Abstract
Drought is considered the main environmental factor limiting productivity in eucalyptus plantations in Brazil. However, recent studies have reported that exposure to water deficit conditions enables plants to respond to subsequent stresses. Thus, this study investigates the ecophysiological acclimatization of eucalyptus clones submitted to recurrent water deficit cycles. Eucalyptus seedlings were submitted to three recurrent water deficit cycles and anatomical, morphological and physiological changes were analyzed. The results were: (1) Eucalyptus seedlings responded to water deficits by directing carbohydrates to root and stem growth; (2) Size and number of stomata were reduced; (3) Stomatal conductance decreased which allowed the plants to reduce water losses through transpiration, increasing instantaneous water use efficiency; (4) The relationship between gas exchanges and available water contents allowed the seedlings to uptake the retained soil water at higher tensions; and, (5) Physiological recovery from subsequent water deficits became faster. As a result of these changes, the eucalyptus seedlings recovered from the same degree of water stress more rapidly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Planted forests play a key role in supplying environmental and economic services worldwide although they represent only 7% of the global forest area (Villar et al. 2011). The genus Eucalyptus provides a major source for plantations in Brazil, occupying 5.63 million hectares, equivalent to 72.2% of the total area planted with trees (IBÁ 2016). Eucalyptus are planted throughout Brazil, exposing the numerous species to a range of abiotic stresses. Among these, drought is considered the main factor limiting productivity (Correia et al. 2014; Zhou et al. 2014).
Plant responses to drought are complex and involve diverse biochemical and physiological processes. A reduction in stomatal and/or mesophyll conductance is cited by several authors as being the main cause of reduced photosynthesis under drought stress (Grassi and Magnani 2005; Warren et al. 2011; Cano et al. 2014). The inhibition of ribulose-1,5-bisphosphate-carboxylase-oxygenase (Rubisco) activity (Tezara et al. 2002), and a reduced capacity for ribulose-1,5-bisphosphate (RuBP) regeneration (Thimmanaik et al. 2002) also play an important role in restricting photosynthesis. With the Eucalyptus genus, responses to low water availability include increases in photorespiration (Thumma et al. 2012), and changes in biomass allocation and cell wall reinforcement (Ladiges 1975). However, exposure of plants to recurring stress allows them to respond and adapt to these conditions, making them more resistant to future stresses (Molinier et al. 2006; Asensi-Fabado et al. 2013).
Some researchers have reported that exposure to drought stress conditions enables plants to respond to a new stress with more rapid morpho-physiological changes contributing to a higher drought stress tolerance (Bruce et al. 2007; Ding et al. 2012; Kinoshita and Seki 2014; Iwasaki et al. 2014). Plants under water deficit or stress display morpho-physiological changes such as: (1) increasing cell wall elasticity which contributes to the maintenance of cell turgor (De Diego et al. 2013); (2) inducing leaf rolling, limiting water loss by restricting the leaf transpiration surface (Hura et al. 2012; Peguero-Pina et al. 2016); and, (3) increasing cellulose synthesis by maintaining cell wall integrity and cell turgor pressure, allowing continuous cell growth under low water potential (Ricardi et al. 2014; Le Gall et al. 2015). However, research investigating the mechanisms related to adaptation and acclimatization are still scarce, leaving this poorly understood, especially its ecophysiological aspects.
The hypothesis of this study is that the exposure of eucalyptus seedlings to water deficits will fine-tune their response to future drought stress. Therefore, the study investigates the ecophysiological acclimatization of eucalyptus clones subjected to recurrent water deficit cycles.
Materials and methods
Plant material and experimental conditions
This study was carried out under greenhouse conditions of controlled temperature and humidity at the Laboratory of Meteorology and Forest Ecophysiology, Federal University of Espírito Santo.
Seedlings of the eucalyptus clone AEC 144 (Eucalyptus grandis x Eucalyptus urophylla), the most widely used clone in plantations in Brazil, were produced in 50 cm3 tubes with a substrate of biostabilized pine bark, vermiculite, milled charcoal and phenolic foam. This substrate is commonly used in the production of seedlings using cuttings. Within 80 days, the seedlings were planted into 50 L pots. Fertilization was carried out according to recommendations of Prezotti et al. (2007).
The seedlings were separated into four treatments, three subjected to water deficit cycles and one was a well-watered control:
First water deficit cycle: Only the seedlings of the 3rd water deficit group (3rd WD) were subjected to water deficit. The 1st WD, 2nd WD and control group (Cgroup) were maintained with soil moisture at field capacity. This cycle started 30 days after planting the seedlings;
Second water deficit cycle: The 2nd WD and 3rd WD groups were subjected to water deficit. The 1st WD group and the control (Cgroup) were maintained with soil moisture at field capacity. This cycle started 74 days after planting;
Third water deficit cycle: The 1st WD, 2nd WD and 3rd WD groups were subjected to water deficits. Only the plants in the Cgroup were maintained with soil moisture at field capacity. This cycle started 109 days after planting the seedlings.
The water deficit cycles started at the same time in all treatment groups. In this way, when the third water deficit cycle started, the 3rd WD, 2nd WD, and 1st WD groups were being subjected to their third, second and first water deficit, respectively. The control group was not subjected to any water deficit cycle. Physiological analyses were performed during the third water deficit cycle, and morphological analyses were carried out after the seedlings had recovered from the third water deficit.
For the purpose of this study, a water deficit cycle was defined as: “the suspension of irrigation until the photosynthetic rate of the seedlings reached zero (0 μmol CO2 m−2 s−1)”. Irrigation (Watering) resumed immediately after zero photosynthesis was recorded.
Microclimatic characterization
An automatic meteorological station was installed for microclimatic characterization of the environment within the greenhouse, and consisted of two temperature and relative humidity sensors, model CS500 (Campbell Scientific, Inc., Logan, UT, USA). The data was stored in a data logger, model CR1000 (Campbell Scientific, Inc., Logan, UT, USA), scanning every 10 s and storing the average values every minute. Vapor pressure deficit (VPD) data was obtained by calculating the difference between the saturation values and the actual vapor pressure.
Monitoring of water stress cycles
This was performed by weighing the pots to determine field capacity (FD = 49%), wilting point (WP = 19%) and bulk density (ρ = 0.43 g·cm−3) according to Donagema et al. (2011). Using these values, the available water capacity (AWC) was determined using the following equation:
The AWC was transformed to AWC in liters by multiplying AWC (mm) by the pot area (m2). The pots containing the plants were sealed with a 10-mm thick Styrofoam sheet, ensuring that only transpiration occurred with no evaporation from the pots. The ratio of the transpired fraction (weighed daily at 6:00 am) to the AWC multiplied by 100 produced the AWC (%).
Gas exchange measurements were performed every day at 8:00 am during the third water deficit cycle to determine the times when the plants should be watered, and a new cycle should begin. The time of 8:00 am was used because it is the moment of greatest photosynthetic potential under the greenhouse conditions employed, since the reduction of photosynthetic capacity throughout the day is a common phenomenon across species (Kosugi et al. 2009; Kamakura et al. 2012). The measurements were carried out on 10 plants, measuring two leaves per plant, and considering each plant as one replicate.
After watering, the seedlings remained under irrigated conditions until a photosynthetic rate of 100% of the Cgroup was achieved. To ensure the total restoration of photosynthesis after the 100% rate was reached, irrigation was maintained for a further 10 days in plants that had undergone water stress. Irrigation was then suspended, and the next water deficit cycle begun. Thus, as the cycles were synchronized at the same time, the beginning of the water deficit cycle was 10 days after the full recovery of the last group.
Gas exchange analysis
Gas exchange evaluations were carried out in two phases: (1) To monitor water stress cycles; and, (2) To identify daily patterns of photosynthesis variations in each group throughout the day on the 1st, 5th, 9th and 13th days from the beginning of the third water deficit cycle. With these analyses, net photosynthetic rate (A, µmol CO2 m−2 s−1), transpiration rate (E, mmol H2O m−2 s−1), stomatal conductance (gs, µmol H2O m−2 s−1), and intercellular CO2 concentration (Ci, µmol CO2 mol−1) were measured. The measurements were made using a portable infrared gas analyzer (IRGA, model Li COR 6400, LI-COR Inc., Lincoln, NE, USA), at a light intensity of 1500 μmol m−2 s−1 (Evans et al. 1993), and a CO2 concentration of 400 µL L−1. Evaluations were done every four hours between 8:00 am and 4:00 pm in fully expanded leaves in the upper third of the plant, using 10 replications per treatment. Each seedling was considered as one replicate, and measurements were recorded for two different leaves on each plant. The instantaneous carboxylation efficiency (EiC) was established through the relationship between the net photosynthetic rate and the intercellular CO2 concentration (A/Ci), and the instantaneous water use efficiency (iWUE) was established through the relationship between net photosynthetic rate and transpiration rate (A/E).
Relative water content (RWC)
The RWC of leaves was determined on the 1st, 5th, 9th and 13th days from the beginning of the third water deficit cycle. Approximately 15-mm circular discs were cut from leaves of eight seedlings in each treatment. The discs were collected at 5:00 am before sunrise to determine fresh weight (FW). Three leaves per plant were used for analysis and each plant was considered as one replicate. After collection, the discs were placed in the dark for 24 h in Petri dishes containing distilled water to determine the turgid weight (TW). For dry weight (DW) determination, samples were oven-dried at 60 °C until a constant weight was reached. The RWC was calculated according to the following:
Fifteen leaf discs with diameters of 15 mm were cut per plant. Each set of 15 discs was considered as one repetition, comprising a total of eight replicates.
Dry weight, diameter and height determinations
After the recovery of the seedlings from the final water deficit cycle, dry mass (DM) was obtained after drying leaves, stems and roots at 75 °C until a constant weight was achieved. Heights were measured with a ruler and diameters with a digital caliper.
Stomata analysis
Anatomical analyses were carried out at the same time as the DM measurements. The printing method was applied which consists of pressing the leaf surface into a layer of adhesive glue. When the glue solidifies on the blade, it retains an image of the pressed surface. A total of 10 blades were prepared for each treatment using the abaxial layers of leaves from the upper third of the plants. One leaf per plant was sampled, being a new leaf grown after the final water deficit cycle. Digital 1-mm2 images of the prints were captured using a digital camera (model AxioCam MRc5, Carl Zeiss Microscopy, Oberkochen, Germany) coupled to a light microscope (model Axio Scope. A1, Carl Zeiss Microscopy, Oberkochen, Germany), with a 50 X objective lens. Four counts and four measurements of stomatal width and length were made for each blade, totaling 40 counts per treatment. Each blade was considered one repetition.
Statistical analysis
A completely random design was used with four treatments (Cgroup, 1st WD, 2nd WD and 3rd WD) and ten replications per treatment, each represented by one seedling. Statistical analyses were carried out using the software R (R Core Team 2017). To analyze the response of each variable to the environments studied, the data were submitted to an analysis of variance (ANOVA) and, when a significant (F-test p < 0.05), the mean values were compared using the Tukey test (p < 0.05). Subsequently, in order to obtain an integrated assessment of cyclic water stress in eucalyptus, the data were subjected to a multivariate analysis of variance (MANOVA) and to canonical discriminant analysis (CDA) using the Candisc package (Friendly and Sigal 2014). From the CDA data, a biplot graphic was generated (Fig. 6) to evaluate the multivariate differences between the treatments and to hierarchize the contribution (weight) of each variable on physiological responses after subjection to cyclic water stress. The center of the circles on the graph represents the mean value of the first two canonical variables. When the ellipses overlap, the treatments are not statistically different. The size of the arrow represents the importance of each variable in the distinction of the treatments, and its direction indicates the positive or negative effect of the variable on the treatments. Further explanation and examples of CDA can be found in Gittins (1985). Considering that EiC results are very collinear to A and Ci, they were not included in the CDA analysis. The variable stomata, used in CDA analysis, was obtained by the multiplication of the variable sizes and widths of the stomata, and the variable shoot/root ratios were obtained from the relationship between the sum of the stem and leave dry mass and root dry mass.
Results
Microclimate conditions during the cyclic water stress
Mean air temperature (Tmean) inside the greenhouse was maintained at 30 °C ± 3 °C throughout the experimental period. Mean vapor pressure deficit (VPD) was 2 kPa ± 0.5 kPa. There were small variations in Tmean and VPD during the water deficit cycles (Table 1).
Table 1 also shows the time required for each treatment to reach zero photosynthesis. Seedlings of the 3rd WD group, when exposed to the first water deficit cycle, took 29 days to reach zero photosynthesis, whereas plants of the 1st WD group when exposed to the first cycle (i.e. in the 3rd water deficit cycle) took 6 days. It is important to note that all plants were planted on the same day and exposed to water deficit cycles in a synchronized way, so plants of the 1st WD group when exposed to the first cycle had a much larger leaf area than the plants of the 3rd WD when exposed to its respective first cycle. Thus, the time required to achieve zero photosynthesis has been reduced over time, mainly due to plant growth.
Plant dry weight, diameter and height
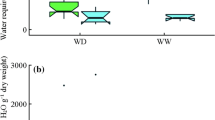
The water deficit cycles affected plant growth. The dry mass of the plant groups 1st WD, 2nd WD and 3rd WD were 40%, 48% and 57% lower than the controls, respectively (Fig. 1). Similar results were found for stem diameters and heights and show that seedlings that experienced a greater number of water deficit periods showed greater reductions in dry mass, diameters and heights (photos of the results are presented in Fig. S2 at the supplementary material).
The dry mass proportion (DM %) is an indicator of how the eucalyptus seedlings acclimatize to a water deficit (Fig. 2). The DM % was different in each water deficit cycle. In the Cgroup, leaves made up a large proportion of dry mass. The situation changed in the 1st WD group where the leaves were a smaller proportion of the dry mass, mainly due to leaf shedding during drought. However, in the 2nd WD and 3rd WD groups, there was almost no leaf abscission after the first water deficit cycle, and it was noted, that there was carbon allocated to the roots and stems as a form of acclimatization to the water deficit.
Stomata
Stomatal number, length and width were other morphological changes observed in eucalyptus under water deficits (Fig. 3). The number of stomata was reduced in the 2nd WD group. In the 3rd WD group, there was also a reduction in length and width.
Gas exchange during the period of water deficit and rewatering
Measurements of leaf gas exchange are fundamental to understanding eucalyptus adaptation to water stress. In this study, plants in the 1st WD group showed a rapid fall in A, and by the 5th day, their assimilation rate was completely impaired (Fig. 4). This result is a consequence of the high rates of E since the 1st day, which caused a rapid reduction of available water and RWC (Fig. S1). On other hand, plants in the 2nd WD and 3rd WD groups showed a reduction of gs from the 1st day of water deficit and this, together with the lowest proportion of leaves, allowed the plants to reduce their E rates, and as a consequence, maintain high RWC and A rates longer.
Diurnal variation of net photosynthesis (A), stomatal conductance (gs), transpiration rates (E) and intercellular CO2 concentration (Ci) for the eucalyptus clone AEC 144, under three water deficit cycles, on the 1st, 5th, 9th, and 13th days of experimentation. Data are mean ± Standard Error. NS: no significance, *: significant (ANOVA, p < 0.05). n = 10. Whole lines represent plants under water deficit and dashed lines indicate plants in recovery period
On the 9th day, the A in the 3rd WD group was higher throughout the day than in the 2nd WD group. With the 8:00 am measurements, this can be explained by the gs, which was higher at 8:00 am in the 3rd WD plants, resulting in a greater Ci value which represents a greater amount of substrate (CO2) for carrying out photosynthesis. However, from 12:00 am, the gs was the same in both treatments. Thus the highest A rates in the 3rd WD plants were due to their higher RWC (see supplementary data).
The recovery of physiological processes after rewatering is another important difference among the treatments. 3 days after rewatering, the 1st WD group (9th day) had not fully recovered their photosynthetic potential. On the other hand, one and 2 days after rewatering (13th day), the 3rd WD and 2nd WD groups, respectively, had A and RWC values similar to their respective well-irrigated controls.
Available water content and its impact on gas exchange
Monitoring of soil moisture verified that seedlings in the 2nd WD and 3rd WD groups could utilize soil moisture even under low water availability. To make this physiological acclimatization more explicit, the relationship between A, gs, E and EiC and the available water content (AWC) was determined (Fig. 5). The 1st WD group showed null values of A, gs, E and EiC (0 µmol m−2 s−1) when the AWC was 40%. On other hand, plants in the 2nd WD and 3rd WD groups reached the same values when the AWC was 20% and 15%, respectively. Figure 6 clearly shows that acclimatized plants better control gs and E, which makes the carboxylation process more efficient, so that the plant becomes able to assimilate the same amount of CO2 while losing less water.
The relationship between photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs) and instantaneous carboxylation efficiency (EiC) with available water content (AWC) for the eucalyptus clone AEC 144 under three water deficit cycles. The polygons correspond to an area of the confidence interval 95%. Statistical analyzes are presented in Table S1 of the supplementary data
Cyclic water stress impacts on eucalyptus seedlings: a multivariate approach
The multivariate approach allowed an integrated assessment of cyclic water stress on the eucalyptus seedlings (Fig. 6). CDA showed that the first two canonical variables (CV) were responsible in explaining 98.2% of the data variance induced by cyclic water stress. This resulted in a reduction of A, Ci, gs and E, as well as the size of the stomata and in the shoot/root ratio. Furthermore, it can be seen that the iWUE increased from the 2nd WD plants. The variables A, Ci, gs and E were more important to distinguish the treatment in the CV1, while the relation shoot/root and iWUE, respectively, were the most relevant variables in the CV2.
All of the adaptations by the eucalyptus seedlings contributed to their ability to withstand water deficits. Because of these adaptations, the duration of the third water deficit cycle varied among the treatments. The 1st WD group reached zero photosynthesis 6 days after the beginning of the third water deficit, while the 2nd WD and 3rd WD groups stopped photosynthesizing 11 and 12 days, respectively, after the beginning of the third water deficit, showing their acquired resistance to periods of drought.
Discussion
Eucalyptus seedlings became increasingly adapted to water deficits in each drought period. This was due to progressive morphological and physiological acclimatization that occurred in the plants. They responded to drought by apportioning biomass differentially to different organs, but did not redistribute existing biomass (Reynolds and Thornley 1982). Our results also show that the seedlings responded to water deficits by directing carbohydrates to root and stem growth, which is in agreement with results of Maseda and Fernández (2015). This change in allocation, promoted by water stress, is a known acclimation response for eucalyptus (Costa et al. 2004).
When water becomes limiting, leaf expansion is reduced before photosynthesis is affected. This reduces carbon and energy consumption and a higher proportion of plant assimilates can be allocated to root and stem growth (Taiz and Zeiger 2013). With the best developed roots and stems, the seedlings tended to optimize water absorption and then to maximize the growth rate under these conditions (Bloom et al. 1985). However, a reduction in leaf dry mass can also have a negative impact on carbon gain in eucalyptus (Fig. 1), damaging the growth volume rate which can lead to an increase in production costs.
The adaptation to water deficits not only involved changes in the dry mass proportions contributed by different organs but also in anatomical and morphological changes. In general, the transpiration rate may be considered to be directly proportional to stomatal size and density, and so to better control transpiration rates, the seedlings reduced the size and number of their stomata (Fig. 3), consistent with previous studies by Xu and Zhou (2008) and Mansouri and Radhouane (2015). Plants with less than half the normal number of stomata conserve moisture and are highly tolerant to drought (Hepworth et al. 2015). Another important aspect is stomatal conductance since this plays a key role in gas exchange between vegetation and the atmospheric interface (Xu et al. 2010).
The reduction of gs or stomatal conductance, which causes a reduction in Ci (intercellular CO2 concentration), is suggested to be the main limiting factor of photosynthesis under water deficits (Bota et al. 2004; Cano et al. 2014). In this study, after undergoing water deficit cycles, the plants had a lower gs, and initially, this did not affect A (net photosynthesis). Thus, with a lower gs, the plants reduce their losses of water to the atmosphere, which guarantees their survival for longer periods under a water deficit. With the intensification of the water deficit, there was a decrease in A, E and RWC and an increase in Ci, (which occurred at different times for each treatment group), which suggests that the photosynthetic machinery was impaired by a water deficit. Some studies have suggested that the deactivation of Rubisco under water stress (Flexas et al. 2006; Cano et al. 2014), and the reduced capacity for ribulose-1,5-bisphosphate (RuBP) regeneration (Thimmanaik et al. 2002) are the main explanation to high Ci values.
Focusing on the relationship between gas exchange and available water content, it may be inferred that exposure to water deficits enables the seedlings to uptake retained water at higher tensions. This capacity may be a function of a change in root water potential (ψw). The adjustment of ψw, either through the absorption of ions from the soil or the translocation of solutes from other organs to the roots, may be responsible for this change in water uptake ability (Steudle 2000a, b; Merchant et al. 2010), which is also documented for eucalyptus (Christina et al. 2015). However, changes in root morphology, structure and anatomy (Kadam et al. 2015; Maseda and Fernández 2015), changes in the transport pattern or mechanism of water flow (Costa et al. 2004), and changes in the activity of water channels (Steudle 2000b) may also be responsible for changed water uptake capacity of the roots.
The cyclic water deficit also influenced the rewatering period. The A (net photosynthesis) and RWC (relative water content) values showed a prompt recovery in all treatments and as the plants were subjected to subsequent water deficits, the recovery period became faster. These results corroborate those obtained by Correia et al. (2014), and show that the ability to recover after a water deficit period increases after passing through previous periods of water deficit. The recovery of A was shown to be different to both gs and E (transpiration rates), in agreement with the results of Galmés et al. (2007). Plants take a longer time to recover gs and E; however, they can quickly recover rates of A. Galmés et al. (2007) hypothesized that, after a severe water deficit, plants can make better use of intercellular CO2 (Ci), making the carboxylation process more efficient and thus allowing the plant to maintain high rates of A.
Several studies have demonstrated that moderate stomatal closure is an extraordinary adaptation to various environmental stresses in plants (Keenan et al. 2013; Li et al. 2014; Adams et al. 2016). When plants encounter drought stress, stomatal conductance usually decreases earlier than photosynthesis, leading to an increase of instantaneous water use efficiency (iWUE) in plants (Condon et al. 2002; Chaves and Oliveira 2004). Our studies show that, after cyclic water stress, the iWUE in eucalyptus seedlings became higher, even if the plant was not under water deficit (Fig. S1), thus contributing to the plant having water available longer.
The canonical discriminant analysis allows an overview of the importance of each morpho-physiological variable in the treatments differentiation (Fig. 6). After they pass through the first water deficit cycle, the main changes occur in the relationship between shoot and root. Therefore, the first changes are related to the reduction of the transpiration area, which are also found for several others species (Baldocchi and Xu 2007; Peguero-Pina et al. 2014). A positive aspect of the transpiration area reduction is the decrease in aerodynamic resistance of leaves. This leads to a better coupling between leaf temperatures and air temperatures (Peguero-Pina et al. 2016), enhancing the control of transpiration by stomata (Jarvis and McNaughton 1986). From the second water deficit cycle, the plants stomatal conductance controls water loss and increases iWUE. This stomatal response may be mediated by non-hydraulic signals (such as abscisic acid) (Davies and Zhang 1991), leading to the maintenance of a relatively stable leaf water potential (Amdt et al. 2001). Thus, even if the plant is not under water stress, it has better control of the transpiration process, allowing water consumption to decrease, which ensures that the plant has water available longer.
According to White et al. (2009), management practices help to mitigate the consequences of water deficits in planted forests. The results in this study might be a first indication that a good way to mitigate the consequences of water stress in eucalyptus would be to subject the seedlings, prior to planting, to moderate water deficits in order to develop mechanisms to survive subsequent drought stress. However, we believe that more studies on the subject are needed to extrapolate these findings to field conditions where different soil and climatic conditions exist. Besides, our studies indicate that there is a loss of biomass accumulation during water deficit cycles which can cause plants to take longer to reach the ideal harvest volume or to be ready for planting in the case of nurseries, causing an increase in production costs. On the other hand, thinking only of gain tolerance to drought, these results are directly applicable to nurseries responsible for the production of eucalyptus seedlings and that use controlled atmospheric conditions and similar substrates as in this work.
The results of this study support the hypothesis that subjecting eucalyptus seedlings to water deficit changes their ecophysiological aspects and makes them more resistant to subsequent water deficits. These changes allow the plants to survive longer periods of water deficit. Acclimatization by the exposure of eucalyptus to water deficit, during initial growth, can contribute to successful plantation establishment, reduce seedling mortality and production and maintenance costs and thus making forest plantations sustainable and profitable.
References
Adams MA, Turnbull TL, Sprent JI, Buchmann N (2016) Legumes are different: leaf nitrogen, photosynthesis, and water use efficiency. Proc Natl Acad Sci 113:4098–4103
Amdt S, Cliford S, Wanek W, Jones H, Popp M (2001) Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progresif drought stress. Tree Physiol 21:705–715
Asensi-Fabado MA, Oliván A, Munné-Bosch S (2013) A comparative study of the hormonal response to high temperatures and stress reiteration in three Labiatae species. Environ Exp Bot 94:57–65
Baldocchi DD, Xu L (2007) What limits evaporation from Mediterranean oak woodlands—The supply of moisture in the soil, physiological control by plants or the demand by the atmosphere? Adv Water Resour 30:2113–2122
Bloom AJ, Chapin SF, Mooney HA (1985) Plants-an economic analogy. Annu Rev Ecol 16:363–392
Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Cano FJ, López R, Warren CR (2014) Implications of the mesophyll conductance to CO2 for photosynthesis and water-use efficiency during long-term water stress and recovery in two contrasting eucalyptus species. Plant, Cell Environ 37:2470–2490
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Christina M, Le Maire G, Battie-Laclau P, Nouvellon Y, Bouillet J, Jourdan C, de Moraes Gonçalves J, Laclau J (2015) Measured and modeled interactive effects of potassium deficiency and water deficit on gross primary productivity and light-use efficiency in eucalyptus grandis plantations. Glob Chang Biol 21:2022–2039
Condon AG, Richards R, Rebetzke G, Farquhar G (2002) Improving intrinsic water use efficiency and crop yield. Crop Sci 42:122–131
Correia B, Pintó-Marijuan M, Neves L, Brossa R, Dias M, Costa A, Castro B, Araújo C, Santos C, Chaves M, Pinto G (2014) Water stress and recovery in the performance of two Eucalyptus globulus clones: physiological and biochemical profiles. Physiol Plant 150:580–592
Costa F, Shvaleva A, Maroco JP, Almeida M, Chaves M, Pereira J (2004) Responses to water stress in two Eucalyptus globulus clones differing in drought tolerance. Tree Physiol 24:1165–1172
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
De Diego N, Sampedro MC, Barrio RJ, Saiz-Fernández I, Moncaleán P, Lacuesta M (2013) Solute accumulation and elastic modulus changes in six radiata pine breeds exposed to drought. Tree Physiol 33:69–80
Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought “train” transcriptional responses in Arabidopsis. Nat Commun 3:740
Donagema GK, de Campos DVB, Calderano SB, Teixeira W, Viana J (2011) Manual de métodos de análise de solo, 2nd edn. Embrapa Solos, Rio de Janeiro
Evans JR, Jakobsen I, Ögren E (1993) Photosynthetic light-response curves–2. Gradients of light absorption and photosynthetic capacity. Planta 189:191–200
Flexas J, Ribas-Carbo M, Bota J, Galmés J, Henkle M, Martínez-Cañellas S, Medrano H (2006) Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol 172:73–82
Friendly M, Sigal M (2014) Recent advances in visualizing multivariate linear models. Rev Colomb Estat 37:261–283
Galmés J, Medrano H, Flexas J (2007) Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol 175:81–93
Gittins R (1985) Canonical analysis: a review with applications in ecology springer-v, vol 12. Spriger, Berlin, p 360
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell Environ 28:834–849
Hepworth C, Doheny-Adams T, Hunt L, Cameron D, Gray J (2015) Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytol 208:336–341
Hura T, Hura K, Dziurka K, Ostrowska A, Baczek-Kwinta R, Grzesiak M (2012) An increase in the content of cell wall-bound phenolics correlates with the productivity of triticale under soil drought. J Plant Physiol 169:1728–1736
IBÁ (2016) Annual Report 2016. In: INDÚSTRIA Bras. ÁRVORES. http://iba.org/images/shared/Biblioteca/IBA_RelatorioAnual2016_.pdf. Accessed 5 Oct 2017
Iwasaki M, Paszkowski J, Freeling M (2014) Epigenetic memory in plants. EMBO J 33:1987–1998
Jarvis PG, McNaughton KG (1986) Stomatal control of transpiration: scaling up from leaf to region. Adv Ecol Res 15:1–49
Kadam NN, Yin X, Bindraban PS, Struik P, Jagadish K (2015) Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water deficit stress than rice? Plant Physiol 167:1389–1401
Kamakura M, Kosugi Y, Takanashi S, Tobita H, Uemura A, Utsugi H (2012) Observation of the scale of patchy stomatal behavior in leaves of Quercus crispula using an Imaging-PAM chlorophyll fluorometer. Tree Physiol 32:839–846
Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger G, Schimid H, Richardson A (2013) Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499:324–327
Kinoshita T, Seki M (2014) Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol 55:1859–1863
Kosugi Y, Takanashi S, Matsuo N, Nik AR (2009) Midday depression of leaf CO2 exchange within the crown of Dipterocarpus sublamellatus in a lowland dipterocarp forest in Peninsular Malaysia. Tree Physiol 29:505–515
Ladiges PY (1975) Some aspects of tissue water relations in three populations of Eucalyptus viminalis labill. New Phytol 75:53–62
Le Gall H, Philippe F, Domon J-M, Gillet F, Pelloux J, Rayon C (2015) Cell wall metabolism in response to abiotic stress. Plants 4:112–166
Li Y, Xu SS, Gao J, Pan S, Wang G (2014) Chlorella induces stomatal closure via NADPH oxidase-dependent ROS production and its effects on instantaneous water use efficiency in Vicia faba. PLoS ONE 9(3):e93290. https://doi.org/10.1371/journal.pone.0093290
Mansouri S, Radhouane L (2015) Is stomatal density an ideal tool to explain drought effect on photosynthesis in tunisian barley varieties? J Chem Biol Phys Sci 5:2865–2876
Maseda PH, Fernández RJ (2015) Growth potential limits drought morphological plasticity in seedlings from six Eucalyptus provenances. Tree Physiol 36:243–251
Merchant A, Peuke AD, Keitel C, Macfarlane C, Warren C, Adams M (2010) Phloem sap and leaf 13C, carbohydrates, and amino acid concentrations in Eucalyptus globulus change systematically according to flooding and water deficit treatment. J Exp Bot 61:1785–1793
Molinier J, Ries G, Zipfel C, Hohn B (2006) Transgeneration memory of stress in plants. Nature 442:1046–1049
Peguero-Pina JJ, Sancho-Knapik D, Barrón E, Camarero J, Vilagrosa A, Gill-Pelegron E (2014) Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann Bot 114:301–313
Peguero-Pina JJ, Sisó S, Sancho-Knapik D, Díaz-Espejo A, Flexas J, Galmés J, Gil-Pelegrín E (2016) Leaf morphological and physiological adaptations of a deciduous oak (Quercus faginea Lam.) to the Mediterranean climate: a comparison with a closely related temperate species (Quercus robur L.). Tree Physiol 36:287–299
Prezotti LC, Gomes JA, Dadalto GG (2007) Manual de recomendação de calagem e adubação para o Estado do Espírito Santo: 5a aproximação., INCAPER. Vitória
R Core Team (2017) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna
Reynolds JF, Thornley JHM (1982) A shoot:root partitioning model. Ann Bot 49:585–597
Ricardi MM, González RM, Zhong S, Domínguez P, Duffy T, Turjanski P, Salter J, Alleva K, Carrai F, Giovannoni J, Estévez J, Iusem N (2014) Genome-wide data (ChIP-seq) enabled identification of cell wall-related and aquaporin genes as targets of tomato ASR1, a drought stress-responsive transcription factor. BMC Plant Biol 14:29
Steudle E (2000a) Water uptake by roots: effects of water deficit. J Exp Bot 51:1531–1542
Steudle E (2000b) Water uptake by plant roots: an integration of views. Plant Soil 226:45–56
Taiz L, Zeiger E (2013) Plant Physiology, 5th edn. Artmed, Porto Alegre
Tezara W, Mitchell V, Driscoll SP, Lawlor DW (2002) Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J Exp Bot 53:1781–1791
Thimmanaik S, Kumar SG, Kumari GJ, Suryanarayana N, Sudhakar C (2002) Photosynthesis and the enzymes of photosynthetic carbon reduction cycle in mulberry during water stress and recovery. Photosynthetica 40:233–236
Thumma BR, Sharma N, Southerton SG (2012) Transcriptome sequencing of Eucalyptus camaldulensis seedlings subjected to water stress reveals functional single nucleotide polymorphisms and genes under selection. BMC Genom 13:364
Villar E, Klopp C, Noirot C, Novaes E, Kirst M, Plomion C, Gion J (2011) RNA-Seq reveals genotype-specific molecular responses to water deficit in eucalyptus. BMC Genom 12:538
Warren CR, Aranda I, Cano FJ (2011) Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant, Cell Environ 34:1609–1629
White DA, Crombie DS, Kinal J, Battaglia M, McGrath J, Mendham D, Walker S (2009) Managing productivity and drought risk in Eucalyptus globulus plantations in south-western Australia. For Ecol Manage 259:33–44
Xu Z, Zhou G (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59:3317–3325
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654
Zhou S, Medlyn B, Sabaté S, Sperlich D, Prectice J (2014) Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiol 34:1035–1046
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was supported by the State of Espírito Santo Research Foundation (FAPES; Grant No. 65768051/14).
The online version is available at http://www.springerlink.com
Corresponding editor: Hu Yanbo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nóia Júnior, R.d., Amaral, G.C., Pezzopane, J.E.M. et al. Ecophysiological acclimatization to cyclic water stress in Eucalyptus. J. For. Res. 31, 797–806 (2020). https://doi.org/10.1007/s11676-019-00926-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00926-9