Abstract
The regulation of plant transpiration is a key factor affecting transpiration efficiency, growth and adaptation of Eucalyptus species to limited water availability in tropical and subtropical environments. However, few studies have related this trait to the performance of Eucalyptus seedlings and none have investigated the influence of vapor pressure deficit (VPD) on transpiration rates and growth. In this study, the transpiration and growth responses of seedlings of Eucalyptus urophylla (S.T. Blake) and Eucalyptus cloeziana (F. Muell.) to progressive soil water deficits were evaluated under semi-controlled conditions using the fraction of transpirable soil water (FTSW) method. In addition, the influence of VPD on seedling transpiration, development and growth was also investigated. The FTSW threshold ranged from 0.40 to 0.99 for the transpiration rate and from 0.32 to 0.97 for the development and growth variables. Little or no changes in the FTSW threshold were detected in response to changes in atmospheric VPD. Both Eucalyptus species presented a conservation strategy under drought stress. In addition, water-conserving mechanisms during the seedling phase were related to rapid stomatal closure, reduced leaf area, and number of leaves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil water availability is a major driver of forest productivity. Recent projections of climate change have indicated, among other possible consequences, variable trends in precipitation and the intensification of drought worldwide (Santos et al. 2017; Llopart et al. 2020). An issue is how plants—cultivated or in natural environments—will respond to changing conditions. Besides the implication to natural ecosystem functioning, the intensification of drought will have a significant negative impact on agriculture and forestry with increased mortality and decreased growth (Scolforo et al. 2019; Elli et al. 2020; Hu et al. 2020).
Brazil has a global role in agricultural production, including cellulose fiber from Eucalyptus as an important forest product. Brazil has the largest area in the world cultivated with Eucalyptus with a mean increment of 36 m3 ha−1 a−1 and rotation of seven years or shorter (IBA 2019; Elli et al. 2020). The Eucalyptus plantation sector plays an important role in the country’s economy and land use. About 7.5 million hectares are intensively cultivated under high technology (Elli et al. 2020) from which a variety of products such as pulp, paper, charcoal, boards and solid wood are obtained (IBA 2019).
Drought constitutes a major limiting factor in the growth of eucalypts in many regions of Brazil (Abreu et al. 2015; Scolforo et al. 2019), especially where water deficits are associated with high temperatures during particular periods of the year such as in Minas Gerais State, located in Brazil’s southeast which has the largest area of eucalypt plantations. Recent climatic studies have shown the possibilities for considerable increases in air temperatures of up to 5 °C, as well as various trends in amounts of precipitation, with projections of reduced rainfall in the northern region and a slight increase in central and south regions of the state (Santos et al. 2017; Martins et al. 2020). Therefore, there will be changes in soil water availability (supply) and in the amount of water consumed by forest plantations (demand).
The imbalance between water supply and demand leads to water-deficit situations (Souza et al. 2014; Abreu et al. 2015), which affects all cellular processes, leaf expansion, stomatal conductance, photosynthetic activities, dry matter remobilization, senescence and foliar abscission, and transpiration rates (TR) (Esmaeilzade-Moridani et al. 2015; King and Purcell 2017; Santos et al. 2017). In forest species, all of these effects are even more drastic when they occur during the seedling phase (Abreu et al. 2015) because they impair seedling establishment in the field, reducing drastically forest potential development and yield (Sinclair et al. 2005; Martins et al. 2008; Elli et al. 2020).
In this context, different indexes have been developed to evaluate plant responses to drought stress: total available soil water (TAW), available water capacity (AWC), available water fraction (AWF), plant-available soil water (PAW), and fraction of transpirable soil water (FTSW). Among these, FTSW has been widely used to detect plant responses to drought stress (Sinclair and Ludlow 1986; Sinclair et al. 2005, 2017; Medina et al. 2017).
FTSW has two distinct stages (Sinclair and Ludlow 1986; Sinclair et al. 2005; Souza et al. 2014). Stage I occurs when soil water is maximum and stomatal conductance and water vapor loss are the highest. Stage II begins when soil water decreases and the rate of water uptake from the soil cannot match the potential TR. At stage II, the plants initiate stomatal closure for conserving water (Medina et al. 2017) as the stomata are gateways for gas exchange between the leaf and the atmosphere (Devi and Reddy 2018).
The threshold between stages I and II (FTSWt) indicates the point at which the transpiration rate is reduced (Martins et al. 2008; King and Purcell 2017; Adiredjo et al. 2018), i.e., when stomatal closure in response to water deficit is initiated (Sinclair and Ludlow 1986; Souza et al. 2014) and the degree of plant tolerance to drought (Souza et al. 2018b). FTSWt is commonly used in crop yield simulation models (Devi et al. 2009; Abreu et al. 2015; Fuentealba et al. 2016; Medina et al. 2019) and is extremely useful for understanding genotypic behavior in the face of water deficits (Medina et al. 2019).
It is reasonable to assume that FTSWt is a result of balancing plant water content (Ray et al. 2002; Bimpong et al. 2011; Pang et al. 2017; Devi and Reddy 2018). Therefore, factors influencing plant water demands could influence FTSWt (Ray et al. 2002; Schoppach and Sadok 2012; Abreu et al. 2015; Hainaut et al. 2016; Ouattara et al. 2018).
Several studies have demonstrated that FTSWt and consequently, plant transpiration are driven by changes in VPD (Bimpong et al. 2011; Schoppach and Sadok 2012; Abreu et al. 2015; Hainaut et al. 2016; Devi and Reddy 2018; Ouattara et al. 2018), which is a function of air temperature and relative humidity (RH) (Abreu et al. 2015; Medina et al. 2019). Theoretically, high vapor pressure deficits, which usually occurs from midday to ~ 4 pm, influence the water balance and result in an increased rate of transpiration (Devi and Reddy 2018). This situation drives a greater hydraulic conductivity, allowing water to be more readily replenished to leaves (Ray et al. 2002; Lago et al. 2011; Gholipoor et al. 2012; Sinclair et al. 2017).
A critical requirement to sustain leaves is to have restricted TR under high VPD by partial stomatal closure so that the rate of transpiration decreases to match water flux into the leaf (Sinclair et al. 2017). However, this response is not universal and differences have been reported among species, cultivars, hybrids and varieties (Sinclair et al. 2017; Medina et al. 2019) such as maize hybrids (Ray et al. 2002), cassava cultivars (Lago et al. 2011) and Eucalyptus saligna Smith. (Martins et al. 2008) for which limitations on transpiration rates under high VPD have not been observed and, consequently, no relationship between FTSWt and VPD.
Although FTSWt is an important indicator of plant responses to water-deficit, few studies have looked in forest species. The limited researches were on Scots pine (Pinus sylvestris L.) (Irvini et al. 1998), beech (Fagus sylvatica L.) (Granier et al. 2000), Norway spruce (Picea abies (L.) H. Karst.) (Lagergren and Lindroth 2002), canoe-cedar (Thuja plicata Donn ex D. Don), red maple (Acer rubrum L.), English holly (Ilex aquifolium L.), black locust (Robinia pseudocacia L.) (Sinclair et al. 2005), Barbados-nut (Jatropha curcas L.) (Ouattara et al. 2018), Lemon-Scented Gum (Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Johnson) (Abreu et al. 2015), and two Eucalyptus species (Martins et al. 2008). All these studies confirmed a two-phase response to soil water availability with little differences in the FTSWt values that represent the transition from stages I to II. However, none considered the influence of VPD on this response.
Therefore, in this study, the following questions arise: (1) Are there Eucalyptus seedling defense strategies under increasing soil water deficits?; (2) What is the water use efficiency by Eucalyptus seedlings under increasing soil water deficits?; and, (3) What is the dry weight allocation by Eucalyptus seedlings under increasing soil water deficits? To answer these questions, this study assessed transpiration rates as well as the development response of Eucalyptus urophylla (S.T. Blake) and Eucalyptus cloeziana (F. Muell.) seedlings to progressive soil water deficits, taking into account differences in VPD. These two species are widely planted in Brazil and studies show differences in their response to drought. Although highly adaptable to a wide range of environmental conditions (Gonçalves et al. 2017; Elli et al. 2020), E. cloeziana is sensitive to soil water reduction (Ngugi et al. 2004), while E. urophylla, has been reported to be more drought tolerant (Elli et al. 2020).
Understanding the physiological responses to the biophysical environment, particularly water stress, is key for the development of silvicultural prescriptions and to anticipate the response of these two important species and also of other tree species of similar behavior to the projected climate changes.
Materials and methods
Observed meteorological data
The daily maximum (Tmax) and minimum (Tmin) air temperatures were measured using conventional thermometers with mercury and alcohol, respectively, and a hygrometer recorded the relative humidity (RH). The instruments were installed in a meteorological station at the greenhouse. Daily VPD was obtained as per Abreu et al. (2015):
where Tmin is the minimum temperature (°C), Tmax is the maximum temperature (°C), VPD is the vapor pressure deficit (kPa), esmin is the saturation water vapor pressure using Tmin (kPa), esmax is the saturation water vapor pressure using Tmax (kPa), esavg is the average between the esmin and esmax (kPa), eamin is the actual water vapor pressure using Tmin (kPa), eamax is the actual water vapor pressure using Tmax (kPa), eaavg is the average between eamin and eamax (kPa), RHmin is the minimum and RHmax is the maximum relative humidity (%).
Experimental design
Two greenhouse experiments (E1 and E2) were carried out in Itajubá, Minas Gerais, southeastern Brazil (SEB) (22.50° S, 45.45° W and altitude of 850 m) following similar protocols as in Sinclair and Ludlow (1986) and Abreu et al. (2015). Itajubá has a typical monsoon climate, with a well-defined dry and cold season in the austral winter (June to August), and rainy and hot in the austral summer (December to February) (Reis et al. 2021). E1 was carried out in the spring and summer (from October to March) of 2012–2013 and E2 in the fall and winter (from April to November) of 2013. Both experiments were installed in a completely random design in a 2:2 factorial scheme with two Eucalyptus species, E. urophylla and E. cloeziana, two soil–water treatments (well-watered, WW, and progressive water deficit, WD), and nine replicates per treatment. The experimental units were pots containing two seedlings.
To establish the experimental units, highly pure seeds (≈ 92%) were obtained from the Society for Forestry Investigations, Minas Gerais. Approximately twenty seeds of both species were sown in each of 36 − 25 cm × 22 cm × 20 cm-polypropylene pots filled with 8 kg of a Rhodic Hapludox Oxisol. The soil chemical characteristics included 2.45 dag kg−1 of organic matter (Walkley–Black), 0.5 mg dm−3 of potassium and 4.0 mg dm−3 of phosphorous, obtained by Mehlich extractor 1. Approximately 90 days before sowing, fertility and acidity were corrected according to Barros and Novais (1999) by applying 10.18 g of simple superphosphate (18%), 0.31 g of potassium chloride (60%), 0.20 g of ammonium sulfate (20%) and 12.32 g of calcium carbonate in each pot. After emergence, the seedlings in each pot were periodically removed until only two remained.
Water regimes and transpiration responses to progressive soil water deficit
Plants were grown under WW conditions until the main stem had formed 25 leaves, which is considered the most proper development plateau for Eucalyptus seedlings (Martins et al. 2008). WW conditions were guaranteed by daily watering to 100% of pot weight capacity, never allowing the water content to fall below 50% of the pot weight capacity, following the procedures of Sinclair and Ludlow (1986), Lago et al. (2011), Abreu et al. (2015) and King and Purcell (2017). When all seedlings had produced 25 leaves on the main stem, WD was developed, which occurred on 01/27/2013 and 03/05/2013 in E1, and on 08/14/2013 and 10/28/2013 in E2. The first date of each experiment refers to E. urophylla and the second to E. cloeziana.
In order to initiate soil–water treatments, all pots were saturated at 04:30 pm, allowed to drain between 24 and 30 h until they reached a constant weight. The pots were then covered with opaque white low-density plastic wrap to avoid water losses by evaporation, so that only water lost by TR was quantified (Sinclair and Ludlow 1986; Bindi et al. 2005; Guha et al. 2018).
All pots were weighed to determine their initial weight, considered as day 1 of E1 and E2 (Sinclair and Ludlow 1986; Guha et al. 2018). For each species, nine pots were assigned to the WW treatment and nine pots to the WD treatment. The pots were weighed daily at 04:30 pm from day 1 to the last day of the experiment which occurred when WD pots reached 10% TR or lower when compared to WW pots (Sinclair and Ludlow 1986; Esmaeilzade-Moridani et al. 2015).
The difference in pot weight for each weighing interval was assumed to be due to water loss through TR (g pot−1) (King and Purcell 2017). The WW pots were watered by adding as much water as the difference between the weight measured on each day and the initial weight (day 1). The WD pots were not watered until the last day of the experiment because, according to Devi et al. (2009), as soil drought occurs slowly, water addition in the WD pots was not necessary. The final weight (weight measured on the last day of the experiment) was obtained for each pot when the TR of the WD pots was less than 10% when compared to WW pots.
To minimize the influence of day-to-day variations in environmental conditions, daily TR values were normalized as described by King and Purcell (2017), Fuentealba et al. (2016) and Cathey et al. (2013), resulting in normalized transpiration (NTR) values ranging approximately from 0 to 1 (Eq. 6). For the normalization procedure, a correction factor (cf) was calculated to adjust for differences in TR among plants within a species under WW treatment (Eq. 7). The cf was calculated while all plants were well-watered by dividing the average water loss for individual plants by the average water loss of the WW treatment (WWavg) of the same species before starting WD. For each weighing interval from day 1 to the last day of the experiment (TR ≤ 10%), water loss for individual pots was divided by average loss for WW pots of the same species. This ratio (g pot−1/average g pot−1) was divided by the cf to give NTR for each pot for a given weighing interval (King and Purcell 2017):
Total transpirable soil water (TTSW, g pot−1) for an individual pot in the WD treatment was defined as the difference between pot-capacity weight and pot weight at the end of the experiment. The FTSW of each pot was calculated according to Sinclair and Ludlow (1986):
where: AWS is the difference between individual pot weight on each day and pot weight at the end of the experiment (g pot−1).
Values of NTR and FTSW obtained during the drying experiment for each pot on each day were combined to determine the FTSWt value and NTR response. The logistic function (Eq. 8) was fitted, relating NTR to FTSW, for each species in each experiment (E1 and E2). To assess the influence of VPD on NTR values, this procedure was carried out considering three cases: (a) NTR values on all days of the experiment, (b) NTR values on days with low vapor pressure deficits (VPDl), and (c) NTR values on days with high vapor pressure deficits (VPDh). The threshold between VPDh and VPDl was considered as 1.5 kPa according to Ray et al. (2002), Lago et al. (2011), and Abreu et al. (2015). The FTSWt at which the normalized transpiration began to decline in response to soil drying was obtained for the three cases:
where ‘a’, ‘b’, and ‘c’ are the coefficients of the logistic function estimated by the Gauss–Newton algorithm. Coefficient ‘a’ represents the maximum expected FTSW value (considered = 1), ‘b’ is related to the rate of curve decrease and ‘c’ is the intercept.
The fit was evaluated with the following statistics: model efficiency (ME, Eq. 10) and root mean square error (RMSE, Eq. 11):
Eucalyptus responses to progressive soil water deficit
To verify plant responses to progressive water deficits under the WW and WD treatments, physiological and growth variables were evaluated. The physiological variables were: initial, final, and total transpiration (TRi, TRf and TRt, g plant−1), total and partitioned dry weight (root, stem, and leaf, g), dry weight allocation, daily water consumption (WC, g plant−1 day−1), and water requirement (WR, g H2O g−1 dry weight). The WR index was used as it indicates water use efficiency in plants under drought conditions (Sinclair 2012; Fuentealba et al. 2016; Souza et al. 2018b).
Because the dry weight was measured by destructive sampling, three extra pots were included in both experiments (E1 and E2). The dry weight was obtained on the first day of the experiment from these extra pots, and at the end of the experiment from all pots under the WW and WD treatments. Roots, stems and leaf samples were dried to constant weight at 65 °C. The dry weight allocation was obtained by the ratio between root (i), stem (ii), and leaf (iii) dry weights, and the total dry weight. The WR represents the amount of water (in L or g) used to produce a unit of dry mass (kg or g), obtained by the ratio between TRt and the dry weight increment (final dry mass minus initial dry mass obtained on day 1). The WR was calculated for WW and WD.
The growth variables measured were: number of leaves (NL), height (H, cm), root collar diameter (D, mm) and leaf area (LA, cm2). Height was measured with a ruler and diameter with a digital caliper. Leaf area was obtained by the product of leaf width (cm), length (cm) and the form factor (KA). The KA of E. urophylla was 0.68 and of E. cloeziana was 0.67 (Abreu 2014).
All physiological (TRi, TRf, TRt, WC and WR), variables except for dry weight allocation and growth variables (NL, H, D, and LA) were analyzed using a two-way analyses of variance (ANOVA) to compare the mean differences between the two species and two soil–water treatments (WW and WD) at 5% probability. Dry weight allocation was analyzed using a three-way ANOVA to evaluate the effect of two species, two soil–water treatments and three dry weight allocation (root, stem and leaf) at 5% probability. When a significant difference was detected in the ANOVA, the treatment means were compared with Tukey’s test at 5% probability. In the Tukey’s test, the following hypotheses were tested: H0 = mean values are equal (P ≥ 0.05) versus H1 = mean values are unequal (P < 0.05). Before performing the ANOVA, the assumption of normality of the data was checked by the Shapiro–Wilk test at 5% probability.
In addition, the growth variables (NL, H, D and LA) were normalized by the same procedure as was performed for NTR and related, one at a time, to FTSW with a logistic function (Eq. 8) for both species and experiments (E1 and E2). The previously described a, b, and c cases were considered individually. The fit was also assessed with the statistics RMSE and ME.
Results
Growth and physiological variables
E. urophylla and E. cloeziana were maintained WW through 100 and 137 days in E1, and 107 and 182 days in E2, respectively. The WD treatment was then applied, and each species responded slightly differently in relation to progressive soil water deficit, resulting in different durations between experiments. The WD for E. urophylla lasted 15 and 21 days, while for E. cloeziana it was 12 and 17 days, in E1 and E2 respectively.
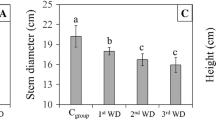
Tables 1 and 2 show, respectively, the responses of the growth and dry weight variables and physiological traits for the WW and WD treatments on day 1 and at the end of the experiments, after the progressive soil water deficits. At the time the soil–water treatments were applied in both experiments, the plants had similar growth, dry weights (Table 1) and TRi (Table 2). This status is desirable as it reduces the differences or errors attributed to WW and WD treatments (Abreu et al. 2015). The effects of soil–water treatments are shown by the final values for leaf area and number, diameters, and heights (Table 1) and TRf, TRt and WC (Table 2). For all these variables, there were significant differences between the WW and WD treatments in both experiments, with lower values in the WD treatment. Leaf area had the greatest reduction under the WD treatment (~ 50% for both species and experiments), followed by, in descending order: root (~ 40%) and leaf (~ 33%) dry weight, diameters (~ 28%), stem dry weight (~ 25%), number of leaves (~ 22%) and height (~ 19%). For the TRf, TRt and WC variables, the reduction was greater than for the growth variables, and ranged from ~ 55% (TRt and WC) to ~ 92% (TRf), with slight differences between species and experiments as shown in Table 2.
A considerable range in TRt and WC was measured between soil–water treatments (Table 2). In WW, the total water transpired throughout the experiments ranged from 1121 to 2215 g plant−1 (E. urophylla) and 907 to 2646 g plant−1 (E. cloeziana). In the WD treatment, TRt water ranged from 519 to 1059 g plant−1. Thus, in WW treatment, the WC ranged from 105 to 53 g plant−1 d−1, while in WD the WC values were around 30 g plant−1 d−1.
Despite the large variability in water requirements between both species in WW, especially for E. cloeziana (Fig. 1), which ranged from 143.98 to 2941.88 g H2O g−1 dry weight, the soil–water treatments did not have a large impact on WR for either species and, except for E. cloeziana in E1, differences in water requirements were not significant (Table 2).
With regards to dry weight allocation (Fig. 2), there was a significant interaction between species, soil–water treatments and dry weight allocation (P < 0.05). In general, E. urophylla and E. cloeziana showed similar dry weight allocation in both experiments and soil–water treatments, prioritizing the dry weight to leaves (except for E. cloeziana in E2 and in the WD treatment), although the percentages differed slightly and higher for E. cloeziana. In E2 and WD treatment, E. cloeziana had greater dry weight allocation to stems compared to roots or leaves. Regarding both species, E. cloeziana had slightly higher dry weight allocation to roots and stems, while E. urophylla had higher allocation to leaves. Therefore, there was no difference in prioritizing dry weight allocation under water deficit conditions, i.e., it is not a conservation strategy under progressive soil water deficit.
Dry weight allocation among root, stem and leaf in soil–water treatments (well-watered -WW and progressive soil water deficit–WD) for Eucalyptus urophylla and Eucalyptus cloeziana in two experiments E1 and E2. Lowercase letters indicate statistically significant differences in soil–water treatments, capital letters indicate statistically significant differences in dry weight allocation, and different numbers indicate statistically significant differences between species; the same letters or numbers do not differ among themselves by Tukey test (5% probability)
Transpiration and growth responses to fraction of transpirable soil water (FTSW)
During the application of the WW and WD treatments, there were differences in meteorological conditions in both greenhouse experiments observed (Fig. 3). Although the meteorological variation was small in terms of average values between E1 and E2, the absolute daily temperatures ranged from 9.9 °C (minimum) to 59 °C (maximum), absolute RH from 52 to 96%, VPD from 0.12 kPa to 19.54 kPa during both experiments.
Daily maximum (Tmax), minimum (Tmin), mean (Tavg) air temperatures, relative humidity (RH) and vapor pressure deficit (VPD) during two greenhouse experiments (E1 and E2) of Eucalyptus urophylla and Eucalyptus cloeziana seedlings; VPD line is the threshold between VPDl (< 1.5 kPa) and VPDh (≥ 1.5 kPa)
The E. urophylla and E. cloeziana species were exposed, respectively, to VPDl (VPDh) conditions on 45% (55%) and 53% (47%) of the days in E1, while in E2, the VPDl (VPDh) conditions occurred on 52% (48%) and 29% (71%) of the days. Therefore, the number of days the species tolerated WD conditions was different between species and experiments. In E1, E. urophylla tolerated water deficits for 15 days and E. cloeziana for 12 days, while in E2, E. urophylla tolerated water deficits for 21 days and E. cloeziana for 17 days.
The logistic function described closely the NTR and the growth responses (NNL, NLA, NH and ND) for FTSW for both species in both experiments, with higher ME values ranging from 0.77 to 0.95 for the NTR, and from 0.32 to 0.90 for the growth variables (Table 3), except in E2 for E. cloeziana (height and diameter). As FTSW decreased, the NTR also varied around the maximum, and after reaching FTSWt, the normalized transpiration started to decrease due to stomatal closure (Schoppach and Sadok 2012; Souza et al. 2014; Abreu et al. 2015; King and Purcell 2017).
In general, E. urophylla showed a large variation in FTSWt values from 0.41 (E1) to 0.89 (E2), while E. cloeziana showed a smaller variation with values from 0.85 (E1) to 0.99 (E2). E. cloeziana also had higher FTSWt values than E. urophylla. At first, this result might indicate greater sensitivity of E. cloeziana to detect soil water deficit and, at the same time, faster stomatal closure to reduce transpiration, even in conditions of available soil water.
Variability was also observed in FTSWt between the species in E1 and E2, especially for normalized height (NH) from 0.42 to 0.85, and normalized leaf area (NLA) from 0.32 to 0.83 (Table 3). In addition, for E. urophylla in E1, the normalized number of leaves (NNL), NH, and normalized diameter (ND) responded to soil water deficit before any change in transpiration rate (FTSWt ≥ 0.75). At the same time, the strategy of E. cloeziana to adjust to soil water deficit was to first reduce TR (FTSWt from 0.85 to 0.99) and NNL (FTSWt from 0.85 to 0.97). However, regarding E. cloeziana in E1 and E2 and E. urophylla in E2, the FTSWt values for NNL, NH (except E. urophylla in E2), ND and NLA (except E. cloeziana in E2) were similar to the FTSWt values for the NTR.
When VPD effect on NTR was considered, slight differences in FTSWt values were observed between both species (Table 3 and Figs. 4 and 5) in the two experiment periods. In general, the FTSWt value was lower on high vapor pressure (VPDh) days, and higher on low vapor pressure (VPDl) days, although this difference was only ~ 0.03 in the FTSWt. For example, considering E. urophylla in E1, the FTSWt was 0.50 (VPDl), 0.32 (VPDh) and 0.41 for all days, and for E. cloeziana in E1, the FTSWt was 0.86 (VPDl), 0.80 (VPDh) and 0.85 for all days. The FTSWt values did not differ for E. cloeziana in E2, with a value of 0.99 for all three tested cases (a, b, and c; see Materials and methods). Despite this, NTR began to decrease earlier or at the same time at higher FTSW values on VPDh days for E. urophylla and E. cloeziana in E2. In other words, the FTSWt from stage I to stage II, which indicates the point when TR is reduced, was anticipated under VPDh conditions.
Normalized transpiration and growth variables responses to fraction of transpirable soil water in Eucalyptus urophylla in two greenhouse experiment periods, E1-Spring/Summer and E2-Fall/Winter considering three cases: all days of the experiment (case a), days with low vapor pressure deficit (VPD < 1.5 kPa) (case b), and days with high vapor pressure deficit (VPD ≥ 1.5 kPa) (case c). FTSWt = threshold values of fraction of transpirable soil water from stage I to II indicates the point when transpiration rate and growth variables are reduced; NTR = normalized transpiration; NNL = normalized number of leaves; NLA = normalized leaf area; NH = normalized height; ND = normalized diameter
Normalized transpiration and growth variable responses to fraction of transpirable soil water in Eucalyptus cloeziana in two greenhouse experiment periods (E1-Spring/Summer and E2-Fall/Winter) considering three cases: all days of the experiment (case a), days with low vapor pressure deficit (VPD < 1.5 kPa) (case b), and days with high vapor pressure deficit (VPD ≥ 1.5 kPa) (case c). FTSWt = threshold values of fraction of transpirable soil water from stage I to II indicates the instant when the transpiration rate, and growth variables are reduced; NTR = normalized transpiration; NNL = normalized number of leaves; NLA = normalized leaf area; NH = normalized height; ND = normalized diameter
With regards to the growth variables, both species had different responses when under VPDl and VPDh (Figs. 4 and 5). The NNL responded earlier in VPDh than VPDl days. The FTSWt values for NLA, NH and ND were similar in VPDh and VPDl days.
Discussion
This study evaluated the transpiration and growth responses of two Eucalyptus species to progressive soil water deficit and verified the influence of daily variations of VPD in these responses. The hypotheses were that physiological plant processes, such as transpiration, are influenced by progressive soil water deficits, that there are genotypic differences in the adaptation of species to drought stress, and that the VPD influences these responses. The transpiration and growth responses (Table 3 and Figs. 4 and 5) showed differences in the FTSWt values and slopes from stage I to II of both species between the two greenhouse experiments (E1 and E2). The response of transpiration, number of leaves, leaf area, height and diameter to FTSW and the influence of VPD on these responses have been studied in several crop species, such as maize (Ray et al. 2002), peanut (Devi et al. 2010), potato (Lago et al. 2012; Souza et al. 2014), cassava (Lago et al. 2011; Pinheiro et al. 2014), cotton (Devi and Reddy 2018), rice (Heinemann et al. 2011), sorghum (Gholipoor et al. 2012) and millet (Esmaeilzade-Moridani et al. 2015; Medina et al. 2017). However, these responses had not been studied in E. urophylla and E. cloeziana species, and this study is the first for these two important commercial species.
The results of the two greenhouse experiments showed that both species reduced transpiration and growth rates under progressive soil water deficit. These results were expected due to reduced soil water availability resulting in lower growth and development, transpiration and dry weight (Souza et al. 2018b; Hubbard et al. 2020). In addition, reductions in heights and diameters are expected in plants under water deficit conditions as observed in this study (Table 1).
The progressive reduction of soil water causes plants to lose their cellular turgidity. With lower turgidity, there is less cell expansion, inhibiting growth and leaf expansion (Shao et al. 2008). When water deficits are prolonged, senescence and leaf abscission take place (Martins et al. 2008; Abreu et al. 2015), resulting in lower photosynthesis and less biomass production (Elli et al. 2020). Furthermore, it has been shown that plants under soil water deficits tend to first reduce their total transpiration and WC in order to maintain plant water content by conserving soil water content early (Sinclair et al. 2017).
There was a difference in normalized transpiration and growth between the two species. E. cloeziana showed higher FTSWt values and started to decrease before the transpiration, number of leaves, height and diameter, and leaf area, compared to E. urophylla. However, both species (except E. urophylla in E1) had higher FTSWt values (≥ 0.84 to NTR) than other species such as Vitis vinifera L. (Bindi et al. 2005) and Thuja plicata Donnex D. Don (0.35), Acer rubrum L. (0.26), Robina pseudoacacia L. (0.37), Hibiscus sp. (0.29), Ilex aquifolium L. (0.33) (Sinclair et al. 2005) and Jatropha curcas L. (0.30 to 0.44) (Ouattara et al. 2018).
Although this study did not determine stomatal index, higher FTSWt values for NTR suggest that the hydraulic signals emitted by leaves cause early stomatal closure (McAdam and Brodribb 2014; Sinclair et al. 2017) to minimize water loss and maintain the plant water status (Souza et al. 2014). In other words, when hydraulic conductance becomes limiting to water transport, the plant is unable to meet transpiration demand, and this results in decreased stomatal conductance. Higher FTSWt values suggest that there is a combination of limited water flux due to soil and plant conductance (early reduction in NTR) to match hydraulic flux (Sinclair 2012; Abreu et al. 2015). Stomatal closure may be the main defense strategy of both Eucalyptus species to progressive (Marrou et al. 2015) and prolonged (Abreu et al. 2015) water deficits.
Since the FTSWt values for normalized transpiration were slightly higher than for the normalized number of leaves (0.64 to 0.89), normalized leaf area (0.30 to 0.86), normalized height (0.31 to 0.84) and normalized diameter (0.63 to 0.88) (except for NLA in E1 and NH in E2, both for E. urophylla) for both species, it was possible to confirm that NTR responded to soil water deficit prior (or close) to the growth variables (NNL, NLA, NH and ND), mainly in VPDh. An early transpiration, number of leaves, leaf area, height, and diameter reduction at higher FTSWt values result in benefits and improvements in water requirement (Souza et al. 2018a), as they aim to maintain soil water (Schoppach and Sadok 2012; Sinclair et al. 2017) and to match the hydraulic flux (Sinclair 2012). These results suggest that both Eucalyptus species have, almost at the same time, stomatal control mechanisms and other strategies such as early leaf senescence and early growth reduction to avoid progressive soil water deficits.
As water requirement is the tradeoff between water use and biomass production, drought tolerant species such as E. urophylla might be expected to use water more conservatively than other species (Martins et al. 2008; Abreu et al. 2015; Hubbard et al. 2020), i.e., with lower WR values. But, this was not the case for these experiments. The hypothesis that water use requirements would be lower for drought tolerant species such as E. urophylla was invalid, similar to the findings of Martins et al. (2008) and Hubbard et al. (2020). Water requirement values were similar between well-watered and water deficit treatments and E. urophylla and E. cloeziana species, demonstrating that both species have similar water use efficiencies under well-watered and water deficit conditions. In addition, water requirements for both species (406 to 1567 g H2O g−1 dry weight) were superior to other eucalypt species such as Eucalyptus grandis (240 to 280 g H2O g−1 dry weight), Eucalyptus saligna (220 to 280 g H2O g−1 dry weight) (Martins et al. 2008), and Corymbia citriodora (100 to 420 g H2O g−1 dry weight) (Abreu et al. 2015).
Our results suggest that E. urophyla and E. cloeziana have a conservative strategy in which plants react to a soil water deficit by: (1) reducing leaf, stem and diameter growth and/or increasing leaf senescence), (2) reducing transpiration rates, TRt, and WC, and (3) by closing stomata when FTSWt is still relatively high under high soil moisture. This is opposite to a productive strategy in which: (1) plants keep growing despite increasing soil water deficits, as observed for example, for E. saligna (Martins et al. 2008) and Corymbia citriodora (Abreu et al. 2015), and in which (2) plants reduce water requirements under water deficit conditions.
Genotypic variability in transpiration within forest species is quite wide: responses of FTSW thresholds range from 0.25 to 0.90 (Sinclair et al. 2005; Martins et al. 2008; Abreu et al. 2015), but in Eucalyptus species, FTSWt ranged from 0.70 to 0.90 (Martins et al. 2008; Abreu et al. 2015). The FTSWt values obtained in this study for NTR declined (from 0.40 to 0.99), similar to what was observed for E. grandis (0.90), E. saligna (0.70) (Martins et al. 2008), and Corymbia citriodora (from 0.32 to 0.85) (Abreu et al. 2015). This demonstrates that early decreases in transpiration during the soil drying cycle promote soil water conservation. In E. urophylla and E. cloeziana, there was first stomatal closure, a first defense strategy under water deficit, followed by disruption of the emission of young leaves, leaf abscission and senescence (FTSWt 0.73 to 0.96), a second defense strategy. Growth (leaf area, height, and diameter) was then reduced (FTSWt 0.31 to 0.86), being the third defense strategy (Shao et al. 2008; Schoppach and Sadok 2012; Kelling et al. 2015).
In this study, the plants were grown in a greenhouse without controlling air temperature and relative humidity. Vapor pressure deficit values were based on daily natural fluctuations, similar that of Casadebaig et al. (2008), Lago et al. (2011, 2012), and Abreu et al. (2015). Despite the weak difference (~ 0.03), the FTSWt value for NTR was slightly higher in VPDl days (0.99 ≥ FTSWt ≥ 0.50 for both species), and slightly lower in VPDh days (0.99 ≥ FTSWt ≥ 0.32) (Table 3 and Figs. 4 and 5), corroborating Ray et al. (2002), Martins et al. (2008) and Lago et al. (2011).
The second hypothesis of this study, in which increased atmospheric vapor pressure deficits may alter the FTSWt to initiate the decline in transpiration and growth was not greatly influenced by VPD. As a rule, it was expected that higher FTSWt values should occur under VDPh conditions, hence with initiated fast regulation of stomata (Ray et al. 2002; Devi and Reddy 2018) and decline in transpiration rate, number of leaves, leaf area, height and diameter variables (Abreu et al. 2015) in wet and dry soils (Devi et al. 2010). In spite of this, an anticipated reduction in transpiration rate in both Eucalyptus species was observed when the evaporative demand was higher (VDPh). This anticipated reduction may occur to match water flux into the leaves (Sinclair et al. 2017) and to conserve soil water (Devi and Reddy 2018). This response is a possible approach for enhanced adaptation to water deficits by Eucalyptus species. However, it is not possible to confirm that VPD changes FTSWt values in both Eucalyptus species.
The responses of FTSW to VPD varied in magnitude and pattern of FTSWt values, which shows genotypic variations. For example, studies carried out by Devi et al. (2010) on peanut genotypes, Lago et al. (2011) for cassava cultivars, Gholipoor et al. (2012) for sorghum genotypes, and Abreu et al. (2015) on Corymbia citriodora, found decreases in FTSWt with increased VPD. However, all these studies showed differences among the genotypes studied. In contrast, Zaman-Allah et al. (2011), Souza et al. (2014) and Devi and Reddy (2020), showed the opposite, i.e., an increase of FTSWt for TR decline under VDPh and a low FTSWt for TR decline under VPDl. Ray et al. (2002), Casadeibag et al. (2008), and Schoppach and Sadok (2012) showed that FTSWt should not be affected by vapor pressure deficit, a similar response to our results. Even so, higher vapor pressure deficit resulted in greater amounts of water transpired regardless of soil–water treatments. At the same time, there was a consistent decrease in dry weight (Fig. 2), with increasing VPD by both Eucalyptus species in well-watered and water deficit treatments.
Although there is no well-defined pattern described in the literature between the FTSWt response under VPDl and VPDh conditions and among genotypes, lower FTSWt values (from 0.30 to 0.60) are suitable under weak to moderate water deficit (short to medium-term water deficit stress) conditions (Abreu et al. 2015). On the other hand, under a prolonged soil water deficit, high FTSWt values (≥ 0.70) (Martins et al. 2008) are expected because a decline in TR at high soil moisture contributes to a conservative water use (Zaman-Allah et al. 2011; Sinclair et al. 2017) and may maintain soil water availability for a longer period, favoring plant survival (Fuentealba et al. 2016; Yan et al. 2017). With species such as E. urophylla and E. cloeziana, which are managed under rotation cycles of 6 to 8 years (Cook et al. 2016; Scolforo et al. 2019), the period in which they may be subjected to soil water deficits may be lengthy, especially in regions such as southeastern Brazil, which has a well-defined dry season (Santos et al. 2017; Martins et al. 2020).
In such regions, higher FTSWt values are desirable and contribute to water conservation in the soil. This conserved water would be useful to plants in the juvenile phase to maturity phase (> 3 years in Eucalyptus species) and therefore, for yield improvement in the dry season. On a daily scale, high FTSWt values may be indicative of rapid stomatal regulation and early closure at critical periods to reduce transpiration, mainly under high vapor pressure deficits (Sinclair 2012; McAdam and Brodribb 2014; Sinclair et al. 2017). Regulation of stomata to vapor pressure deficits is a process by which plants adjust their transpiration during daytime (Devi and Reddy 2018) and according to soil water content (Casadebaig et al. 2008; Sinclair et al. 2017).
In spite of several studies, the mechanisms of stomatal responses to vapor pressure deficits are poorly understood (McAdam and Brodribb 2014) and highly variable among crops. Some studies have suggested limited transpiration rates due to hydraulic limitations (Sinclair 2012; Souza et al. 2014; Abreu et al. 2015; Sinclair et al. 2017), and the involvement of water channel proteins (Sinclair et al. 2017). Stomatal regulation of leaf water balance has been proposed to be controlled by active metabolic processes along with a passive hydraulic process (McAdam and Brodribb 2014). Recent studies observed the expression of abscisic acid (ABA) synthesizing genes in guard cells and regulating stomatal responses to VPD (McAdam and Brodribb 2016; Devi and Reddy 2018). According to Devi and Reddy (2018), these metabolic processes are prompted by low leaf water potential due to ABA (McAdam and Brodribb 2016) and might result in limiting the transpiration rate. For this reason, both Eucalyptus species in this study had a reduction in stomatal conductance due to early stomatal closure at high FTSW for both high and low VPD in order to maintain leaf potential to reduce leaf damage. Moreover, both species showed an early reduction in growth (Table 3 and Figs. 4 and 5).
In summary, both Eucalyptus species showed a conservative strategy to progressive soil water deficits. Water-conserving mechanisms during the seedling phase showed that three traits contributed to water savings under progressive soil drought: (1) higher FTSW thresholds for the decline in transpiration to avoid rapid soil water depletion, (2) higher FTSW thresholds for the decline in NL to reduce the contact area and to avoid the water and water vapor loss to the atmosphere, and (3) higher growth rates (LA, H, and D) when soil moisture was non-limiting and a restriction of growth under progressive soil water deficits (Tables 1 and 3).
Conclusions
The results indicate a range in the response by transpiration and growth variables under progressive soil water deficits for E. urophylla and E. cloeziana. The threshold values of fraction of transpirable soil water ranged from 0.40 to 0.99 for transpiration rate, 0.78 to 0.97 for number of leaves, 0.32 to 0.83 for leaf growth, 0.42 to 0.85 for height growth, and 0.80 to 0.95 for diameter growth, with the highest values for E. cloeziana. The limitation in transpiration rate and growth contributes to soil water savings. There was little to no change in the fraction of transpirable soil water threshold detected in response to changes in atmospheric vapor pressure deficits. These results indicate that the general decline in transpiration rate may be assumed without major concern for the vapor pressure deficits. Nevertheless, future studies need to confirm the limited transpiration in the field under a wide range of vapor pressure deficits.
Abbreviations
- FTSW:
-
Fraction of transpirable soil water
- FTSWt:
-
Threshold fraction of transpirable soil water
- TR:
-
Transpiration rate
- NTR:
-
Normalized transpiration
- NNL:
-
Normalized number of leaves
- NLA:
-
Normalized leaf area
- NH:
-
Normalized height
- ND:
-
Normalized diameter
- WW:
-
Well- watered
- WD:
-
Water deficit
- VPD:
-
Vapor pressure deficit
- VPDh:
-
High vapor pressure deficit
- VPDl:
-
Low vapor pressure deficit
- Tavg :
-
Mean temperature
- Tmax :
-
Maximum temperature
- Tmin :
-
Minimum temperature
- RH:
-
Relative humidity
- esavg :
-
Average saturation water vapor pressure using Tmin and Tmax
- esmin :
-
Saturation water vapor pressure using Tmin
- esmax :
-
Saturation water vapor pressure using Tmax
- eaavg :
-
Average actual water vapor pressure using Tmin and Tmax
- eamin :
-
Actual water vapor pressure using Tmin
- eamax :
-
Actual water vapor pressure using Tmax
- E1:
-
Experiment 1
- E2:
-
Experiment 2
References
Abreu MC (2014) Transpiração e parâmetros de crescimento e desenvolvimento em mudas de espécies florestais submetidas a deficiência hídrica. Dissertation, Universidade Federal de Itajubá
Abreu MC, Martins FB, Freitas CH, Pereira RAA, Melloni EGP (2015) Valores limítrofes para transpiração desenvolvimento e crescimento de Corymbia citriodora (Hook) KD Hill and LAS Johnson em resposta à deficiência hídrica no solo. Rev Árvore 39(5):841–852
Adiredjo AL, Casadebaig P, Langlade N, Lamaze T, Grieu P (2018) Genetic analysis of the transpiration control in sunflower (Helianthus annuus L.) subjected to drought. Vegetos 31(1):1–6
Barros NF, Novais RF (1999) Eucalipto. In: Recomendações para o uso de corretivos e fertilizantes em Minas Gerais: 5ª aproximação. Ribeiro et al. (eds) CFSEMG/ UFV Viçosa, MG, pp 303–305
Bimpong IK, Serraj R, Chin JH, Serraj R, Ramos J (2011) Determination of genetic variability for physiological traits related to drought tolerance in African rice (Oryza glaberrima). J Plant Breed Crop Sci 3(4):60–67
Bindi M, Bellesi S, Orlandini S, Fibbi L, Moriondo M, Sinclair TR (2005) Influence of water deficit stress on leaf area development and transpiration of sangiovese grapevines grown in pots. Am J Enol Vitic 56:68–72
Casadebaig P, Debaeke P, Lecoeur L (2008) Thresholds for leaf expansion and transpiration response to soil water deficit in a range of sunflower genotypes. Eur J Agron 28(4):646–654
Cathey SE, Kruse JK, Sinclair TR, Dukes MD (2013) Transpiration and visual appearance of warm season turf grasses during soil drying. Environ Exp Bot 89:36–43
Cook RL, Binkley D, Stape JL (2016) Eucalyptus plantation effects on soil carbon after 20 years and three rotations in Brazil. For Ecol Manage 359(1):92–98
Devi MJ, Reddy VR (2018) Transpiration response of cotton to vapor pressure deficit and its relationship with stomatal traits. Front Plant Sci 871:1–12
Devi MJ, Reddy VR (2020) Stomatal closure response to soil drying at different vapor pressure deficit conditions in maize. Plant Physiol Biochem 154:714–722
Devi MJ, Sinclair TR, Vadez V (2010) Genotypic variation in peanut for transpiration response to vapor pressure deficit. Crop Sci 50:191–196
Devi MJ, Sinclair TR, Vadez V, Krishnamurthy L (2009) Peanut genotypic variation in transpiration efficiency and decreased transpiration during progressive soil drying. Field Crop Res 114(2):280–285
Elli EF, Sentelhas PC, Bender FD (2020) Impacts and uncertainties of climate change projections on Eucalyptus plantations productivity across Brazil. For Ecol Manage 474(15):118365
Esmaeilzade-Moridani M, Kamkar B, Galeshi S, Ghaderi-Far F, Silva JAT (2015) Leaf expansion and transpiration responses of millet species to soil water deficit. Pedosphere 25(6):834–843
Fuentealba MP, Zhang J, Kenworthy K, Erickson J, Kruse J, Trenholm L (2016) Transpiration responses of warm-season turf grass in relation to progressive soil drying. Sci Hortic 198(26):249–253
Gholipoor M, Sinclair TR, Prasad PVV (2012) Genotypic variation within sorghum for transpiration response to drying soil. Plant Soil 357:35–40
Gonçalves JLM, Alvares CAA, Rocha JHT, Brondani CB, Hakamada R (2017) Eucalypt plantation management in regions with water stress. Southern For 79(3):169–183
Granier A, Biron P, Lemoine D (2000) Water balance transpiration and canopy conductance in two beech stands. Agric for Meteorol 100(4):291–308
Guha A, Chhajed SS, Choudhary S, Sunny R, Jansen S, Barua D (2018) Hydraulic anatomy affects genotypic variation in plant water use and shows differential organ specific plasticity to drought in Sorghum bicolor. Environ Exp Bot 156:25–37
Hainaut P, Remacle T, Decamps C, Lambert R, Sadok W (2016) Higher forage yields under temperate drought explained by lower transpiration rates under increasing evaporative demand. Eur J Agron 72:91–98
Heinemann AB, Stone LF, Fageria NK (2011) Transpiration rate response to water deficit during vegetative and reproductive phases of upland rice cultivars. Sci Agric 68(1):24–30
Hu YB, Xie W, Chen BD (2020) Arbuscular mycorrhizal symbiosis improves drought tolerance of maize seedlings by altering photosystem II efficiency and the levels of key metabolites. Chem Biol Technol Agric 7:20. https://doi.org/10.1186/s40538-020-00186-4
Hubbard RM, Carneiro RL, Campoe O, Alvares CA, Figura MA, Moreira GG (2020) Contrasting water use of two Eucalyptus clones across a precipitation and temperatures gradient in Brazil. For Ecol Manag 475(1):118407
IBÁ (2019). Brazilian Industry of Trees Report 2019. www.https://ibaorg/datafiles/publicacoes/relatorios/iba-relatorioanual2019.pdf. Accessed on 28 May 2020
Irvini J, Perks MP, Magnani F, Grace J (1998) The response of Pinus sylvestris to drought: stomatal control of transpiration and hydraulic conductance. Tree Physiol 18:393–402
Kelling CRS, Reichardt K, Streck NA, Lago I, Zanon AJ, Rodrigues MA (2015) Transpiração e crescimento foliar de crisântemo em função da fração de água transpirável no substrato. Pesqui Agropec Bras 50(9):735–744
King CA, Purcell LC (2017) Evaluation of methods for estimating transpiration response to soil drying for container-grown plants. Crop Sci 57:2143–2148
Lagergren F, Lindroth A (2002) Transpiration response to soil moisture in pine and spruce trees in Sweden. Agric for Meteorol 112(2):67–85
Lago I, Streck NA, Bisognin DA, Souza AT, Silva MR (2011) Transpiração e crescimento foliar de plantas de mandioca em resposta ao deficit hídrico no solo. Pesq Agropec Bras 46(11):1415–1423
Lago I, Streck NA, Zanon AJ, Hanauer JG, Bisognin DA, Silva MR (2012) Transpiração e crescimento foliar de clones de batata em resposta à fração de água transpirável no solo. Rev Bras Cienc Solo 36(3):745–754
Llopart M, Reboita MS, Rocha RP (2020) Assessment of multi-model climate projections of water resources over South America CORDEX domain. Clim Dyn 54(6):99–116
Marrou H, Vadez V, Sinclair TR (2015) Plant survival of drought during Establishment: an interspecific comparison of five grain legumes. Crop Sci 55(3):1264–1273. https://doi.org/10.2135/cropsci2014.11.0760
Martins FB, Pereira RA, Torres RR, Santos DF (2020) Climate projections of chill hours and implications for olive cultivation in Minas Gerais, Brazil. Pesqui Agropec Bras 55:e01852
Martins FB, Streck NA, Da Silva JC, Morais WW, Susin F, Navroski MC, Vivian MA (2008) Deficiência hídrica no solo e seu efeito sobre transpiração crescimento e desenvolvimento de mudas de duas espécies de eucalipto. Rev Bras Cienc Solo 32(3):1297–1306
McAdam SAM, Brodribb TJ (2014) Separating active and passive influences on stomatal control of transpiration. Plant Physiol 164:1578–1586
McAdam SAM, Brodribb TJ (2016) Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiol 171:2008–2016
Medina S, Gupta SK, Vadez V (2017) Transpiration response and growth in pearl millet parental lines and hybrids bred for contrasting rainfall environments. Front Plant Sci 8:1–16
Medina S, Vicente R, Nieto-Taladriz MT, Aparicio N, Chairi F, Vergara-Diaz O, Araus JL (2019) The plant-transpiration response to vapor pressure deficit (VPD) in durum wheat is associated with differential yield performance and specific expression of genes involved in primary metabolism and water transport. Front Plant Sci 9:1–19
Ngugi MR, Doley D, Hunt MA, Ryan P, Dart P (2004) Physiological responses to water stress in Eucalyptus cloeziana and E. argophloia seedlings. Trees 18:381–389
Ouattara B, Diédhiou I, Belko N, Cissé N (2018) Growth and transpiration of Jatropha curcas L. seedlings under natural atmospheric vapor pressure deficit and progressive soil drying in semi-arid climate. Agric Sci 9(6):639–654
Pang J, Turner NC, Khan T, Du YL, Xiong JL, Colmer TD, Devilla R, Stefanova K, Siddique KHM (2017) Response of chickpea (Cicer arietinum L.) to terminal drought: Leaf stomatal conductance pod abscisic acid concentration and seed set. J Exp Bot 68(8):1973–1985
Pinheiro DG, Streck NA, Richter GL, Langner JA, Winck JEM, Uhlmann LO, Zanon AJ (2014) Limite crítico de água no solo para transpiração e crescimento foliar em mandioca em dois períodos com deficiência hídrica. Rev Bras Cienc Solo 38(6):1740–1749
Ray JD, Gesch RW, Sinclair TR, Hartwell AL (2002) The effect of vapor pressure deficit on maize transpiration response to a drying soil. Plant Soil 239:113–121
Reis FY, Martins FB, Torres RR, Florêncio GWL, Cassemiro JM, Monteiro VFC, Ferreira MC (2021) Climate change impact on the initial development of tropical forest species: a multi-model assessment. Theor Appl Climatol 145:533–547
Santos DF, Martins FB, Torres RR (2017) Impacts of climate projections on water balance and implications on olive crop in Minas Gerais. Rev Bras Eng Agric e Ambient 21(2):77–82
Schoppach R, Sadok W (2012) Differential sensitivities of transpiration to evaporative demand and soil water deficit among wheat elite cultivars indicate different strategies for drought tolerance. Environ Exp Bot 84:1–10
Scolforo HF, McTague JP, Burkhart H, Roise JP, Alvares CA, Stape JL (2019) Modeling whole-stand survival in clonal eucalypt stands in Brazil as a function of water availability. For Ecol Manag 432:1002–1012
Shao HB, Chu LY, Jaleel CA, Zhao CX (2008) Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol 331(3):215–225
Sinclair TR (2012) Is transpiration efficiency a viable plant trait in breeding for crop improvement? Funct Plant Biol 39(5):339–365
Sinclair TR, Devi J, Shekoofa A, Choudhary S, Sadok W, Vadez V, Riar M, Rufty T (2017) Limited-transpiration response to high vapor pressure deficit in crop species. Plant Sci 260:109–118
Sinclair TR, Holbrook NM, Zwieniecki MA (2005) Daily transpiration rates of woody species on drying soil. Tree Physiol 25(11):1469–1472
Sinclair TR, Ludlow MM (1986) Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust J Plant Physiol 13(3):329–341
Souza AF, Oliveira E, Junior R (2018a) Desenvolvimento inicial e eficiência de uso de água e nitrogênio por mudas de Calophyllum brasiliense, Eucalyptus urograndis, Tabebuia impetiginosa e Toona ciliata. Cienc Florestal 28(4):1465–1477
Souza AT, Streck NA, Heldwein AB, Bisognin DA, Winck JEM, Rocha TSM, Zanon AJ (2014) Transpiration and leaf growth of potato clones in response to soil water deficit. Sci Agric 71(2):96–104
Souza PU, Lima LKS, Soares TL, Jesus ON, Coelho Filho MA, Girardi EA (2018b) Biometric physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci Hortic 229:77–90
Yan F, Li X, Liu F (2017) ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ Exp Bot 139:99–104
Zaman-Allah M, Jenkinson DM, Vadez V (2011) Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Funct Plant Biol 38(4):270–281
Acknowledgements
The first author thank the Coordination for the Improvement of Higher Education Personnel (Capes, process No. 1115820/1090909) for the scholarship granted and the fourth author thank the Minas Gerais Research Founding (FAPEMIG) for financially supporting the projects APQ-01392-13 and APQ 01258-17.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was supported by Minas Gerais Research Founding (FAPEMIG – projects APQ-01392–13 and APQ 01,258–17).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yanbo Hu.
Rights and permissions
About this article
Cite this article
Abreu, M.C., Soares, A.A.V., de Freitas, C.H. et al. Transpiration and growth responses by Eucalyptus species to progressive soil drying. J. For. Res. 33, 1529–1543 (2022). https://doi.org/10.1007/s11676-021-01448-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01448-z