Abstract
Ethiopian moist evergreen Afromontane forests are vital for the conservation of plant diversity, including genetic pools of Coffea arabica L. However, these forests are continuously shrinking and need empirical scientific studies for their effective conservation and sustainable management. This study was, therefore, conducted to assess woody species composition, diversity, endemicity, structure, plant communities, and environmental determinants in Sirso moist evergreen Afromontane forest of southern Ethiopia. Environmental variables such as altitude, slope, aspect, disturbance levels, and geographical location were recorded for each main plots. Vegetation structure and importance value index (IVI), diversity, vegetation classification, and correlation with environmental factors were analyzed. A total of 74 woody species representing 70 genera and 34 families were identified. Seven species were endemic. Rhus glutinosa A. Rich subsp. glutinosa and Prunus africana (Hook.f.) Kalkm. were species categorized as vulnerable according to the IUCN red list category. The highest number of species was recorded for the Rubiaceae family (8), followed by Euphorbiaceae (6), and Asteraceae (6). Five plant communities were identified: Maesa lanceolata–Dombeya torrida, Galiniera saxifraga–Cyathea manniana, Vepris dainellii–Triumfetta tomentosa, Dracaena fragrans–Rytigynia neglecta, and Arundinaria alpina. The distribution of these communities depends on altitude, slope, and disturbance factors. Syzygium guineense (Wild.) DC., Schefflera abyssinica (Hochst. ex A. Rich.) Harms, and Galiniera saxifraga (Hochst.) Bridson were species with the highest IVI, while P. africana, Cordia africana Lam., Hagenia abyssinica (Bruce) J.F.Gmelin, Bridelia micrantha (Hochst.) Baill., and C. arabica had the lowest. Population structure of woody species showed an inverted J-shape, a J-shape, a bell-shape, and an irregular shaped pattern. These latter three population structure revealed signs of disturbances, and were negatively correlated with altitude and slope. The study showed that the Sirso moist evergreen Afromontane forest is species rich and should be given conservation priority to maintain endemic and native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethiopia is known for its tropical floral diversity. Its complex topography and environmental heterogeneity with a wide range of terrestrial and aquatic ecosystems offer suitable environments for numerous life-forms (Friis and Demissew 2001; Soromessa et al. 2004). Vegetation types range from tropical rain and cloud forests in the southwest to desert scrub lands in the east and north (Teketay et al. 2010). The country contains two of the world’s 34 biodiversity hotspots, namely the Eastern Afromontane and the Horn of Africa hotspots (Lemenih and Woldemariam 2010; Hegazy and Lovett-Doust 2016). The greatest extent of the Afromontane environments is found in Ethiopia, supporting exceptional biodiversity, including much local endemism (Young et al. 2017), and are the headwaters of several major African rivers (Stickler et al. 2013).
Moist evergreen Afromontane forests are the major forest fragments remaining in the country (Senbeta et al. 2014). The majority of these forests are important for the conservation of plant diversity such as the genetic pool of wild populations of Coffea arabica and highland forest bird species (Woldemariam 2003; Senbeta et al. 2005; Schmitt et al. 2010). Plant communities in the forest ecosystems are relatively uniform, with recognizable floristic composition and structure, and are relatively distinct from surrounding vegetation (van der Eddy and Janet 2013). The species composition and distribution of the communities express their relationships to one another and to environmental characteristics (Kent and Coker 1992), as well as realistic and accurate insight of the type of ecosystems (Grabherr and Kojima 1993).
Tropical forests face serious problems, including the irreversible loss of diversity due to deforestation and fragmentation (Lyaruu et al. 2000). Likewise, Ethiopia’s moist evergreen Afromontane forest fragments are continuously shrinking and are only intact in limited areas (Senbeta et al. 2005; Friis et al. 2010). Specifically, in this study area, deforestation and degradation are associated with agricultural expansion, illegal tree harvesting, and over grazing, all of which are practiced in and nearby the forests. This is due to farmland shortages, the absence of other alternative livelihoods for rural–urban migrants, rampant rural poverty, and unemployment (Debebe and Ayele 2013). For instance, forest and bush land coverage of Melokoza district, which encompasses the study area, decreased from 50,456 to 42,432 ha between 2005 and 2012 (Debebe and Ayele 2013). Consequently, understanding ecological and anthropogenic factors affecting composition and structure of this forest type is vital in the face of ever-increasing threats to the ecosystem (Bane et al. 2007; Senbeta et al. 2014). The assessment of moist evergreen Afromontane resources is also necessary for their sustainable use as well as to facilitate the formulation of effective and integrated environmental and economic policies (Nune et al. 2013). However, empirical scientific information is lacking on their floristic composition, diversity, endemicity, structure, type of plant communities, and environmental determinants. This study addresses this lack of information by studying the Sirso moist evergreen Afromontane forest in southern Ethiopia. It also identifies the environmental determinants affecting woody species composition of communities. The results would be helpful for future conservation, management practices, and sustainable utilization by identifying important elements of plant diversity to protect threatened and endemic plant species.
Materials and methods
Study area
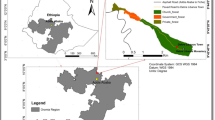
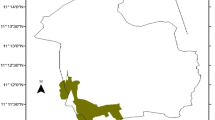
The study was conducted in the Sirso moist evergreen Afromontane forest of the Melokoza district located in the Gamo Gofa Zone, South Nation Nationality People’s Regional (SNNPR) State of Ethiopia (Fig. 1). The area is situated approximately 660 km southwest of Addis Ababa, Ethiopia’s capital city. The district is located between 6°18′ to 6°42′ N and 36°00′ to 37°00′ E. Elevation ranges from 700 to 3200 m a.s.l. with a monthly average temperature of 22 °C and annual rainfall 1200–1300 mm. The vegetation types consist of wood-lands mixed with lowland bamboo at lower altitudes and moist forests at upper altitudes (Denu and Desissa 2013). The Sirso forest is one of the moist evergreen Afromontane forest types in southern Ethiopia (Friis et al. 2010) and consists of economically important plants such as coffee (Coffea arabica), spices (Aframomum corrorima (A. Braun) P.C.M. Jansen), honey (Schefflera abyssinica), food (Ensete ventricosum (Welw.) Cheesman), and medicine (Vepris dainellii (Pic. Serm.) Kokwaro and Hagenia abyssinica). The total area of the Sirso forest is approximately 3501 ha and located between 1547 and 2707 m a.s.l.
Sampling design
A systematic sampling technique was used to collect data. Five transects were laid out north to south and aligned parallel at 2-km intervals. The length of the transects extended from 1.7–5.7 km. Fifty 20 × 20 m sample plots were systematically laid down at 100-m altitudinal intervals along the transect lines for recording and measuring diameter at breast height/diameter at stump height (DBH/DSH ≥ 2.5 cm), and height ≥ 1.5 m. Within the main plots, five 2 m × 2 m sub-plots were established, four in each corner and one in the middle, for seedling and sapling inventory. A 100- m altitudinal difference was considered as altitude affects species distribution through temperature, rainfall, and incoming radiation (Gamachu 1988; Yirdaw et al. 2015).
Data collection
Woody species were recorded and measured in each sample plot and growth habits described. Individual trees DBH ≥ 2.5 cm and height ≥ 1.5 m were measured. Shrub diameters were measured at stump height. Saplings were DBH < 2.5 cm and height ≥ 1.5 m; seedlings with root collar diameters < 2.5 cm and heights < 1.5 m. Geographic location, altitude, and aspect (the direction of the slope the sample plot faces) were recorded using a Garmin GPS 72 (± 3-m accuracy). The slopes (%) were recorded using a Suunto Clinometer. Main anthropogenic activities in or near sample plots, such as clearing for agricultural expansion, tree and shrub cutting for different purposes, grazing, and tracking (roads for people and cattle inside the forest) were recorded. The disturbance level was estimated and categorized under a zero to five ordinal scale, where zero is sample plots without any disturbance and five to highly disturbed plots. Plant specimens were labeled, pressed, dried, identified, and deposited in the herbarium of the Ethiopian Biodiversity Institute.
Data analysis
Species richness and diversity
The status of native and endemic woody species were determined based on the International Union for Conservation of Nature (IUCN) red list category (Vivero et al. 2005), rarity (Magurran 2004), and local criteria (Bekele et al. 1999). In an assemblage, the 25% of species with the lowest abundance would be defined as rare. For the local criteria, species which had < 100,000 individuals in the country would be categorized as the least number of individuals. Woody species diversity, classification of community types, and their correlation with environmental variables were determined by multivariate analyses using R version 3.4.3 vegan package (Oksanen et al. 2013). Hierarchical agglomerative cluster analysis techniques using similarity ratios were used to classify plant community types. Compound indices are preferred and effective for diversity analysis and interpretation (Wilsey et al. 2005; Heino et al. 2008; Morris et al. 2014). Therefore, Shannon’s diversity index, Simpson’s diversity index (1-D), and Shannon’s evenness for diversity analysis were used (Pielou 1966; Kent and Coker 1992). Shannon’s diversity index places more weight on the rare species in the sample, whereas Simpson’s diversity index gives more weight to the most abundant species (Krebs 1999). Sorensen’s similarity index was also used to examine the floristic similarities among plant community types (Sorensen 1948).
Community–environmental factors relation
A Canonical Correspondence Analysis (CCA) was carried out to evaluate the influence of environmental variables on community composition and structure (Legendre and Legendre 1998). CCA was used since it is a direct gradient analysis displaying the variation of vegetation in relation to environmental factors (Kent and Coker 1992; Chahouki 2013). CCA scaling type 1 was used because it is the most appropriate in the ordination of sites–species composition (Legendre and Legendre 1998). Vegetation data and factors were analyzed using the Adonis function from the vegan package which fits linear models to distance matrices and uses 999 permutation tests with Pseudo F-ratios. The Adonis function implements a multivariate analysis of variances (MANOVA) using distance matrices and draws a CCA plot with only those environmental variables showing significant (in our case P ≤ 0.05) effects on species composition of communities (Oksanen et al. 2013). The relationship between environmental factors and specific communities depends on the relative distance between them (Chahouki 2013). Strong predictors have longer arrows than weak predictors (Oksanen et al. 2013).
Forest structure
Population structure, regeneration status, and importance value index (IVI) were analyzed using Microsoft Excel program (Lulekal et al. 2008; Mwavu and Witkowski 2009). The population structure was analyzed on 16 defined DBH and height classes. DBH classes (cm) were 2.5–6.4, 6.5–10.4, 10.5–14.4, 14.5–18.4, 18.5–22.4, 22.5–26.4, 26.5–30.4, 30.5–34.4, 34.5–38.4, 38.5–42.4, 42.5–46.4, 46.5–50.4, 50.5–54.4, 54.5–58.4, 58.5–62.4, and ≥ 62.5. Height classes (m) were 1.5–5.4, 5.5–9.4, 9.5–13.4, 13.5–17.4, 17.5–21.4, 21.5–25.4, 25.5–29.4, 29.5–33.4, 33.5–37.4, 37.5–41.4, 41.5–45.4, 45.5–49.4, 49.5–53.4, 53.5–57.4, 57.5–61.4, and ≥ 61.5. Similarly, the population structure of each woody species was analyzed based on five DBH classes: 2.5–14.4, 14.5–26.4, 26.5–38.4, 38.5–50.4, and > 50.4 cm. The number of individuals in each DBH and height classes were determined and histograms constructed to show population patterns. Representative woody species for each population pattern were displayed. The regeneration status of the whole forest and for each species was analyzed by the ratios of seedling to sapling to mature individuals. The name of each community type was based on its importance value of tree and/or shrub species (Bruun and Ejrnæs 2000; Roberts 2016). The importance value index (IVI) was calculated by summing the percentage values of the relative density, relative frequency, and relative dominance (Curtis 1959). Species with high IVIs have less priority for conservation while those with low IVI values have higher priority:
where RD is the relative density of a species and Ni is number of individuals of the ith species (s);
where RF is the relative frequency of a species and Fi is number occurrences of the ith species (s);
where RDO is the relative dominance of a species and Bi is total basal area of the ith species (s);
where BA is the basal area, d is the diameter of the tree/shrub.
Results
Woody species composition and endemicity
Seventy-four woody species, representing 70 genera and 34 families, were identified. The highest species richness was recorded for the Rubiaceae family (8), followed by the Euphorbiaceae (6), the Asteraceae (6), and the Fabaceae (5). Among the species, tree species were 69%, shrubs 27%, and lianas 4% (Appendix S1). There were seven woody species that are endemic to Ethiopia (Table 1).
Plant communities
Five plant communities were identified from the classification of 74 woody species at 1.5–2 dissimilarity levels (Fig. 2). The name for each community was based on the importance value of tree and/or shrub species.
- (1)
Maesa lanceolata–Dombeya torrida community: This consisted of 10 plots and 46 species between 1706–2606 m a.s.l. with slopes of 5%–65%. It is represented by Maesa lanceolata Forssk, Dombeya torrida (J. F. Gmel.) P. Bamps, Vernonia myriantha Hook.f., and Allophylus spp. Hippocratea africana (Willd.) Loes. was the commonest liana. Coelospermum paniculatum F. Muell. and Embelia schimperi Vatke were also found in this community. Ensete ventricosum and several grass species occupied the herbaceous layer.
- (2)
Galiniera saxifraga–Cyathea manniana community: This had 16 plots and 51 species situated at mid-elevation (1747–2400 m a.s.l. and slope 5–56%). It is represented by G. saxifraga and C. manniana. H. africana, C. paniculatum, and E. schimperi were in this community. The herbaceous layer was dominated by Aframomum corrorima.
- (3)
Vepris dainellii–Triumfetta tomentosa community: This consisted of 46 species in 14 plots distributed between 1547 and 2400 m a.s.l. with slopes 20–65%. The community was represented by tree species Vepris dainellii (Pic. Sem.) Kokwaro and Teclea nobilis Delile and the shrub Triumfetta tomentosa Bojer. Aframomum corrorima dominated the herbaceous layer, similar to community two.
- (4)
Dracaena fragrans–Rytigynia neglecta community: This community comprised seven plots and 29 species distributed between 2107 and 2407 m a.s.l. with slopes of 35%–75%. Dracaena fragrans (L.) Ker Gawl. and Rytigynia neglecta (Hiern) Robyns were characteristic species, with Ensete ventricosum dominating the herbaceous layer.
- (5)
Arundinaria alpina community: This community was the least species rich, containing only three plots and seven species distributed between 2507 and 2707 m a.s.l. with slopes of 10%–30%. This community was characterized by the highland bamboo, Arundinaria alpina K. Schum. The grass Oplismenus hirtellus (L.) P. Beauv. dominated the herbaceous layer.
Community diversity
Species richness, diversity, and Shannon’s evenness were used to interpret the variation among communities (Table 2). Community two had the highest species richness (51 species), followed by communities one and three (46 each). Community five had the lowest species richness (7) and lowest diversity and evenness. Community three had the highest diversity and evenness followed by communities one and two.
Community similarities
Sorenson’s similarity coefficients showed that the species composition of communities two and three had the highest similarity (43%); the lowest similarity was between communities four and five (14%). Community five showed low similarity to any of the other four communities (Table 3). Community five is at a higher altitude than the other communities and explains why the species assemblage is so different. It is also shown by the Canonical Correspondence Analysis (CCA) where the community is excluded from the other four communities.
DBH and height distribution
The analysis of DBH and height distribution showed that the majority of species had more individuals in the lower DBH and height classes (Fig. 3a, b).
Regeneration status and population structure
From 74 woody species, the number of seedlings, saplings, and mature trees/shrubs were estimated at 7880, 6950, and 1265 ha−1, respectively. The ratio of seedlings and saplings to mature individuals was 6.2:1 and 5.5:1, respectively. The highest numbers of saplings and seedlings were G. saxifraga, C. manniana, V. dainellii, Syzygium guineense, D. fragrans, and V. myriantha. Twenty-eight percent of the species had neither seedlings nor saplings.
The analysis of population structure revealed four general patterns (Fig. 4). The first was a positively skewed or inverted J-shape representing a high number of individuals in the lower DBH classes and a gradual reduction in the higher classes. G. saxifraga, Croton macrostachyus Del., Macaranga capensis (Baill.) Benth. ex Sim, Millettia ferruginea (Hochst.) Bak., C. manniana, D. torrida, Bersama abyssinica Fresen., and Canthium oligocarpum Hiern belonged to this population pattern (Fig. 4a–d). The second pattern of Schefflera abyssinica and S. guineense showed a J-shape distribution (Fig. 4e, f) in which there were fewer numbers of individuals in the lower DBH classes. The third pattern was bell-shaped of Sapium ellipticum (Krauss) Pax and Albizia gummifera (J. F. Gmel.) C. A. Sm. showing a high number of individuals in the middle DBH classes and fewer in the lower and higher DBH classes (Fig. 4g, h). The fourth was an irregular distribution of Polyscias fulva (Hiern) Harms and Cordia africana Lam. (Figure 4i, j).
Importance value index (IVI) of species
Syzygium guineense had the highest IVI followed by S. abyssinica, G. saxifraga, and Ficus sur, Forssk and accounted for 29% of the total species (Table 4). Prunus africana, C. africana, Hagenia abyssinica, B. micrantha, and C. arabica were species with lower IVI. However, even though P. africana, C. africana, and H. abyssinica are prohibited species by government proclamation due to facing high risk of threatening, they are still preferred and harvested by local communities.
Influence of environmental factors
Altitude, slope, and disturbance factors significantly influenced plant communities of the study forest, (P ≤ 0.001 for altitude and slope; P ≤ 0.05 for disturbance). Aspect had no significant effect. The canonical correspondence analysis (CCA) showed that community five is distinct from the other four communities due to altitude. Slope had the most influence on community four and disturbance factors on communities one, two, and three. The study showed that disturbance was negatively correlated with altitude and slope (Fig. 5).
Canonical Correspondence Analysis (CCA) ordination plots of five community types and determinant factors. Significant environmental variables (P ≤ 0.05) are shown as vectors. Ellipses represent the different community types. The eigenvalues of axis 1 (CCA1) and axis 2 (CCA2) are 0.5 and 0.4, respectively. (C-1 Maesa lanceolata–Dombeya torrida community; C-2 Galiniera saxifraga–Cyathea manniana community; C-3 Vepris dainellii–Triumfetta tomentosa community; C-4 Dracaena fragrans–Rytigynia neglecta community; C-5 Arundinaria alpina community)
Discussion
Woody species composition and endemicity
The Sirso moist evergreen Afromontane forest is home to a diversity of 74 woody species. A relatively large range in altitudes, an array of suitable environments, and diverse topography are reasons for the high species richness. The number of woody species recorded was higher than for similar forest types in different parts of the country. For example, the Komba-Daga moist evergreen forest in southwest Ethiopia had 62 woody species (Kidane et al. 2015), the Agama tropical Afromontane forest also in the southwest had 39 species (Addi et al. 2016), and the Wondo Genet Afromontane forest in the central highlands had 72 species (Kebede et al. 2013). The highest species richness of the Rubiaceae family in our study was similar to the Maji forest and the Berhane-Kontir forest (Senbeta et al. 2014). However, Rubiaceae is a common and well-adapted family for low to mid elevation humid forests; the largest diversity is predominantly in the humid tropics and subtropics (Christian 2003). The second largest families recorded were Euphorbiaceae and Asteraceae, which are known for their diverse species and numbers in the tropics (Gilbert 1995; Tadesse 2004). Likewise, in the Yayu (Senbeta et al. 2014) and Gelesha forests (Alemu et al. 2015) of Ethiopia and the Shervarayan hills of the Eastern Ghats of India (Kadavul and Parthasarathy 1999), Euphorbiaceae was dominant, and in the Wondo Genet (Kebede et al. 2013), Gendo (Gemechu et al. 2015), and Komba-Daga (Kidane et al. 2015) moist Afromontane forests of Ethiopia, the Asteraceae was dominant. Seven endemic woody species were also recorded in this study. Rhus glutinosa subsp. glutinosa and Prunus africana were found in the Sirso forest and are categorized as vulnerable according to the IUCN red list category (Vivero et al. 2005), implying that the species face a high risk of extinction in the wild and need conservation action. The status of Pycnostachys abyssinica Fresen. and Senecio myriocephalus Sch.Bip.ex A. Rich. have not yet been evaluated in the IUCN red list. The number of endemic woody species recorded in the study forest (seven) is higher than the Wondo Genet forest (five) (Kebede et al. 2013) and the Magada forest (three) of Ethiopia (Tura and Reddy 2015).
Plant community diversities and similarities
The highest species richness and diversity of Galiniera saxifraga–Cyathea manniana, Maesa lanceolata–Dombeya torrida, and Vepris dainellii–Triumfetta tomentosa communities is related to elevation range, slope variation, soil characteristics, climate variability, and the adaptive and distribution nature of the species. The communities were found between 1547 and 2607 m a.s.l. and on slopes from 5% to 65%, optimal conditions for vegetation growth (Reddy et al. 2011). Species in these communities are adapted to a wide range of elevations and slopes. The highest evenness was for the Vepris dainellii–Triumfetta tomentosa community, indicating little dominance by any single species, the Arundinaria alpina and Dracaena fragrans–Rytigynia neglecta communities had lower diversity and evenness. The species in these communities adapted to high altitudes (2107–2707 m a.s.l.) depending on their physiological needs (Austrheim 2002). These characteristics result in communities dominated by a few species such as Maesa lanceolata, Syzygium guineense, and Arundinaria alpina in the Dracaena fragrans–Rytigynia neglecta community or by a single species such as Arundinaria alpina in community type 5.
The communities showed relatively low similarities with each other. This might be due to the fact that many of the species are not distributed throughout the communities except for Maesa lanceolata, Syzygium guineense, Croton macrostachyus, Galiniera saxifraga, Bersama abyssinica, and Dracaena steudneri Engl. These were the most common species recorded in four community types. These species are shade tolerant and generalist species adapted to a wide range of altitudes, a variety of soil and climate conditions, and to interspecific interactions in moist evergreen vegetation ecosystems. The low similarity among communities is also supported by our CCA results, where altitude, slope, and disturbance were the main factors for community composition and distribution.
Regeneration status, population structure, and important value index of woody species
The results show the presence of more seedlings than saplings and more saplings than mature individuals. This implies that most of woody species in the study forest have good potential to regenerate ensure the perpetuation of the species (Melese and Ayele 2017; Goncalves et al. 2018). The highest number of saplings and seedlings were G. saxifraga, C. manniana, V. dainellii, S. guineense, D. fragrans, and V. myriantha. This confirms that these species have high seedling survival rates in moist natural forests. Except for V. myriantha, the seeds of those species are recalcitrant and the moisture of the forest supports immediate germination and growth. In addition, these species are more competitive and less affected by disturbance factors. Species with no seedlings and saplings are under threat of local extinction (Gurmessa et al. 2012). Therefore, management and conservation priority should be given to species with no or few seedlings and saplings and for the whole forest to insure regeneration of all woody species.
The reverse J-shape distribution of diameter and height classes indicates that the forest has favorable regeneration and recruitment potential (Tesfaye et al. 2002). However, the J-shape, bell-shape, and an irregular shaped pattern were also found for individual species. The J-shape and bell-shape patterns imply poor regeneration and recruitment due to germination constraints, seedling mortality, and recruitment limitation (Goncalves et al. 2018). This could be a result of activities such as illegal harvesting and livestock grazing (Zegeye et al. 2006), coupled with biophysical factors of climatic variability, seed predation, and intense competition (Lulekal et al. 2008; Kidane et al. 2015). The irregular pattern of species such as P. fulva and C. africana could be due to harvesting for house construction, timber, farm tools, and firewood (Kala 2015; Goncalves et al. 2018). For instance, the absence of Polyscias fulva in diameter class 4 (38.5–50.4 cm) could be due to harvesting of this size for construction, whereas larger sized individuals are left purposely for hanging beehives. The absence of C. africana in DBH class 5 is possibly a consequence of selective harvesting for timber by the local community.
The Importance Value Index can be used for prioritizing species for conservation (Zegeye et al. 2006; Tadele et al. 2013; Berhanu et al. 2016). Higher IVI were recorded for S. guineense, S. abyssinica, G. saxifraga, and F. sur. This is due to their relatively higher density, frequency, and dominance in the forest and are considered as the most ecologically important woody species. Similar results have been reported for Syzygium guineense and Ficus sur in Komto moist Afromontane forest, western Ethiopia (Gurmessa et al. 2012), and for S. abyssinica and S. guineense in Belete moist evergreen Montane forest, southwestern Ethiopia (Gebrehiwot and Hundera 2014). However, Prunus africana, C. africana, H. abyssinica, B. micrantha, and C. arabica had lower IVI values and hence require conservation priority.
Influence of environmental factors
Altitude, slope, and disturbance were influential in community structure and composition, whereas aspect was not. The high altitude differences influenced temperature, humidity, and soil characteristics. This affects species distribution and diversity patterns among the forest communities (Trejo and Dirzo 2002; La Torre et al. 2007; Sambare et al. 2011). Similarly, other studies have reported altitude as a main factor for woody species distribution (Wale et al. 2012; Zhang et al. 2016; Pala et al. 2017; Sinha et al. 2018). According to Gonmadje et al. (2018), altitude and slope are the most important variables determining tree species composition patterns at a local scale. Disturbance had the most influence on communities one, two, and three. The significant influence of disturbance on these communities could be related to high local preference of species for timber, fuel wood, and non-timber products. Moreover, the negative correlation of disturbance with altitude and slope could be attributed to less extraction as increasing altitude and slope make these species less accessible.
Conclusions
The Sirso natural forest is one of a few remaining moist evergreen Afromontane forests in southern Ethiopia. It is rich in woody and endemic species, and contains five plant communities: Maesa lanceolata–Dombeya torrida, Galiniera saxifraga–Cyathea manniana, Vepris dainellii–Triumfetta tomentosa, Dracaena fragrans–Rytigynia neglecta, and Arundinaria alpina. The distributions of these communities are influenced by altitude, slope, and disturbance factors. Communities closest to local inhabitants are affected by disturbance, whereas, those relatively inaccessible for exploitation are affected by altitude and slopes. This determines the variations in species richness, diversity, and evenness among communities. Analysis of population structure and regeneration status showed a reversed J-shape, a J-shape, a bell-shape, and an irregular pattern. These latter three revealed signs of some disturbances, indicating that the Sirso moist evergreen Afromontane forest is in a stage of risk for biodiversity degradation. Consequently, the entire forest ecosystem, as well as threatened species, needs to be protected by establishing in situ conservation and restoration activities as well as education and awareness programs for local communities.
References
Addi A, Soromessa T, Kelbessa E, Dibaba A, Kefalew A (2016) Floristic composition and plant community types of Agama Forest, an Afromontane Forest in Southwest Ethiopia. J Ecol Nat Environ 8(5):55–69
Alemu B, Hundera K, Abera B (2015) Floristic composition and structural analysis of Gelesha forest, Gambella regional State, southwest Ethiopia. J Ecol Nat Environ 7(7):218–227
Austrheim G (2002) Plant diversity patterns in semi-natural grasslands along an elevational gradient in southern Norway. Plant Ecol 161(2):193–205
Bane J, Nune S, Mekonnen A, Bluffstone R (2007) Policies to increase forest cover in Ethiopia. In: Proceedings of a policy workshop. Environmental Economics Policy Forum for Ethiopia (EEPFE) and Ethiopian Development Research Institute (EDRI), Addis Ababa, pp 18–19
Bekele T, Haase G, Soromessa T, Edward S, Demissie A, Bekele T, Haase G (1999) Forest genetic resources of Ethiopia: Status and proposed actions. Institute of Biodiversity Conservation and Research (IBCR), Addis Ababa, pp 1–48
Berhanu A, Demissew S, Woldu Z, Didita M (2016) Woody species composition and structure of Kuandisha afromontane forest fragment in northwestern Ethiopia. J For Res 28(2):343–355
Bruun HH, Ejrnæs R (2000) Classification of dry grassland vegetation in Denmark. J Veg Sci 11(4):585–596
Chahouki MAZ (2013) Classification and ordination methods as a tool for analyzing of plant communities. Multivariate analysis in management, engineering and the sciences. InTech Open, London, pp 1–34
Christian P (2003) Flora of Ethiopia and Eritrea Volume 4 Part 1. Uppsala University, Uppsala, pp 266–267
Curtis JT (1959) The vegetation of Wisconsin: an ordination of plant communities. University of Wisconsin Press, Wisconsin, pp 1–660
Debebe D, Ayele T (2013) Land use patterns and its implication for climate change: the case of Gamo Gofa, Southern Ethiopia. Int J Sci Res Rev 2(3):155–173
Denu D, Desissa D (2013) Abundance and use of Vepris dainellii (Pichi-Serm.) Kokwaro, an Ethiopian endemic plant, in Melokoza woreda, Southern Ethiopia. Ethiop J Educ Sci 8(2):1–10
Friis IB Demissew S (2001) Vegetation maps of Ethiopia and Eritrea. A review of existing maps and the need for a new map for the Flora of Ethiopia and Eritrea. In: Biodiversity research in the horn of Africa Region. Danske Videnskabernes Selskab Biol Skr, Copenhagen, pp 399–439
Friis IB, Sebsebe D, Breugel PV (2010) Atlas of the potential vegetation of Ethiopia. Det Kongelige Danske Videnskabernes Selskab, Copenhagen, pp 1–307
Gamachu D (1988) Some patterns of altitudinal variation of climatic elements in the mountainous regions of Ethiopia. Mt Res Dev 8:131–138
Gebrehiwot K, Hundera K (2014) Species composition, plant Community structure and natural regeneration status of Belete Moist Evergreen Montane Forest, Oromia Regional state, southwestern Ethiopia. MEJS 6(1):97–101
Gemechu T, Soromessa T, Kelbessa E (2015) Structure and regeneration of Gendo moist montane forest, East Wellega Zone, Western Ethiopia. J Environ Earth Sci 5(15):148–168
Gilbert MG (1995) Flora of Ethiopia and Eritrea Volume 2 Part 2. Uppsala University, Uppsala, pp 265–266
Goncalves FM, Revermann R, Cachissapa MJ, Gomes AL, Aidar MP (2018) Species diversity, population structure and regeneration of woody species in fallows and mature stands of tropical woodlands of southeast Angola. J For Res 29(6):1569–1579
Gonmadje C, Doumenge C, Sunderland T, McKey D (2018) Environmental filtering determines patterns of tree species composition in small mountains of Atlantic Central African forests. Acta Oecol 1:1. https://doi.org/10.1016/j.actao.2018.04.001
Grabherr G, Kojima S (1993) Vegetation diversity and classification systems. In: Solomon AM, Shugart HH (eds) Vegetation dynamics and global change. Chapman & Hall, New York, pp 218–232
Gurmessa F, Soromessa T, Kelbessa E (2012) Structure and regeneration status of Komto Afromontane moist forest, East Wollega Zone, west Ethiopia. J For Res 23(2):205–216
Hegazy A, Lovett-Doust J (2016) Plant ecology in the Middle East. Oxford University Press, Oxford, pp 1–225
Heino J, Mykra H, Kotanen J (2008) Weak relationships between landscape characteristics and multiple facets of stream macroinvertebrate biodiversity in a boreal drainage basin. Landscape Eco 23(4):417–426
Kadavul K, Parthasarathy N (1999) Plant biodiversity and conservation of tropical semi-evergreen forest in the Shervarayan hills of Eastern Ghats, India. Biodivers Conserv 8(3):419–437
Kala CP (2015) Forest structure and anthropogenic pressures in the Pachmarhi biosphere reserve of India. J For Res 26(4):867–874
Kebede M, Yirdaw E, Luukkanen O, Lemenih M (2013) Plant community analysis and effect of environmental factors on the diversity of woody species in the moist Afromontane forest of Wondo Genet, South Central Ethiopia. Biodiv Res Conserv 29(1):63–80
Kent M, Coker P (1992) Vegetation description and analysis: a practical approach, 2nd edn. Wiley, New York, pp 1–363
Kidane KG, Hundera K, Dalle G (2015) Species diversity and structural analysis of Komba-Daga moist evergreen forest, Kaffa Zone, Southwest Ethiopia. Res Rev: Life Sci 5(3):14–23
Krebs CJ (1999) Ecological Methodology, 2nd edn. Addison-Wesley Educational Publishers, Benjamin Cummings, Menlo Park, pp 1–620
La Torre MDLÁ, Herrando-Pérez S, Young KR (2007) Diversity and structural patterns for tropical montane and premontane forests of central Peru, with an assessment of the use of higher-taxon surrogacy. Biodivers Conserv 16(10):2965–2988
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam, pp 1–870
Lemenih M, Woldemariam T (2010) Review of Forest, woodland and bushland resources in Ethiopia up to 2008. In: Edwards S (ed) Ethiopian environment review. Forum for Environment, Addis Ababa, pp 131–186
Lulekal E, Kelbessa E, Bekele T, Yineger H (2008) Plant species composition and structure of the Mana Angetu moist montane forest, south-eastern Ethiopia. J East Af Nat Hist 97(2):165–185
Lyaruu HV, Eliapenda S, Backéus I (2000) Floristic, structural and seed bank diversity of a dry Afromontane forest at Mafai, central Tanzania. Biodivers Conserv 9(2):241–263
Magurran AE (2004) Measuring biological diversity. Blackwell, Oxford, pp 1–256
Melese SM, Ayele B (2017) Woody plant diversity, structure and regeneration in the Ambo State Forest, South Gondar Zone, Northwest Ethiopia. J For Res 28(1):133–144
Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, Meiners T, Müller C, Obermaier E, Prati D, Socher SA (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol 4(18):3514–3524
Mwavu EN, Witkowski ET (2009) Population structure and regeneration of multiple-use tree species in a semi-deciduous African tropical rainforest: implications for primate conservation. For Ecol Manag 258(5):840–849
Nune S, Kassie M, Mungatana E (2013) Forest Resource Accounts for Ethiopia. Implementing environmental accounts. Springer, New York, pp 103–142
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara RB, Simpson GL, Solymos P, Stevens MH, Wagner H, Oksanen MJ (2013) Package ‘vegan’. Community ecology package, version. 2(9): 1–295
Pala NA, Mathur M, Iqbal K, Negi AK, Shukla G, Chakravarty S (2017) Community dynamics: competition and facilitation studies of tree species in temperate forests of the Indian Himalaya. Indian J Ecol 44(2):279–288
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Reddy CS, Babar S, Amarnath G, Pattanaik C (2011) Structure and floristic composition of tree stand in tropical forest in the Eastern Ghats of northern Andhra Pradesh, India. J For Res 22(4):491–500
Roberts DW (2016) labdsv: Ordination and Multivariate Analysis for Ecology. R package version 1.7–0.2015. http://ecology.msu.montana.edu/labdsv/R/labs/lab3/lab3.html
Sambare O, Bognounou F, Wittig R, Thiombiano A (2011) Woody species composition, diversity and structure of riparian forests of four watercourses types in Burkina Faso. J For Res 22(2):145–158
Schmitt CB, Senbeta F, Denich M, Preisinger H, Boehmer HJ (2010) Wild coffee management and plant diversity in the montane rainforest of southwestern Ethiopia. Afr J Ecol 48(1):78–86
Senbeta F, Schmitt C, Denich M, Demissew S, Velk PL, Preisinger H, Teketay D (2005) The diversity and distribution of lianas in the Afromontane rain forests of Ethiopia. Divers Distrib 11(5):443–452
Senbeta F, Schmitt C, Woldemariam T, Boehmer HJ, Denich M (2014) plant diversity, vegetation structure and relationship between plant communities and environmental variables in the Afromontane forests of Ethiopia. Ethiop J Sci 37(2):113–130
Sinha S, Badola HK, Chhetri B, Gaira KS, Lepcha J, Dhyani PP (2018) Effect of altitude and climate in shaping the forest compositions of Singalila National Park in Khangchendzonga Landscape, Eastern Himalaya, India. J Asia Pac Biodivers 11(2):267–275
Sorensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biol Skr 5:1–34
Soromessa T, Teketay D, Demissew S (2004) Ecological study of the vegetation in Gamo Gofa zone, southern Ethiopia. Trop Ecol 45(2):209–222
Stickler CM, Coe MT, Costa MH, Nepstad DC, McGrath DG, Dias LC, Rodrigues HO, Soares-Filho BS (2013) Dependence of hydropower energy generation on forests in the Amazon Basin at local and regional scales. Proc Natl Acad Sci 110(23):9601–9606
Tadele D, Lulekal E, Damtie D, Assefa A (2013) Floristic diversity and regeneration status of woody plants in Zengena Forest, a remnant montane forest patch in northwestern Ethiopia. J For Res 25(2):329–336
Tadesse M (2004) Flora of Ethiopia and Eritrea Volume 4 Part 2. Uppsala University, Uppsala, pp 1–426
Teketay D, Lemenih M, Bekele T, Yemshaw Y, Feleke S, Tadesse W, Nigussie D (2010) Forest resources and challenges of sustainable forest management and conservation in Ethiopia. Degraded forests in Eastern Africa: management and restoration. Earthscan, New York, pp 19–63
Tesfaye G, Teketay D, Fetene M (2002) Regeneration of fourteen tree species in Harenna forest, southeastern Ethiopia. Flora 197(6):461–474
Trejo I, Dirzo R (2002) Floristic diversity of Mexican seasonally dry tropical forests. Biodivers Conserv 11(11):2063–2084
Tura GB, Reddy PR (2015) Study of Vegetation Composition of Magada Forest, Borana Zone, Oromia, Ethiopia. Univ J Plant Sci 3(5):87–96
van der Eddy M, Janet F (2013) Vegetation ecology, 2nd edn. Wiley, Hoboken, pp 1–572
Vivero JL, Demissew S, Kelbessa E (2005) The red list of endemic trees et shrubs of Ethiopia and Eritrea. Fauna and Flora International, Cambridge, pp 1–23
Wale HA, Bekele T, Dalle G (2012) Plant community and ecological analysis of woodland vegetation in Metema Area, Amhara National Regional State, Northwestern Ethiopia. J For Res 23(4):599–607
Wilsey BJ, Chalcraft DR, Bowles CM, Willig MR (2005) Relationships among indices suggest that richness is an incomplete surrogate for grassland biodiversity. Ecology 86(5):1178–1184
Woldemariam TG (2003) Vegetation of the Yayu forest in SW Ethiopia: impacts of human use and implications for in situ conservation of wild Coffea arabica L. populations. ZEF Bonn, Gottingen, pp 1–8
Yirdaw E, Starr M, Negash M, Yimer F (2015) Influence of topographic aspect on floristic diversity, structure and treeline of Afromontane cloud forests in the Bale Mountains Ethiopia. J For Res 26(4):919–931
Young NE, Romme WH, Evangelista PH, Mengistu T, Worede A (2017) Variation in population structure and dynamics of montane forest tree species in Ethiopia guide priorities for conservation and research. Biotropica 49(3):309–317
Zegeye H, Teketa D, Kelbessa E (2006) Diversity, regeneration status and socio-economic importance of the vegetation in the islands of Lake Ziway, south-central Ethiopia. Flora 201(6):483–498
Zhang C, Li X, Chen L, Xie G, Liu C, Pei S (2016) Effects of topographical and edaphic factors on tree community structure and diversity of subtropical mountain forests in the lower Lancang River Basin. Forests 7(10):222–239
Acknowledgements
Our special thanks go to Dr. Debisa Lemessa, senior researcher and director of forest and range land plants diversity Directorate in the Ethiopian Biodiversity Institute for his unreserved assistance during methodology development and data analysis. We are grateful to Melese Bekele, Sisay Alemu, and Kedir Erbo, junior researchers in the Ethiopian Biodiversity Institute for their assistance during data collection and data analysis. We would also like to show our gratitude to Gorfu Goshu, forester in environmental protection and forest office of Melokoza district and Gido Gigo and Habtamu Chanko, farmers in Melokoza district for their assistance during data collection. We also extend our gratitude to the Ethiopian Biodiversity Institute and to Hawassa University for logistic and financial support for field data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The project was fully funded by the Ethiopian Biodiversity Institute and NORAD project, Hawassa University.
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mewded, B., Negash, M. & Awas, T. Woody species composition, structure and environmental determinants in a moist evergreen Afromontane forest, southern Ethiopia. J. For. Res. 31, 1173–1186 (2020). https://doi.org/10.1007/s11676-019-00894-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00894-0