and Kooch 2018) pointed out that mineralization of soil C and N by N-fixing tree species is significantly higher than for non-N-fixing species. In general, some habitat characteristics such as the quality of litter and soil physicochemical characteristics influence the variability of soil C and N mineralisation under various tree species. In general, A. subcordata provides better conditions for the mineralization of soil C and N due to the more alkaline soil.
Relationship among tree litter types and soil properties
This study is the first scrutiny that quantifies the impacts of typical trees in the Hyrcanian mixed beech forest on litter and soil properties. The PCA revealed a clear difference in the specific litter and soil properties among the studied trees. The type of broadleaf species also affects spatial variations in nutrient cycling. The better quality of organic matter under A. subcordata plots increased the activity of soil fauna/flora, increasing soil fertility. Our comparisons indicate that soil quality increases in order of F. orientalis < Q. castaneifolia < P. fraxinifolia < T. begonifolia < Z. carpinifolia < A. cappadocicum. < A. velutinum. < F. excelsior < C. betulus < A. subcordata. Also, C. betulus and F. excelsior serve an essential role in modifying microbial flora and soil nutrient content. Thus, a mixed natural forest is fundamental to providing soil services in temperate ecosystems and is a pivotal for sustainable forest care. Since the tree species influences litter quantity and quality, forest habitats provide various functions. Therefore, forestry management should be in the direction of ecosystem tolerance and select the most suitable tree species to ensure proper forest care.
Conclusions
The tree species in the Hyrcanian mixed beech forests—F. orientalis, Q. castaneifolia, P. fraxinifolia, T. begonifolia, Z. carpinifolia, A. cappadocicum, A. velutinum, F. excelsior, C. betulus, and A. subcordata—strongly influence litter and soil properties. Our findings revealed that the differences in basic characteristics of these 10 species resulted in a distinct effect on microbial flora and affected soil nutrient cycling. Soil fertility and microbial hotspots in these forests were clearly species-specific confirming our research hypothesis of soil fertility and microbial hotspots governed by tree species and litter properties. According to our data, A. subcordata (as N-fixing species), C. betulus and F. excelsior species are the main drivers of microbial activities related to nutrient cycling in old-growth beech forests. In fact, the admixture of valuable broad-leaved species with beech stands at fertile sites served as a significant silvicultural system for maintaining soil quality via natural or human-induced soil acidification. These findings improve our knowledge of the impacts of different tree species on the litter and soil properties of deciduous mixed forests and subsequent productivity of the relevant ecosystem.
References
Alameda D, Villar R, Iriondo JM (2012) Forest ecology and management spatial pattern of soil compaction: trees’ footprint on soil physical properties. For Ecol Manag 283:128–137. https://doi.org/10.1016/j.foreco.2012.07.018
Alef K (1995) Estimating of soil respiration. In: Alef K, Nannipieri P (eds) Methods in soil microbiology and biochemistry. Academic Press, New York, pp 464–470
Allison LE (1975) Organic carbon. In C. A. Black (Ed.), Methods of soil analysis. Madison, W. I: American Society of Agronomy, Part 2. pp. 1367–1378.
Anderson TH, Domsch KH (1990) Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol Biochem 22:251–255. https://doi.org/10.1016/0038-0717(90)90094-G
Andrade E, Aquino D, Chaves L (2017) Water as capital and its uses in the caatinga. In: Silva JMC, Leal IR, Tabarelli M (eds) Caatinga. Springer, Cham
De Andres EG (2019) Interactions between climate and nutrient cycles on forest response to global change: the role of mixed forests. Forests 10:609. https://doi.org/10.3390/f10080609
Anonymous N (2018) Golband watershed management planning. Islamic Republic of Iran, Organization of forest and rangelands and watershed management, p 376p
Augusto L, Ranger J, Binkley D, Rothe A (2002) Impact of several common tree species of temperate forest on soil fertility. Ann for Sci 59:233–254. https://doi.org/10.1051/forest:2002020
Azaryan M, Abrari Vajari K, Amanzadeh B (2021) Variations in humus and fine root properties related to development stages in a temperate natural beech forest. Eur J for Res 140:307–316. https://doi.org/10.1007/s10342-020-01331-2
Berry R, Livesley SJ, Aye L (2013) Tree canopy shade impacts on solar irradiance received by building walls and their surface temperature. Build Environ 69:91–100. https://doi.org/10.1016/j.buildenv.2013.07.009
Blake GR, Hartge KH (1986) Particle density. In: Klute A (Ed.), Methods of soil analysis. Part 1. Physical and mineralogical methods, 2nd ed. SSSA Book Ser. 5. ASA and SSSA, Madison, WI, 377–382
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils. Agron J 54:464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Bower CA, Reitemeier RF, Fireman M (1952) Exchangeable cation analysis of saline and alkali soils. Soil Sci 73:251–262. https://doi.org/10.1097/00010694-195204000-00001
Bremner JM, Mulvaney CS (1982). Nitrogen-total. Methods of soil analysis. Part 2. Chemical and microbiological properties, (methodsofsoilan2), pp.595–624.
Brockett BF, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven bio-geoclimatic zones in western Canada. Soil Biol Biochem 44:9–20. https://doi.org/10.1016/j.soilbio.2011.09.003
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Cambardella CA, Elliott ET (1992) Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci Soc Am J 56:777–783. https://doi.org/10.2136/sssaj1992.03615995005600030017x
Cao JB, He XX, Chen YQ, Chen YP, Zhang YJ, Yu SQ, Zho LX, Liu ZF, Zhang CL, Fu SL (2020) Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci Total Environ 704:135341. https://doi.org/10.1016/j.scitotenv.2019.135341
Catenazzi A, Donnelly MA (2007) Distribution of geckos in northern Peru: long-term effect of strong ENSO events. J Arid Environ 71:327–332. https://doi.org/10.1016/j.jaridenv.2007.05.003
Charley JL, West NE (1977) Plant-induced soil chemical patterns in some shrub-dominated semi-desert ecosystems of Utah. J Ecol 63:945. https://doi.org/10.2307/2258613
Chaudhuri PS, Paliwal SNR (2008) Earthworm population of rubber plantations (Hevea brasiliensis) in Tripura, India. Trop Ecol 49:225–234
Chen YY, Cao JB, He XX, Liu T, Shao YH, Zhang CL, Zhou QQ, Li F, Mao P, Tao LB, Liu ZF, Lin YB, Zhou LX, Zhang WX, Fu SL (2020) Plant leaf litter plays a more important role than roots in maintaining earthworm communities in subtropical plantations. Soil Biol Biochem 144:107777. https://doi.org/10.1016/j.soilbio.2020.107777
Cheng WX, Zhang QL, Coleman DC, Carroll CR, Hoffman CA (1996) Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol Biochem 28:1283–1288. https://doi.org/10.1016/S0038-0717(96)00138-1
Cheng XL, Yang YH, Li M, Dou XL, Zhang QF (2013) The impact of agricultural land use changes on soil organic carbon dynamics in the danjiangkou reservoir area of China. Plant Soil 366:415–424. https://doi.org/10.1007/s11104-012-1446-6
Chodak M, Sroka K, Woś B, Pietrzykowski M (2021) Chemical and microbial properties of post-mining and post-fire soils afforested with different tree species. Appl Soil Ecol 171:104321. https://doi.org/10.1016/j.apsoil.2021.104321
Crumsey J, Le Moine JM, Vogel CS, Nadelhoffer KJ (2013) Historical patterns of exotic earthworm distributions inform contemporary associations with soil physical and chemical factors across a northern temperate forest. Soil Biol Biochem 68:503–514. https://doi.org/10.1016/j.soilbio.2013.10.029
Dechoum MS, Zenni RD, Castellani TT, Zalba SM, Rejmánek M (2015) Invasions across secondary forest successional stages: effects of local plant community, soil, litter, and herbivory on Hovenia dulcis seed germination and seedling establishment. Plant Ecol 216:823–833. https://doi.org/10.1007/s11258-015-0470-z
Dijkstra FA, Smits MM (2002) Tree species effects on calcium cycling: the role of calcium uptake in deep soils. Ecosystems 5:385–398. https://doi.org/10.1007/s10021-001-0082-4
Drouin M, Bradley R, Lapointe L (2016) Linkage between exotic earthworms, understory vegetation and soil properties in sugar maple forests. For Ecol Manag 364:113–121. https://doi.org/10.1016/j.foreco.2016.01.010
Eickenscheidt T, Heinichen J, Augustin J, Freibauer A, Drösler M (2014) Nitrogen mineralization and gaseous nitrogen losses from waterlogged and drained organic soils in a black alder (Alnus glutinosa (L.) Gaertn.) forest. Biogeosciences 11:2961–2976. https://doi.org/10.5194/bg-11-2961-2014
Fenn ME, Baron JS, Allen EB, Rueth HM, Nydick KR, Geiser L, Bowman WD, Sickman JO, Meixner T, Johnson DW, Neitlich P (2003) Ecological effects of nitrogen deposition in the Western United States. Bioscience 53:404–420. https://doi.org/10.1641/0006-3568(2003)053[0404:EEONDI]2.0.CO;2
Glaser K, Baumann K, Leinweber P, Mikhailyuk T, Karsten U (2018) Algal richness in BSCs in forest under different management intensity with some implications for P cycling. Biogeosciences 15:4181–4192. https://doi.org/10.5194/bg-15-4181-2018
Goh KM, Totua SS (2004) Effect of organic and plant residue and orchard management practice on decomposition rates of residues. Commun Soil Sci Plant Anal 35:441–460. https://doi.org/10.1081/CSS-120029724
Guo YJ, Han JG (2008) Soil biochemical properties and arbuscular mycorrhizal fungi as affected by afforestation of rangelands in northern China. J Arid Environ 72:1690–1697. https://doi.org/10.1016/j.jaridenv.2008.04.001
Homer CD, Pratt PF (1961) Methods of analysis for soils, plants and waters. University of California, Agricultural sciences press, berkeley, p 309p
Houle D, Marty C, Duchesne L (2015) Response of canopy nitrogen uptake to a rapid decrease in bulk nitrate deposition in two eastern Canadian boreal forests. Oecologia 177:29–37. https://doi.org/10.1007/s00442-014-3118-0
Hughes P, Mc Bratney AB, Huang J, Minasny B, Micheli E, Hempel J (2017) Comparisons between USDA soil taxonomy and the Australian soil classification system i: data harmonization, calculation of taxonomic distance and inter-taxa variation. Geoderma 307:198–209. https://doi.org/10.1016/j.geoderma.2017.08.009
Insam H, Domsch KH (1988) Relationship between soil organic carbon and microbial biomass on chronosequence of reclamation sites. Microb Ecol 15:177–188. https://doi.org/10.1007/BF02011711
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999. https://doi.org/10.1016/j.soilbio.2005.08.012
Kemner JE, Adams MB, McDonald LM, Peterjohn WT, Kelly CN (2021) Fertilization and tree species influence on stable aggregates in forest soil. Forests 12:39. https://doi.org/10.3390/f12010039
Kemper WD, Rosenau RC (1986) Aggregate stability and size distribution. In: Klute A (ed) Methods of soil analysis, Part 1: Physical and mineralogical methods, 2nd edn. American Society of Agronomy, Madison, Wisconsin, pp 383–411
Kim S, Axelsson EP, Girona MM, Senior JK (2021) Continuous-cover forestry maintains soil fungal communities in norway spruce dominated boreal forests. For Ecol Manag 480:118659. https://doi.org/10.1016/j.foreco.2020.118659
Knops JMH, Bradley KL, Wedin DA (2002) Mechanism of plant species impacts on ecosystem nitrogen cycling. Ecol Lett 5:454–466. https://doi.org/10.1046/j.1461-0248.2002.00332.x
Kooch Y, Bayranvand M (2019) Labile soil organic matter is sensitive to forest floor quality of tree species mixtures in oriental Beech forests. Ecol Indic 107:105598. https://doi.org/10.1016/j.ecolind.2019.105598
Kooch Y, Hosseini SM, Zaccone C, Jalilvand H, Hojjati SM (2014) Soil organic carbon sequestration as affected by afforestation: the Darab Kola forest (north of Iran) case study. Environ Monit Assess 14:2438–2446. https://doi.org/10.1039/C2EM30410D
Kooch Y, Ehsani S, Akbarinia M (2020) Stratification of soil organic matter and biota dynamics in natural and anthropogenic ecosystems. Soil Tillage Res 200:104621. https://doi.org/10.1016/j.still.2020.104621
Kooch Y (2012) Soil variability related to pit and mound, canopy cover and individual trees in a Hyrcanian Oriental Beech stand. PhD Thesis, Tarbiat Modares University, 203p
Koupar SAM, Hosseini SM, Tabari M, Modirrahmati A, Golchin A, Rad FH (2011) Effects of pure and mixed plantations of Populous deltoides with Alnus glotinosa on growth and soil properties: a case study of foman region. Iran Afr J Agric Res 6:5261–5265. https://doi.org/10.5897/AJAR11.163
Kuffner M, Hai B, Rattei T, Melodelima C, Schloter M, Zechmeister-Boltenstern S, Jandl R, Sessitsch SA, A, (2012) Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyro sequencing. FEMS Microbiol Ecol 82(3):551–562
Lagerlof J, Goffre B, Vincent C (2002) The importance of field boundaries for earthworms (Lumbricidae) in the Swedish agricultural landscape. Agric Ecosyst Environ 89:91–103. https://doi.org/10.1016/S0167-8809(01)00321-8
Levula J, Ilvesniemi H, Westman CJ (2003) Relation between soil properties and tree species composition in a Scots pine-Norway spruce stand in southern Finland. Silva Fenn 37(2):205–218
Li YT, Rouland C, Benedetti M, Li FB, Pando A, Lavelle P, Dai J (2009) Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol Biochem 41:969–977. https://doi.org/10.1016/j.soilbio.2009.01.021
Li M, Zhou XH, Zhang QF, Cheng XL (2014) Consequences of afforestation for soil nitrogen dynamics in central China. Agric Ecosyst Environ 183:40–46. https://doi.org/10.1016/j.agee.2013.10.018
Li WT, Hu XF, Liu QH, Yin CY (2021) Soil fungi are more sensitive than bacteria to short-term plant interactions of Picea asperata and Abies faxoniana. Eur J Soil Biol 106:103348. https://doi.org/10.1016/j.ejsobi.2021.103348
Lin H, Li YN, Bruelheide H, Zhang SR, Ren HB, Zhang NL, Ma KP (2021) What drives leaf litter decomposition and the decomposer community in subtropical forests – the richness of the above-ground tree community or that of the leaf litter? Soil Biol Biochem 160:108314. https://doi.org/10.1016/j.soilbio.2021.108314
Ling N, Sun YM, Ma JH, Guo JJ, Zhu P, Peng C, Yu GH, Ran W, Guo SW, Shen QR (2014) Response of the bacterial diversity and soil enzyme activity in particle size fractions of mollisol after different fertilization in a long-term experiment. Biol Fertil Soils 50:901–911. https://doi.org/10.1007/s00374-014-0911-1
Lozano-Parra J, Schnabel S, Ceballos-Barbancho A (2015) The role of vegetation covers on soil wetting processes at rainfall event scale in scattered tree woodland of mediterranean climate. J Hydrol 529:951–961. https://doi.org/10.1016/j.jhydrol.2015.09.018
Majasalmi T, Rautiainen M (2020) The impact of tree canopy structure on understory variation in a boreal forest. For Ecol Manag 466:118100. https://doi.org/10.1016/j.foreco.2020.118100
Mayzlish E, Steinberger Y (2004) Effects of chemical inhibitors on soil protozoan dynamics in a desert ecosystem. Biol Fertil Soils 39:415–421. https://doi.org/10.1007/s00374-004-0723-9
Mc Cune B, Mefford M (1999) Multivariate analysis of ecological data version 4.17. MJM Software. Gleneden Beach, Oregon, USA, p 233
Mueller K, Eissenstat DM, Hobbie SE, Oleksyn J, Jagodzinski AM, Reich PB, Chadwick OA, Chorover J (2012) Tree species effects on coupled cycles of carbon nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 111:601–614. https://doi.org/10.1007/s10533-011-9695-7
Neatrour MA, Jones RH, Golladay SW (2005) Correlations between soil nutrient availability and fine-root biomass at two spatial scales in forested wetlands with contrasting hydrological regimes. Can J For Res 35(12):2934–29417
Nguemezi C, Tematio P, Yemefack M, Tsozue D, Silats TBF (2020) Soil quality and soil fertility status in major soil groups at the tombel area, South-West Cameroon. Heliyon 97:423–436. https://doi.org/10.1016/j.heliyon.2020.e03432
Niknam Gh (1991) Study and identification of plant parasitic nematodes in the farms of Moghan’s Agroindastry Co. A. Thesis Presented for the Master of Science Degree in Plant Pathology. Collage of Agriculture, Tarbiat Modares University. 140 pp.
Nuutinen V, Butt KR (2009) Worms from the cold: lumbricid life stages in boreal clay during frost. Soil Biol Biochem 41:1580–1582. https://doi.org/10.1016/j.soilbio.2009.04.019
Ortiz C, Fernández-Alonso MJ, Kitzler B, Díaz-Pinés E, Saiz G, Bento M, Rubio A (2022) Variations in soil aggregation, microbial community structure and soil organic matter cycling associated to long-term afforestation and woody encroachment in a Mediterranean alpine ecotone. Geoderma 405:115450. https://doi.org/10.1016/j.geoderma.2021.115450
Osborne BB, Nasto MN, Soper FM, Asner GP, Balzotti CF, Cleveland CC, Taylor PG, Townsend AR, Porder S (2020) Leaf litter inputs reinforce islands of nitrogen fertility in a lowland tropical forest. Biogeochemistry 147:293–306. https://doi.org/10.1007/s10533-020-00643-0
Page AL (1986) Methods of soil analysis, part 2. 2nd edn. Wisconsin, U.S.A.
Parhizkar M, Shabanpour M, Miralles I, Antonio Zema A, Esteban Lucas-Borja M (2021) Effects of plant species on soil quality in natural and planted areas of a forest park in northern Iran. Sci Total Environ 778:146310. https://doi.org/10.1016/j.scitotenv.2021.146310
Parsapour MK, Kooch Y, Hosseini SM, Alavi SJ (2018) Litter and topsoil in Alnus subcordata plantation on former degraded natural forest land: A synthesis of age-sequence. Soil Tillage Res 179:1–10. https://doi.org/10.1016/j.still.2018.01.008
Pires LF, Brinatti AM, Saab SC, Cássaro FA (2014) Porosity distribution by computed tomography and its importance to characterize soil clod samples. Appl Radiat Isot 92:37–45
Plaster EJ (1985) Soil science and management. Delmar Publishers Inc., Albany, N.Y., p 124
Preusser S, Poll C, Marhan S, Angst G, Mueller CW, Bachmann L, Kandeler E (2019) Fungi and bacteria respond differently to changing environmental conditions within a soil profile. Soil Biol Biochem 137:107543. https://doi.org/10.1016/j.soilbio.2019.107543
Qin HY, Wang ZH (2022) Biogeochemistry of dominant plants and soils in shewushan gold lateritic deposit China. Plants 11:38. https://doi.org/10.3390/plants11010038
Raiesi F (2012a) Land abandonment effect on N mineralization and microbial biomass N in a semi-arid calcareous soil from Iran. J Arid Environ 76:80–87. https://doi.org/10.1016/j.jaridenv.2011.08.008
Raiesi F (2012b) Soil properties and C dynamics in abandoned and cultivated farmlands in a semi-arid ecosystem. Plant Soil 351:161–175. https://doi.org/10.1007/s11104-011-0941-5
Rathore AP, Chaudhary DR, Jha B (2022) Assessing the effects of Salicornia brachiata roxb. growth on coastal saline soil quality over temporal and spatial scales. Appl Soil Ecol 169:10419
Rehschuh S, Jonard M, Wiesmeier M, Rennenberg H, Dannenmann M (2021) Impact of European beech forest diversification on soil organic carbon and total nitrogen stocks–a meta-analysis. Front for Glob Change 4:2. https://doi.org/10.3389/ffgc.2021.606669
Ren CJ, Zhang W, Zhong ZK, Han XH, Yang GH, Feng YZ, Ren GX (2018) Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci Total Environ 610–611:750–758. https://doi.org/10.1016/j.scitotenv.2017.08.110
Sackett O, Petrou K, Reedy B, De Grazia A, Hill R, Doblin M, Beardall J, Ralph P, Heraud P (2013) Phenotypic plasticity of Southern Ocean diatoms: key to success in the sea ice habitat? PLoS ONE. https://doi.org/10.1371/journal.pone.0081185
Sagheb-Talebi K, Sajedi T, Pourhashemi M (2014) Forests of Iran: ‘a treasure from the past, a hope for the future.’ Springer Verlag, Publishing, pp 15–65
Sasongko PE, Purwanto P, Dewi WS, Hidayat R (2019) Soil microbial communities below decomposing plant litter from different land uses in Tutur Village. The 9th International Conference on Global Resource Conservation (ICGRC) and AJI from Ritsumeikan University AIP Conf Proc
Sayyad E (2009) The effects of afforested stands on nutrition, nutrients return and soil fauna. PhD thesis of Forestry, Tarbiat Modares University, pp. 96.
Schinner F, Von Mersi W (1990) Xylanase-, CM-cellulose-and invertase activity in soil: an improved method. Soil Biol Biochem 22:511–515. https://doi.org/10.1016/0038-0717(90)90187-5
Schlesinger WH, Raikes JA, Hartley AE, Cross AF (1996) On spatial pattern of soil nutrients in desert ecosystems. Ecology 77:364–374. https://doi.org/10.2307/2265615
Sefidi K, Esfandiary Darabad F, Azaryan M (2016) Effect of topography on tree species composition and volume of coarse woody debris in an oriental beech (Fagus orientalis Lipsky) old growth forest, northern Iran. Iforest 9:658–665. https://doi.org/10.3832/ifor1080-008
Sigurdsson BD, Guoleifsson BE (2014) Impact of afforestation on earthworm populations in Iceland. Icel Agric Sci 26:21–36
Silva DKA, de Oliveira FN, de Souza RG, da Silva FSB, de Araujo ASF, Maia LC (2012) Soil microbial biomass and activity under natural and regenerated forests and conventional sugarcane plantations in Brazil. Geoderma 189:257–261. https://doi.org/10.1016/j.geoderma.2012.06.014
Singh R, Bhardwaj DR, Pala NA, Kaushal R, Rajput BS (2018) Soil microbial characteristics in sub-tropical agro-ecosystems of North Western Himalaya. Curr Sci 115:1956–1959
Suthar S (2012) Seasonal dynamics in earthworm density, casting activity and soil nutrient cycling under Bermuda grass (Cynodon dactylon) in semiarid tropics, India. Environmentalist 32:503–511. https://doi.org/10.1007/s10669-012-9419-0
Taleshi SAR, Dhumal KN, Alipour A, Espahbodi K, Ghasemi O (2009) Impact of alder (Alnus subcordata) in fertility of forest soil. Res J Environ Sci 3:640–644. https://doi.org/10.3923/rjes.2009.640.644
Tardy V, Mathieu O, Leveque J, Terrat S, Chabbi A, Lemanceau P, Ranjard L, Maron PA (2014) Stability of soil microbial structure and activity depends on microbial diversity. Environ Microbiol Rep 6:173–183. https://doi.org/10.1111/1758-2229.12126
Tarighat FS, Kooch Y (2018) The effect of four types of broad-leaved trees on soil C and N storage and mineralization in forest areas of Noor City. Iran J Soil Water Res 22(2):175–188
Tucker Serniak L (2017) The effects of earthworms on carbon dynamics in forest soils. Biol Invasions 12:213–229. https://doi.org/10.1016/B978-0-12-409548-9.10670-0
Uri V, Aosaar J, Varik M, Becker H, Ligi K, Padari A, Kanal A, Lõhmus K (2014) The dynamics of biomass production, carbon and nitrogen accumulation in grey alder (Alnus incana (L.) Moench) chronosequence stands in Estonia. For Ecol Manag 327:106–117. https://doi.org/10.1016/j.foreco.2014.04.040
Vesterdal L, Elberling B, Christiansen JR, Callesen I, Schmidt IK (2012) Soil respiration and rates of soil carbon turnover differ among six common European tree species. For Ecol Manag 264:185–196. https://doi.org/10.1016/j.foreco.2011.10.009
De Vries W, de Jong A, Kros J, Spijker J (2021) The use of soil nutrient balances in deriving forest biomass harvesting guidelines specific to region, tree species and soil type in the Netherlands. For Ecol Manag 479:118591. https://doi.org/10.1016/j.foreco.2020.118591
Wang QK, Xiao FM, He TX, Wang SL (2013) Responses of labile soil organic carbon and enzyme activity in mineral soils to forest conversion in the subtropics. Ann for Res 70:579–587. https://doi.org/10.1016/j.still.2021.104946
Wang RZ, Creamer CA, Wang X, He P, Xu ZW, Jiang Y (2016) The effects of a 9-year nitrogen and water addition on soil aggregate phosphorus and sulfur availability in semi-arid grassland. Ecol Indic 61:806–814. https://doi.org/10.1016/j.ecolind.2015.10.033
Wang Q, Kwak JH, Choi WJ, Chang SX (2018) Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: Effects of litter chemistry and forest floor microbial properties. For Ecol Manag 412:53–61
Wang CQ, Xue L, Jiao RZ (2021) Soil phosphorus fractions, phosphatase activity, and the abundance of phoC and phoD genes vary with planting density in subtropical Chinese fir plantations. Soil Tillage Res 209:104946. https://doi.org/10.1016/j.still.2021.104946
Wang ZH, Bai Y, Hou JF, Li F, Li XQ, Cao R, Deng YY, Wang HB, Jiang YR, Yang WQ (2022) The changes in soil microbial communities across a Subalpine forest successional series. Forests 13:289. https://doi.org/10.3390/f13020289
West PC, Gerber JS, Clark JM, Adhya TR, Scholes J, Scholes MC (2015) Biogeochemical cycles and biodiversity as key drives of ecosystem services provided by soils. Soil 1:665–685. https://doi.org/10.5194/soil-1-665-2015
Whitehead AG, Hemming JR (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 55:25–38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Wieder RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litterbags. Ecology 63:1636–1642. https://doi.org/10.2307/1940104
Yao YF, Shao MA, Fu XL, Wang X, Wei XR (2020) Effects of shrubs on soil nutrients and enzymatic activities over a 0–100 cm soil profile in the desert-loess transition zone. CATENA 174:362–370. https://doi.org/10.1016/j.catena.2018.11.031
Zancan S, Trevisan R, Paoletti M (2006) Soil algae composition under different agro-ecosystems in North-Eastern Italy. Agric Ecosyst Environ 112:1–12. https://doi.org/10.1016/j.agee.2005.06.018
Zeng DH, Hu YL, Chang SX, Fan ZP (2014) Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil 317:121–133. https://doi.org/10.1007/s11104-008-9793-z
Zhang WX, Li JX, Guo MF, Liao CH, He XX, Lin YB, Fu SL (2020) The presence of earthworm Pontoscolex corethrurus rather than organic matter sources indirectly controls N2O flux in tropical plantation soils. Eur J Soil Biol 96:103150
Zheng Y, Wang S, Bonkowski M, Chen XY, Griffiths B, Hu F, Liu MQ (2018) Litter chemistry influences earthworm effects on soil carbon loss and microbial carbon acquisition. Soil Biol Biochem 123:105–114. https://doi.org/10.1016/j.soilbio.2018.05.012
Zhong S, Zeng HC, Jin ZQ (2015) Soil microbiological and biochemical properties as affected by different long-term banana-based rotations in the tropics. Pedosphere 25:868–877. https://doi.org/10.1016/S1002-0160(15)30067-9
Žifčáková L, Větrovský T, Howe A, Baldrian P (2016) Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ Microbiol 18(1):288–301
Acknowledgement
The authors express their thanks to Tarbiat Modares University for the financial support of the study reported in this paper.
Author information
Authors and Affiliations
Contributions
Y K conceived and designed the experiments and analyzed the data; N G and S H performed the experiments; Markus Egli provided editorial advice.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The online version is available at http://www.springerlink.com.
Corresponding editor: Yanbo Hu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kooch, Y., Ghorbanzadeh, N., Hajimirzaaghaee, S. et al. Soil functional indicators in mixed beech forests are clearly species-specific. J. For. Res. 34, 1033–1049 (2023). https://doi.org/10.1007/s11676-022-01548-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01548-4