Abstract
Understanding woody plant species composition and structure is fundamental to design and optimize the needed conservation measures for Ethiopian church forests. The aim of this study was to describe the composition, structure, and regeneration status of woody species in church forests in southeast of Lake Tana, Ethiopia. Data were collected from twenty-four church forests. Four plots (20 m × 20 m) were established in each church forest. Plots were located in four cardinal directions (north, east, west, and south) at different distances from the forest center. Four subplots (5 m × 5 m) were established in each plot to assess seedlings and canopy cover. In each plot, all woody plants were identified and counted, and diameter at breast height (DBH) was measured. Species and family importance values were computed to characterize the species composition. Additionally, population structural features were analyzed through the variation of tree size classes. Species richness (SR), Pilou evenness (Jʹ), and Shannon–Wiener index (Hʹ) were used to determine species diversity. A total of 115 woody species representing 53 families and 97 genera were found. Of these, 62% were trees, 36% shrubs, 1.89% climber, and 0.06% reed species. Species richness differed among forests, ranging between 16 and 38 species. Fabaceae, Sapotaceae, and Rubiaceae were the dominant families with a high family importance values of 41, 28, and 22, respectively. The church forests have relatively high indices of species diversity (SR = 26 ± 1.25), (Jʹ = 0.75 ± 0.02), and (Hʹ = 2.42 ± 0.07), indicating that they play a major role in the conservation of woody species. However, a relatively high densities of Eucalyptus spp. ranging from 13 to 1925 individuals ha−1 were recorded, and these exotic tree species, thus, form a potential threat to the conservation of native species. The diameter class distribution of some selected keystone and dominant species formed four main shape types, of which the irregular-shaped pattern was most predominant, which suggests missing cohorts and regeneration problems for most species. Higher densities of Eucalyptus plantations were recorded in more recently established than old church forests. Therefore, effective measures should be taken to address the major pressures, such as plantation of exotic species that negatively affect the species composition and vegetation structure of these church forests, which, in turn, affect their ecosystem functions and services.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Forests play a significant role in providing multiple ecosystem services, such as climate change mitigation. However, due to anthropogenic and natural factors, forest cover has drastically declined worldwide (Contreras-Hermosilla 2000). Similarly, Ethiopia has suffered drastic historical deforestation. Logan (1946) reported that only 5% of the Ethiopian highlands were forested in 1946, suggesting that deforestation started a long time ago. The forest cover, particularly in the highlands of northern Ethiopia, has continued to decline (Darbyshire et al. 2003; Nyssen et al. 2004). For example, in Lake Tana Basin, during the mid-twentieth century, about 20% of the area was covered with woody vegetation, while only about 10% remained by 2014–16 (Frankl et al. 2019). The rapid human population growth is the main driving force that is responsible for the loss of forests (Bishaw 2001). Agricultural expansion, urbanization, free grazing, and unsustainable development activities are the major pressures that intensify deforestation and forest degradation (Hailu et al. 2015). Consequently, the forest coverage is fragmented to small patches, particularly in the central and northern highlands of Ethiopia. In most parts of the northern highlands, patchy remnants of natural forests are found almost only surrounding churches (Gashaw et al. 2015).
Ethiopian church forests provide essential ecosystem services and support diverse plant and animal species, including endangered indigenous tree species (Aerts et al. 2006; Bongers et al. 2006; Reynolds et al. 2017; Morgan et al. 2018). Furthermore, church forests serve as a habitat for many insects and animals, such as birds and mammals (Wassie et al. 2005a, b; Scull et al. 2017). Several studies (Nyssen et al. 2004; Aerts et al. 2006, 2016; Wassie et al. 2009a, b; Wassie et al. 2010; Aynekulu et al. 2011; Woldemedhin and Teketay 2016; Abiyot et al. 2017; Morgan et al. 2018) have investigated the species composition and tree community structure of Ethiopian church forests, their role for biodiversity conservation, and the associated conservation challenges. For example, in Amhara region, Woldemedhin and Teketay (2016) documented 56 woody species from nine church forests in West Gojjam, while Morgan et al. (2018) and Wassie et al. (2010) reported 47 from 11 church forests and 168 woody species from 28 church forests in the northern part of the region, respectively. The recorded species were mainly indigenous trees, indicating the high conservation value of Ethiopian church forests. Reynolds et al. (2015) reported that there are more than 8000 church forests across the Amhara National Regional State (NRS); however, if also small church forests are included, the current number of church forests in the NRS could be much higher than this report. Therefore, there is no doubt that church forests in the NRS are playing a significant role in woody species conservation.
Many indigenous trees and shrubs, which are exterminated in some localities, are still found in the compounds of churches (Wassie et al. 2005a). Protection of a large network of small church forests can be more effective for biodiversity conservation compared with the conservation of a few large patches of an equivalent area (Bhagwat and Rutte 2006; Dudley et al. 2009; Hokkanen et al. 2009; Aerts et al. 2016). This is due to the fact that the large network of small church forests covers a wide variety of habitat, located in different agroecological zones and managed by the respective parish. The significant role of small forest patches, such as church forests to the conservation of overall species diversity and structure, was also described by Teketay et al. (2018). However, pressures, such as free grazing, fuelwood harvesting, woody species removal to construct church buildings or expand burial sites, have been negatively affecting the species composition, community structure, and natural regeneration rate of church forests in northern Ethiopia (Reynolds et al. 2017; Wassie et al. 2009a, 2010). Furthermore, plantation of fast-growing and ornamental exotic species inside the church forests, such as Cupressus lusitanica, Citrus spp., Eucalyptus spp., Grevillea robusta, Jacaranda mimosifolia, Melia azedarach, Pinus spp., is strongly affecting species composition and community structure of church forests (Aerts et al. 2016; Cardelu’s et al. 2019).
In general, these pressures could not only alter the ecological processes and functions of the church forests ecosystem, but also affect the ecosystem services that these church forests provide to the local community. Thus, to combat these pressures and maintain the existing ecosystem services, church forests should be supported in different ways. While stonewall construction around church forests can be a very effective management strategies to overcome the effect of free grazing (Wassie et al. 2009a; Woods et al. 2017), a proper understanding of species composition, structure, and regeneration status of church forests is required to draft long-term management plans.
In the southeast of Lake Tana located in the Amhara NRS, there is little information about the species composition and conservation status in the church forests, except for four church forests (i.e., Emashenekure Giworegis, Gebesiwit Mariyam, Wej Aregawi, and Zahara Mikael) that were assessed by Wassie et al. (2010). Furthermore, almost a decade has passed since the assessment made by Wassie et al. (2010), and, hence, the species composition and structure of the church forests have likely changed over time. To fill this important knowledge gap, we studied the composition, structure, and the regeneration status of woody species, in a network of 24 church forests located in the southeast of Lake Tana.
2 Materials and Methods
2.1 Description of the Study Area
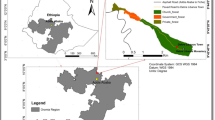
The Lake Tana Basin has an area of 15,089 km2 with an elevation ranging from 1782 to 4109 m above and located in the northwest highlands of Ethiopia (Lemma et al. 2018). This study was conducted in the lower elevations in southeast of Lake Tana between Bahir Dar and Debre Tabor (1794–2204 m). The main land use and land cover types are natural forests, bushland, plantation forest, urban, village, cultivated land, waterbody, wetland, woodlands, and grassland (Song et al. 2018). Cultivated land (62%) is the major land use type, followed by water bodies (20%). However, the forest cover, including plantation and woodlands, is only 5% (Song et al. 2018). This area experiences a strong seasonal rainfall regime (about 70–90% of the total rainfall occurs during June–September), but a very even monthly temperature regime (Peel et al. 2007). The annual average rainfall varies between 1250 and 1500 mm, and the mean annual temperature varies between 15.3 and 19.6 °C (Lemma et al. 2018). A total of 24 church forests located between 37° 27′ E–37° 55′ E and 11° 39′ N–11° 56′ N were selected in the southeast of Lake Tana (Table 10.1; Fig. 10.1). Church forest selection was based on accessibility, distance between the consecutive church forests (a minimum of 4 km) and variability of the surrounding matrix in the radius of 3 km. The surface area of the church forests ranged from 2 to 13 ha, and the churches in the forests were established between 340 and 2010 (EOTC, no date).
2.2 Sampling and Data Collection Methods
2.2.1 Vegetation Composition and Structure Survey
In each church forest, four sampling plots were systematically established in four cardinal directions (north, east, west, and south), but at different distances along the axis from the church buildings to edge of the compound (Fig. 10.2). The starting direction was selected randomly and continued in a clockwise direction. A total of 96 plots with a size of 20 m × 20 m were established to identify mature and saplings individuals of the woody species. In each of the sampling plots, all woody species were identified, counted, and measured. Diameter at breast height (DBH) was measured using a diameter tape. When the height of the plant was greater than 1.6 m, DBH was measured at 1.3 m above ground level, but for smaller plants greater than 1 m, the diameter was measured at 10 cm above the ground. For trees containing buttressed stems, the DBH was measured above the buttress, and for multiple stems, all stems were counted and measured (Wassie et al. 2010). Woody plants less than 1 m height were considered as seedlings, and to score their presence and abundance, 5 m × 5 m plots were established at each corner of the large sampling plots. Identification was done to species level using identification keys (Bekele-Tesemma 2007). Species that were difficult to identify were identified in the National Herbarium Museum at Addis Ababa University. Plant nomenclature used in this article is based on the published guidelines of the Flora of Ethiopia and Eritrea, Vol. 1–8 (1989–2009) (Edwards et al. 1995, 1997; Edwards 1997; Hedberg et al. 2003). The recently updated nomenclature for Acacia spp. was also used (Kyalangalilwa et al. 2013). Lastly, the percent of canopy cover was measured using a convex mirror densiometer at four points within each of the main plots, and the average recording was used for each sampling plot. Maximum plant height within a church forest was taken as maximum forest height.
2.3 Data Analyses
The species diversity was analyzed using species richness (SR), evenness (Jʹ), and Shannon–Wiener (Hʹ) diversity index (Peet 1974). The relative dominance, density, frequency (Appendix 1), and diversity of each woody species and family at each church forest were computed using the equations described in Table 10.2. These values were used to calculate the importance value index (IVI) and family importance values (FIV) of woody species of each church forest in southeast of Lake Tana (Table 10.2). The IVI and FIV values range between 0 and 300. Species and families with high IVI and FIV values are considered more important ecologically than those with low IVI and FIV values, respectively.
In addition, the demographic structure of some selected keystone and dominant species was assessed using the frequency of individuals per diameter class (1–11 classes). The diameter classes were established with the range of 5 cm DBH. Demographic structural shapes such as I shape, J shape, and irregular shape show unfavorable forest structure, while broken reversed J shape indicates a healthy forest structure with continuous regeneration and ingrowth of cohorts (Alelign et al. 2007; Zegeye et al. 2011; Tadele et al. 2014). The dominant species are species that are very well represented in the church forests, whereas the keystone species are those which have social and ecological importance to local people (Wassie et al. 2009b; Gebeyehu et al. 2019): Juniperus procera, Olea europaea, Podocarpus falcatus, Prunus africana, Ekebergia capensis and Mimusops kummel.
3 Results
3.1 Species Richness, Evenness, and Diversity
A total of 115 woody species belonging to 53 families and 97 genera were identified in the 24 church forests studied, and the number of woody species ranged from 16 to 38 species per church forest (Tables 10.3 and 10.4). Of these species, 62% (n = 6268) were trees, 36% (n = 3571) were shrubs, 1.89% (n = 190) were climbers, and 0.06% (n = 6) were reed. The Kudese Minas (Kmi, 3 in Fig. 10.1) church forest had the highest number of species and genus, while Deber Kusekuam (DK, 18 in Fig. 10.1) church forest contained the lowest number of species and genus (Table 10.3).
The Kudese Minas (Kmi, 3 in Fig. 10.1) church forest had the highest number of families, while Qere Giweregis (QG, 16 in Fig. 10.1) church forest contained the lowest number of families (Table 10.3). The families with the highest number of species were Fabaceae (n = 17) followed by both Rubiaceae (n = 8) and Euphorbiaceae (n = 8). Oleaceae was represented by five species, whereas the remaining families were represented by ≤ 4 species (Table 10.4).
The overall evenness (J′ = 0.75 ± 0.02) and Shannon diversity index (H′ = 2.42 ± 0.07) of woody species were recorded in all church forests (Table 10.5). Both Delemo Tekelehayemanot (DT, 13 in Fig. 10.1) and Gebesiwit Mariya (Gma, 10 in Fig. 10.1) church forests had higher evenness than others, while church forest Kudese Minas (Kmi, 3 in Fig. 10.1) had higher Shannon diversity than other church forests. The lowest evenness and diversity were recorded at Wenechet (W, 9 in Fig. 10.1) church forest (Table 10.5).
3.2 Stand Structure of the Church Forests
Species and family importance ranged from 2 to 118% and 3% to 117%, respectively. The most ecologically important woody species (IVI) and families (FIV) differed between church forests (Tables 10.6 and 10.7). For example, the top three woody species in Aba Gerima Mariyam (AG, 2 in Fig. 10.1) church forest were Millettia ferruginea (IVI = 46%), Ficus thonningii (IVI = 39%), and Grevillea robusta (IVI = 25%), while Mimusops kummel (IVI = 97%), Diospyros abyssinica (IVI = 22%), and Ocimum lamiifolium (IVI = 21%) were the top three woody species in Zahara Mikael (ZM, 11 in Fig. 10.1) church forest (Table 10.6). Similarly, Fabaceae (63%), Moraceae (40%), and Proteaceae (24%) had the highest FIV values at Aba Gerima Mariyam (AG, 2 in Fig. 10.1) church forest while, Sapotaceae (93%), Rubiaceae (39%), and Fabaceae (26%) had the highest FIV values at Zahara Mikael (ZM, 11 in Fig. 10.1) church forest (Table 10.7).
The canopy cover of woody tree species at each church forest ranged from 37 to 83% with a mean of 59%. Kudese Minas (Kmi, 3 in Fig. 10.1) and Robit Bat (RB, 1 in Fig. 10.1) church forests had the highest canopy cover, while Tiwaz Abo (TA, 21 in Fig. 10.1) church forest had the lowest canopy cover. More than half of the church forests had a canopy cover greater than 59% (Table 10.3).
The diameter class distribution of some selected keystone and dominant species revealed four types of demographic structures. The four types of structures were broken reversed J shape, I shape, J shape, and irregular shape. The broken reversed J-shaped pattern was composed of a high number of individuals in the lowest diameter classes and progressively declining numbers in the highest diameter classes with an almost complete absence in the highest diameter classes. This pattern was, for example, exhibited by Juniperus procera at Delemo Tekelehayemanot (DT, 13 in Fig. 10.1), Mimusops kummel at Hager Selam Mariyam (HSM, 19 in Fig. 10.1) and Weyebela Kidanemehert (WK, 8 in Fig. 10.1), Prunus africana at Kudese Minas (Kmi, 3 in Fig. 10.1), and Ekebergia capensis at Kudese Minas (Kmi, 3 in Fig. 10.1) church forests.
The I-shaped pattern was formed when the numbers of individuals of a species were only presented in one of the eleven classes. Juniperus procera at Fisa Mikael (FM, 5 in Fig. 10.1), Hager Selam Mariyam (HSM, 19 in Fig. 10.1), Meneguzer Eyesus (ME, 17 in Fig. 10.1), Wenechet (W, 9 in Fig. 10.1) church forests, and Mimusops kummel at Emashenekure Giworegis (ES, 7 in Fig. 10.1) church forest were some of the species that showed I-shaped pattern.
J shape was composed of small numbers of individuals at the lowest classes and gradually increasing numbers at the highest diameter classes, and this pattern was represented by Juniperus procera at Wej Aregawi (WA, 22 in Fig. 10.1), Olea europaea at Deber Kusekuam (DK, 18 in Fig. 10.1), and Gebesiwit Mariyam (Gma, 10 in Fig. 10.1) church forests. The irregular shape was formed when there was a complete absence of individuals in some diameter classes and a fair representation of individuals in other classes. Juniperus procera exhibited irregular-shaped pattern at Emashenekure Giworegis (ES, 7 in Fig. 10.1), Gebesiwit Mariyam (Gma, 10 in Fig. 10.1), Kirekus (K, 12 in Fig. 10.1), Kulela Mesekel (KM, 4 in Fig. 10.1), Siraba Mariyam (SiM, 24 in Fig. 10.1), Shena Tekelehayemanot (ST, 14 in Fig. 10.1), Zajor Mikael (ZA, 6 in Fig. 10.1), and Zahara Mikael (ZM, 11 in Fig. 10.1) church forests. Generally, of the total 57 species assessed, 28 and 21 revealed irregular-shaped and I-shaped structures, respectively, while only five and three showed broken reversed J-shaped and J-shaped structures, respectively. However, a single species exhibited different diameter class distribution patterns across the different church forests. For example, the diameter class distribution for Mimusops kummel was I shaped at Emashenekure Giworegis (ES, 7 in Fig. 10.1), broken reversed J shaped at Hager Selam Mariyam (HSM, 19 in Fig. 10.1), and irregular shaped at Wenechet (W, 9 in Fig. 10.1) church forests (Fig. 10.3).
3.3 Species Richness and Stand Structure of Exotic Species
Of the total 115 woody species, 18 were exotic species (one climber, three shrubs, and 14 trees). Exotic species occurred at 19 church forests with the range from one to four species per church forest. Eucalyptus camaldulensis was recorded at nine church forests (38%), followed by Cupressus lusitanica at seven church forests (29%), and Grevillea robusta at six church forests (25%). The remaining exotic species occurred at ≤ 3 church forests. Relatively high densities of Eucalyptus spp. ranging from 13 to 1925 individuals ha−1 were recorded. Of the exotic species, Eucalyptus camaldulensis had the highest IVI in Kirekus (K, 12 in Fig. 1, IVI = 66%), Qere Mikael (QM, 15 in Fig. 1, IVI = 59%), Shena Tekelehayemanot (ST, 14 in Fig. 1, IVI = 118%), and Tiwaz Abo (TA, 21 in Fig. 1, IVI = 109%) church forests. Grevillea robusta (IVI = 25%) and Cupressus lusitanica (IVI = 21%) exhibited the highest IVI values in Aba Gerima Mariyam (AG, 2 in Fig. 10.1) and in Kirekus (K, 12 in Fig. 10.1) church forests, respectively.
3.4 Species Richness of Seedlings
The seedlings of a total of 62 species (57 indigenous and five exotic) were identified, in the 24 church forests. Of these, 80%, 16%, and 4% were seedlings of trees, shrubs, and climbers, respectively. Species richness of seedlings varied among the 24 church forests, ranging from 2 to 31 species per church forest with a mean and standard error of 18 ± 1.61. Ruta chalepensis was the only species found at the seedling stage. Zahara Mikael (ZM, 11 in Fig. 10.1) church forest had the highest total number of seedlings (391,200 ha−1), while the lowest number of seedlings (200 ha−1) was recorded in Delemo Tekelhayemanot (DT, 13 in Fig. 10.1) church forest (Fig. 10.4). Among the total woody species recorded in the church forests, Diospyros abyssinica had the highest number of seedlings (38%), followed by Mimusops kummel (13%). In general, thirty-three species (29 indigenous and four exotic) exhibited low densities of seedlings 100–1700 ha−1 (53%), whereas four (three indigenous and one exotic), seven (all indigenous), and eighteen (all indigenous) species had 2100–3700 (7%), 4200–5900 (11%) and ≥7600 (29%) individuals ha−1, respectively.
Total number of seedlings recorded in each studied church forest in southeast of Lake Tana (see full names of the churches in Table 10.1)
4 Discussion
4.1 Species Richness, Evenness, and Diversity
In the present study, a total of 115 woody species were identified in the 24 church forests. Of the 24 church forests, four church forests (Emashenekure Giworegis, Gebesiwit Mariyam, Wej Aregawi, and Zahara Mikael) were already assessed by Wassie et al. (2010). The assessment methods were relatively similar to this study. However, except for Zahara Mikael (ZM, 11 in Fig. 10.1) church forest, the numbers of families and woody species we recorded were lower than what Wassie et al. (2010) found ten years earlier (Fig. 10.5). The continued presence of relatively high species richness at Zahara Mikael church forest was likely due to construction of stone wall since 2014, and this could avoid disturbance and increase the probability of recruitment of new seedlings. The reduction in number of woody species in the remaining three church forests could be a sign of increased disturbance from anthropogenic pressures. Among the different pressures, expansion of graveyard, second church buildings inside the church forest, plantation of exotic species, and free grazing were the major factors.
Comparison of the number of families and species between results from Wassie et al. (2010) and the current study. The four church forests were Emashenekure Giworegis (ES, 7 in Fig. 10.1), Gebesiwit Mariyam (Gma, 10 in Fig. 10.1), Wej Aregawi (WA, 22 in Fig. 10.1), and Zahara Mikael (ZM, 11 in Fig. 10.1)
The negative effects of anthropogenic disturbances, including free grazing on woody species of church forests, particularly on seedlings, were reported by Wassie et al. (2009a). The level of disturbances, such as construction of additional church buildings, grave houses, and small house buildings where people organize themselves into associations to celebrate a chosen patron saint together (mehabirs) and grazing, has continuously increased in the study area (Cardelús et al. 2017; Orlowska and Klepeis 2018). Although, some species have always been rare, the presence of many species with IVI < 5% per church forest suggests that the disturbance is still ongoing. However, the presence of old remnant trees (i.e., rarely recorded trees with low IVI values) and those which are economically and ecologically important species, such as Juniperus procera, Olea europaea, Prunus africana, Podocarpus falcatus, Ekebergia capensis, Mimusops kummel, and Cordia africana in the compounds of churches, highlights the importance of church forests in southeast of Lake Tana for indigenous tree species and wildlife conservation similar to other sacred forests in the world (Bhagwat and Rutte 2006).
The Pilou evenness value ranges from 0 to 1, and values close to 1 indicate even representation of individuals of the occurring species in the area (Help et al. 1998). Except for Wenechet (W, 9 in Fig. 10.1) (J = 0.52) and Wej Aregawi (WA, 22 in Fig. 10.1) (J = 0.58) church forests, all church forests in the present study had evenness values (0.63 to 0.91), indicating even representation of the individuals of the occurring species in each church forest. Moreover, except for Qere Mikael (H′ = 1.78), Wenechet (H′ = 1.64), and Wej Aregawi (H′ = 1.97) church forests, all church forests had Shannon diversity greater than two, which indicates medium to high species diversity (Giliba et al. 2011). The highest and lowest Shannon diversity values were recorded in Kudese Minas (Kmi, 3 in Fig. 10.1) and Wenechet (W, 9 in Fig. 10.1) church forests, respectively. In the present study, when more church forests were included, the number of newly described species increased (Fig. 10.6). Therefore, since we studied only 24 church forests out of many available church forests in the larger region of Lake Tana, more species richness and diversity could be recorded when more church forests are studied, owing to increases in the environmental heterogeneity. A similar explanation was also given by Wassie (2007).
4.2 Demographic Structure of Woody Species in Church Forests
Investigating the IVI and FIV values are significant to understand the ecological importance of the species and families, respectively. The IVI and FIV values are good indicators to understand the current condition of the church forests since they provide important insights into the basal area, abundance, frequency, and relative diversity of the species in a particular forest area. In this study, fleshy-fruited tree species had the highest IVI in most church forests. The fruits of these species are indehiscent and consumed by a variety of birds and mammals. As these animals, particularly birds, move from one church forest to another before defecating the seeds, they could further promote seed dispersal between church forests. Therefore, this might be the probable reason why the fleshy-fruited tree species had high IVI values in different church forests. Furthermore, the highest IVI values of these species were due to their high frequency, abundance, and basal area values. The importance of woody species in a given area can be better explained by their basal area than simple stem count (Lamprecht 1989; Bekele 1994; Abyot et al. 2014; Meragiaw et al. 2018). The species that had the highest IVI values had large basal areas and, hence, could play a significant contribution to biodiversity conservation by providing habitat and food for frugivore birds and mammals as well as bee forage. However, several woody species at each church forest had low IVI values, indicating that most species had small basal areas, small numbers of individuals, and are generally rare in the church forests. For example, ≥50% of the woody species in seven church forests had IVI < 5%. The different anthropogenic disturbances in the study area could be the main reasons for the presence of many species with low IVI values, and this could likely affect their ecological significance. The rarity of these species could also be caused by other factors, such as their poor dispersal ability and competition for nutrients or other resources (Hubbell et al. 2001; Engelbrecht et al. 2007). Thus, woody species with a low IVI values require high conservation priority to maintain their composition and diversity.
The Fabaceae family had the highest FIV at five church forests, Sapotaceae at four church forests, and Rubiaceae at two church forests. Teketay et al. (2018) also reported that Fabaceae had the highest FIV value. The main reason why this family had the highest FIV value is likely due to its wide range of ecological adaptations. Although it was represented by only one species and small numbers of individuals, Sapotaceae had the second-highest FIV value in the study area. This is due to the fact that most individuals of this species had large basal area. Species with a large basal area (even though the density of the species is low) had more structural complexity and provide a lot of habitat for various species and also serve as shade for animals and human beings. Hence, Sapotaceae species could have significant ecological importance. They could also provide vital social values, as large trees are culturally and spiritually important in Ethiopian (Orlowska and Klepeis 2018).
The difference in canopy cover among the church forests was due to the relatively higher dominance of big old canopy tree species in some church forests than others. For example, the high canopy covers at Kudese Minas (Kmi, 3 in Fig. 10.1) and Robit Bat (RB, 1 in Fig. 10.1) church forests were due to the relative dominance of big old canopy trees such as Albizia schimperiana, Croton macrostachyus, Millettia ferruginea, and Mimusops kummel. However, Tiwaz Abo (TA) church forest had small canopy cover due to the relative dominance of Eucalyptus camaldulensis individuals, kept small by frequent harvesting or coppicing. According to Sabine and Miehe (1994), forests with > 80% canopy cover are considered as closed forests. Therefore, Kudese Minas (Kmi, 3 in Fig. 10.1) and Robit Bat (RB, 1 in Fig. 10.1) church forests are closed forests. However, in the present study, the majority of church forests had canopy cover <80% with a mean canopy cover of 59%, which implies that most church forests in the study area are open forests. The presence of low canopy cover in the church forests is an indication of the degradation of the primary forests to a shrubland. This could be due to anthropogenic disturbances, such as selective cutting of large canopy trees or natural disturbance due to the death of large canopy trees without any replacement. Although the death of large canopy trees in a natural system can be filled in by young trees, this was not the case in the studied church forests, probably, due to the succession being blocked by grazing or other disturbances, such as soil degradation. On the other hand, canopy cover is an indicator of the microclimate conditions that determine the species composition and structure. For example, temperature decreases with increasing canopy cover, with the greatest cooling when canopy cover exceeds 40% (Ziter et al. 2019), while light, soil water, and airflow exchange increases with decreasing canopy cover (Muscolo et al. 2014). Therefore, understanding the canopy cover is significant to conserve the old growth forests.
Based on the diameter class distribution analyses of the selected species, four types of demographic structure were revealed. Of the total 57 species structure, 49%, 37%, 9%, and 4% showed irregular-shaped, I-shaped, broken reversed J-shaped, and J-shaped patterns, respectively. The irregular-shaped, I-shaped, and J-shaped patterns represent abnormal demographic structure due to the removal of woody species at different diameter classes. The underlying reasons for such kind of demographic structure could be related to overgrazing at young stages of plants and removal of vegetation for burial activities. Celebration of some spiritual activities inside the church forests could have a negative effect on the recruitment of seedlings due to trampling. Additionally, activities such as selective cutting of trees for construction of church buildings, poor reproduction of old trees, and loss of seeds to predators could likely cause the abnormal population structure (Abyot et al. 2014). However, broken reversed J-shaped demographic structures represent relatively a healthy forest.
4.3 Stand Structure of Exotic Species
Eucalyptus camaldulensis had the highest IVI value in 17% of church forests in the study area. This species is mainly planted inside or around the church forests matrix for different purposes, such as construction materials, firewood, timber, ornamental, and also windbreak (Bekele-Tesemma 2007). Similarly, Burkhard et al. (2012) reported that this species was planted in the agricultural matrix around the church forests due to its multiple benefits for local communities. Eucalyptus camaldulensis is a strong competitor within the native tree community due to its fast growth and resilience to disturbances. Thus, the relatively high dominance and density of Eucalyptus spp. in the study area could negatively affect the ecological diversity and structure of the indigenous woody species. As a backlash to its strong wood provisioning performance, it can cause threats to the ecological conditions due to decreased understory cover which lead to increased soil loss rates (Nyssen et al. 2004). It also has a high water consumption rate compared with the other forest communities (Bekele-Tesemma 2007) and also reduces the regeneration potential of native tree species compared with other tree canopies (Thijs et al. 2014a). Plantations of Eucalyptus spp. were higher in recently established church forests compared with the old church forests within the study area (field observation). For example, the relative density of Eucalyptus camaldulensis was high (41%) in Tiwaz Abo (TA, 21 in Fig. 10.1), which was established in 2010 and 0.3% in Meneguzer Eyesus (ME, 17 in Fig. 10.1), which was established in 1682. This might be because most of the recently established churches are constructed at most degraded areas, and the local people prefer to plant exotic and fast-growing species than indigenous plants. Therefore, considering the ecological threat of exotic species plantation, in general, and Eucalyptus camaldulensis plantation, in particular, emphasis should be given for re-introduction of indigenous species in recently established church forests.
4.4 The Regeneration Status of Church Forests in Southeast of Lake Tana
The regeneration status of each species can be determined by looking at the pattern of their seedlings, saplings, and matured stage (Malik and Bhatt 2016). To sustain their future regeneration status, woody species should successfully complete their life cycle. When a species is present in both mature and sapling or seedling stages, it indicates the good regenerating status of the species, whereas when the species is present either in mature stage or sapling or seedling stages only, it may show an extinction debt or a colonization credit, respectively (Thijs et al. 2014b). In the present study, most woody species showed poor regeneration status, which exhibit potential extinction debt. Of the total 115 woody species, seedlings were recorded for 62 woody species with 33 species represented only by 100–1700 seedlings ha−1. However, Zahara Mikael (ZM, 11 in Fig. 10.1) church forest had a large number of seedlings (391,200 ha−1). The possible reason for this is because Zahara Mikael (ZM, 11 in Fig. 10.1) has been fenced with stone walls since 2014, and, thus, this could avoid grazing, creation of new paths and clearings, which are the major factors for seedling regeneration.
Of the species that had the highest IVI values, only few species at few church forests showed good regeneration status. For example, Mimusops kummel had higher numbers of seedlings and saplings than mature trees in Gebesiwit Mariyam (Gma, 10 in Fig. 10.1), Meneguzer Eyesus (ME, 17 in Fig. 10.1), Robit Bat (RB, 1 in Fig. 10.1), and Zahara Mikael (ZM, 11 in Fig. 10.1) church forests, suggesting that the species has a good regeneration status. However, one or two of the growth stages were missed or lower numbers of seedlings and saplings than the number of mature trees were recorded in many woody species, such as Ficus vasta in Gebesiwit Mariyam (Gma, 10 in Fig. 10.1), Kulela Mesekel (KM, 4 in Fig. 10.1), Seneko Medaniyalem (SeM, 23 in Fig. 10.1), Tiwaz Abo (TA, 21 in Fig. 10.1), Wenechet (W, 9 in Fig. 10.1), and Wej Aregawi (WA, 22 in Fig. 10.1) church forests, which indicate their poor regeneration status.
The presence of low numbers of seedlings could pose a threat by reducing the viable population size, which, in turn, affects the ecological function of church forests. The poor regeneration status of woody species was also corroborated by our demographic analysis. Of the selected species for structural analyses, only 5% exhibited the broken reversed J-shaped structure, which presents relatively a healthy forest with good natural regeneration and recruitment potential while the rest (95%) of the species showed abnormal population structures that confirmed the poor regeneration status of the species. The poor regeneration status of woody species in church forests in southeast of Lake Tana is primarily attributed to anthropogenic pressures, such as free grazing, vegetation removal for different purposes, and plantation of fast-growing exotic species. However, climate change and other natural factors, such as physical and chemical soil degradation, could also be considered responsible for the poor regeneration status of the woody species in the study area. Effects of anthropogenic and natural disturbance on the regeneration status of forests have been reported by previous studies (Dai et al. 2002; Tesfaye et al. 2002; Wassie et al. 2009b; Abyot et al. 2014; Meragiaw et al. 2018; Maua et al. 2020).
5 Conclusions
Church forests in the study area are important for regional biodiversity conservation since they are the only remnant forests and the last option to harbor woody species and their associated fauna and flora. This study characterized the species and structural composition of woody species at 24 church forests in southeast of Lake Tana. The diversity indices revealed that most of the church forests contained high species diversity. The ecologically and culturally most important woody species, such as Juniperus procera, Olea europaea, Ficus vasta, and families in the church forests, which were identified by means of their IVI and FIV values, have to be prioritized for conservation. Furthermore, conservation activities are also urgently required for the rare indigenous species and families that had low IVI and FIV values in all forests. For these species, the church forests are often the only safe haven of survival, and action is required to prevent their regional extermination.
The demographic structure for majority of the species showed I-shaped, irregular-shaped, and J-shaped patterns, suggesting poor regeneration status. Human influence, such as plantation of exotic species, affects the structural composition and regeneration status of church forests. Additionally, there were several species at different church forests that had lower numbers of seedlings and saplings than mature trees, which confirm the low regeneration and recruitment potential of the forests. Woody species with low IVI values and those with poor regeneration status should be prioritized for conservation. Since other patch-level and landscape-level factors could possibly affect species and structural composition of church forests, due attention should be given to such factors in future studies. Additionally, the causes of poor regeneration status of woody species and the possible options to increase their natural regeneration should be studied.
References
Abiyot B, Sebsebe D, Zerihun W, Motuma D (2017) Woody species composition and structure of Kuandisha afromontane forest fragment in northwestern Ethiopia. J for Res 28(2):343–355
Abyot D, Teshome S, Ensermu K, Abiyou T (2014) Diversity, structure and regeneration status of the woodland and riverine vegetation of Sire Beggo in Gololcha District Eastern Ethiopia. Momona Ethiop J Sci 6(1):70–96
Aerts R, Van Overtveld K, Haile M, Hermy M, Deckers J, Muys B (2006) Species composition and diversity of small Afromontane forest fragments in northern Ethiopia. Plant Ecol 187(1):127–142
Aerts R, Van Overtveld K, November E, Wassie A, Abiyu A, Demissew S, Daye DD, Giday K, Haile M, TewoldeBerhan S, Teketay D, Teklehaimanot Z, Binggeli P, Deckers J, Friis I, Gratzer G, Hermy M, Heyn M, Honnay O, Paris M, Sterck FJ, Muys B, Bongers F, Healey JR (2016) Conservation of the Ethiopian church forests: threats, opportunities and implications for their management. Sci Total Environ 551–552:404–414
Alelign A, Teketay D, Yemshaw Y, Edwards S (2007) Diversity and status of regeneration of woody plants on the peninsula of Zegie, Northwestern Ethiopia. Trop Ecol 48(1):37–49
Aynekulu E, Denich M, Tsegaye D, Aerts R, Neuwirth B, Boehmer HJ (2011) Dieback affects forest structure in a dry Afromontane forest in Northern Ethiopia. J Arid Environ 75(5):499–503
Bekele T (1994) Phytosociology and ecology of a humid Afromontane forest on the Central Plateau of Ethiopia. J Veg Sci 5(1):87–98
Bekele-Tesemma A (2007) Useful trees and shrubs of Ethiopia: Identification, Propagation and Management for 17 Agroclimatic Zones. In: Bo T, Ensermu K, Sebsibe D, Maundu P (eds) RELMA in ICRAF Project, Nairobi Kenya
Bhagwat SA, Rutte C (2006) Sacred groves: potential for biodiversity management. Front Ecol Env 4(10):519–524
Bishaw B (2001) Deforestation and land degredation in the Ethiopian highlands: a strategy for physical recovery. Northeast Afr Stud 8(1):7–25
Bongers F, Wassie A, Sterck F, Bekele T, Teketay D (2006) Ecological restoration and church forests in Northern Ethiopia. J. Dry Lands 1(1):35–44
Burkhard B, Kroll F, Nedkov S, Müller F (2012) Mapping ecosystem service supply, demand and budgets. Ecol Indic 21:17–29
Cardelús CL, Scull P, Wassie Eshete A, Woods CL, Klepeis P, Kent E, Orlowska I (2017) Shadow conservation and the persistence of sacred church forests in Northern Ethiopia. Biotropica 49(5):726–733
Cardelu’s C, Woods C, Bitew MA, Dexter S, Scull P, Tsegay B (2019) Human disturbance impacts the integrity of sacred church forests, Ethiopia. PLoS One 14(3):1–14
Contreras-Hermosilla (2000) The underlying causes of forest decline the CGIAR system. In CIFOR Occas. Paper, pp 1–25
Dai N, Kenji S, Akiko S (2002) Seedling establishment of deciduous trees in various topographic positions. J Veg Sci 13:35–44
Darbyshire I, Lamb H, Umer M (2003) Forest clearance and regrowth in northern Ethiopia during the last 3000 years. Holocene 13(4):537–546
Dudley N, Higgins-Zogib L, Mansourian S (2009) The links between protected areas, faiths, and sacred natural sites. Conserv Biol 23(3):568–577
Edwards S, Tadesse M, Hedberg I (1995) Flora of Ethiopia and Eritrea Canellaceae to Euphorbiaceae. In: Sue E, Mesfin T, Inga H (eds) Natl. Herb. Ethiop. The National Herbarium Addis Ababa University, Addis Ababa, and Department of Systematic Botany, Uppsala University,Uppsala, Sweden
Edwards S, Demissew S, Hedberg I (1997) Flora of Ethiopia and Eritrea Hydrocharitaceae to Arecaceae. In: Sue E, Sebsebe D, Inga H (eds) The National Herbarium Addis Ababa University, Addis Ababa, and Department of Systematic Botany, Uppsala University,Uppsala, Sweden
Edwards S (1997) Flora of Ethiopia. In: Inga H, Sue E (eds) The national herbarium Addis Ababa University, Addis Ababa, and Department of Systematic Botany, Uppsala University,Uppsala, Sweden
Engelbrecht BMJ, Comita LS, Condit R, Kursar TA, Tyree MT (2007) Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447
EOTC (no date) Metsahef Senkesar and Areganone holy books
Frankl A, Nyssen J, Adgo E, Wassie A, Scull P (2019) Can woody vegetation in valley bottoms protect from gully erosion? Insights using remote sensing data (1938–2016) from subhumid NW Ethiopia. Reg Environ Change 19(7):2055–2068
Gashaw T, Terefe H, Soromessa T, Ahmed S (2015) Riparian areas rehabilitation and restoration: an overview. Point J Agric Biotechnol Res 1(2):55–63
Gebeyehu G, Soromessa T, Bekele T, Teketay D (2019) Species composition, stand structure, and regeneration status of tree species in dry Afromontane forests of Awi Zone, Northwestern Ethiopia. Ecosyst Health Sustain 5(1):199–215
Giliba RA, Boon EK, Kayombo CJ, Musamba EB (2011) Species composition, richness and diversity in Miombo Woodland of Bereku Forest Reserve Tanzania. Biodiversity 2(1):1–7
Hailu BT, Maeda EE, Heiskanen J, Pellikka P (2015) Reconstructing pre-agricultural expansion vegetation cover of Ethiopia. Appl Geogr 62:357–365
Hedberg I, Edwards S, Nemomissa S (2003) Flora of Ethiopia and Eritrea Apiaceae to Dipsaceae. In: Inga H, Sue E, Sileshi N (eds) The National Herbarium Addis Ababa University, Addis Ababa, and Department of Systematic Botany, Uppsala University,Uppsala, Sweden
Help CHR, Herman PMJ, Soetaert K (1998) Indices of diversity and evenness. Oceanis 24(4):61–87
Hokkanen PJ, Kouki J, Komonen A (2009) Nestedness, SLOSS and Conservation Networks of Boreal Herb-Rich Forests Nestedness, SLOSS and conservation networks of boreal herb-rich forests. Appl Veg Sci 12(3):295–303
Hubbell SP, Ahumada JA, Condit R, Foster RB (2001) Local neighborhood effects on long-term survival of individual trees in a neotropical forest. Ecol Res 16:859–875
Kyalangalilwa B, Boatwright JS, Daru BH, Maurin O, Bank MVD (2013) Phylogenetic position and revised classification of Acacia s. l. ( Fabaceae: Mimosoideae ) in Africa, including new combinations in Vachellia and Senegalia. Bot J Linn Soc 172:500–523
Lamprecht H (1989) Silviculture in the Thopics. In: Tropical forestry ecosystems. Their Tree species possibilities methods their long-term utilization. Technical Cooperation Federal Republic of Germany
Lemma H, Admasu T, Dessie M, Fentie D, Deckers J, Frankl A, Poesen J, Adgo E, Nyssen J (2018) Revisiting lake sediment budgets: how the calculation of lake lifetime is strongly data and method dependent. Earth Surf Process Landforms 43(3):593–607
Logan WEM (1946) An introduction to the forests of central and southern Ethiopia. Imp For Inst 24:65
Malik ZA, Bhatt AB (2016) Regeneration status of tree species and survival of their seedlings in kedarnath wildlife sanctuary and its adjoining areas in Western Himalaya. Trop Ecol 54(4):677–690
Maua JO, MugatsiaTsingalia H, Cheboiwo J, Odee D (2020) Population structure and regeneration status of woody species in a remnant tropical forest: a case study of South Nandi forest Kenya. Glob Ecol Conserv 21:e00820. https://doi.org/10.1016/j.gecco.2019.e00820
Meragiaw M, Woldu Z, Martinsen V, Singh BR (2018) Woody species composition and diversity of riparian vegetation along the Walga River Southwestern Ethiopia. PLoS ONE 13(10):1–18
Morgan R, KarimAly K, Asfaw Z (2018) Human ecology of sacred space: Church forests in the highlands of Northwestern Ethiopia. Enviromental Conserv 45(3):291–300
Mori SA, Boom Baian M, de Carvalho AM, dos Santos TS (1983) Sothern Bahian moist forests. New York Bot Gard 49(2):155–232
Mueller DD, Ellenberg H (1975) The count-plot method and plotless sampling techniques. In: Aims methods vegetation ecology, pp 158–159
Muscolo A, Bagnato S, Sidari M, Mercurio R (2014) A review of the roles of forest canopy gaps. J for Res 25(4):725–736
Nyssen J, Poesen J, Moeyersons J, Deckers J, Haile M, Lang A (2004) Human impact on the environment in the Ethiopian and Eritrean highlands—a state of the art. Earth-Science Rev 64(3–4):273–320
Orlowska I, Klepeis P (2018) Ethiopian church forests: a socio-religious conservation model under change. J. East. African Stud 12(4):674–695
Peel MC, Finlayson BL, Mcmahon TA, Peel MC, Finlayson BL, Updated TAM (2007) Updated world map of the Köppen-Geiger climate classification To cite this version: HAL Id: hal-00298818 Updated world map of the K oppen-Geiger climate classification. Hydrol Earth Syst Sci Discuss 4(2):439–473
Peet RK (1974) The measurement of species diversity. Annu Rev Ecol Syst 5(1):285–307
Reynolds T, Collins CD, Wassie A, Liang J, Briggs W, Lowman M, Sisay TS, Adamu E (2017) Sacred natural sites as mensurative fragmentation experiments in long-inhabited multifunctional landscapes. Ecography (cop.) 40:144–157
Reynolds T, Sisay ST, Wassie A, Lowman M (2015) Sacred natural sites provide ecological libraries for landscape restoration and institutional models for biodiversity conservation. Policy Br 2015 U.N. Glob Sustain Dev Report, pp 1–4. Available at: https://sustainabledevelopment.un.org/content/documents/614059-Sacrednaturalsitesprovideecologicallibrariesforlandscaperestorationandinstitutionalmodelsforbiodi.pdf
Sabine G, Miehe G (1994) Ericaceous forests and heathlands in the Bale Mountains of South Ethiopia: ecology and man’s impact. T. Warnke Verlag, Hamburg
Scull P, Cardelús CL, Klepeis P, Woods CL, Frankl A, Nyssen J (2017) The resilience of Ethiopian Church forests: interpreting aerial photographs. Land Degradation Dev 1938–2015458:450–458
Song C, Nigatu L, Beneye Y, Abdulahi A, Zhang L (2018) Mapping the vegetation of the Lake Tana basin, Ethiopia, using Google Earth images, pp 2033–2041
Tadele D, Lulekal E, Damtie D, Assefa A (2014) Floristic diversity and regeneration status of woody plants in Zengena Forest, a remnant montane forest patch in northwestern Ethiopia. J For Res 25(2):329–336
Teketay D, Kashe K, Madome J, Kabelo M, Neelo J, Mmusi M (2018) Enhancement of diversity , stand structure and regeneration of woody species through area exclosure: the case of a mopane woodland in northern Botswana. Ecol Process 7(5). https://doi.org/10.1186/s13717-018-0116-x
Tesfaye G, Teketay D, Fetene M (2002) Regeneration of fourteen tree species in Harenna forest, southeastern Ethiopia. Flora 197:461–474
Thijs KW, Aerts R, Van de Moortele P, Musila W, Gulinck H, Muys B (2014a) Contrasting cloud forest restoration potential between plantations of different exotic tree species. Restor Ecol 22(4):472–479
Thijs KW, Aerts R, Musila W, Siljander M, Matthysen E, Lens L, Pellikka P, Gulinck H, Muys B (2014b) Potential tree species extinction, colonization and recruitment in Afromontane forest relicts. Basic Appl Ecol 15(4):288–296
Wassie A, Teketay D, Powell N (2005) Church forests provide clues to restoring ecosystems in the degraded highlands of Northern Ethiopia. Ecol Restor 23(2):115–144
Wassie A, Teketay D, Powell N (2005) Church forests in north gonder administrative zone, northern Ethiopia. For Trees Livelihoods 15:349–373
Wassie A, Sterck FJ, Teketay D, Bongers F (2009) Effects of livestock exclusion on tree regeneration in church forests of Ethiopia. For Ecol Manage 257(3):765–772
Wassie A, Sterck FJ, Teketay D, Bongers F (2009) Tree regeneration in church forests of Ethiopia: effects of microsites and management. Biotropica 41(1):110–119
Wassie A, Sterck FJ, Bongers F (2010) Species and structural diversity of church forests in a fragmented Ethiopian Highland landscape. J Veg Sci 21(5):938–948
Wassie (2007) Ethiopian church forests opportunities and challenges for restauration. Doctoral Dissertation, Wageningen University Wageningen, Netherlands. https://doi.org/10.1017/S0014479702003046
Woldemedhin T, Teketay D (2016) Forest conservation tradition of the Ethiopian Orthodox Tewahdo Church: a case study in West Gojjam Zone, north-western Ethiopia. Symb Bot Ups 38(16):57–73
Woods CL, Cardelús CL, Scull P, Wassie A, Baez M, Klepeis P (2017) Stone walls and sacred forest conservation in Ethiopia. Biodivers Conserv 26(1):209–221
Zegeye H, Teketay D, Kelbessa E (2011) Diversity and regeneration status of woody species in Tara Gedam and Abebaye forests, northwestern Ethiopia. J for Res 22(3):315–328
Ziter CD, Pedersen EJ, Kucharik CJ, Turner MG (2019) Scale-dependent interactions between tree canopy cover and impervious surfaces reduce daytime urban heat during summer. Proc Natl Acad Sci USA 116(15):7575–7580
Acknowledgements
This study received financial support from VLIR-UOS, Belgium, through the VLIR-IUC Interuniversity cooperation with Bahir Dar University, Ethiopia (BDU-IUC). We are grateful to all EOTC priests, monks, students, and local communities for access to the church forests, technical support and field assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix 1: Species Name, Family, and the Relative Frequency of Occurrence in 24 Church Forests Southeast of Lake Tana, Ethiopia
Appendix 1: Species Name, Family, and the Relative Frequency of Occurrence in 24 Church Forests Southeast of Lake Tana, Ethiopia
Species name | Family | Relative frequency of occurrence (%) |
|---|---|---|
Capparis tomentosa L | Capparidaceae | 92 |
Justicia schimperiana (Hochst.ex Nees) T. Anders | Acanthaceae | 92 |
Cordia africana Lam | Boraginaceae | 83 |
Croton macrostachyus Del | Euphorbiaceae | 83 |
Grewia ferruginea Hochst. ex A. Rich | Tiliaceae | 83 |
Millettia ferruginea (Hochst.) Bak | Fabaceae | 79 |
Maytenus arbutifolia (A.Rich.) Wilczek | Celastraceae | 71 |
Mimusops kummel A. DC | Sapotaceae | 71 |
Albizia schimperiana Oliv | Fabaceae | 67 |
Calpurnia aurea (Ait.) Benth | Fabaceae | 63 |
Carissa spinarum L | Apocynaceae | 63 |
Celtis africana Burm.f | Ulmaceae | 63 |
Pavetta abyssinica Fresen | Rubiaceae | 63 |
Juniperus procera Hochst. ex Endl | Cupressaceae | 58 |
Teclea nobilis Del | Rutaceae | 58 |
Ficus thonningii Blume | Moraceae | 54 |
Premna schimperia Engl | Lamiaceae | 54 |
Clausena anisata (Willd.) Benth | Rutaceae | 50 |
Vernonia myriantha Hook.f | Asteraceae | 50 |
Acanthus sennii Chiov | Acanthaceae | 42 |
Ocimum lamiifolium Hochst. ex Benth | Lamiaceae | 42 |
Olea europaea L | Oleaceae | 42 |
Coffea arabica L | Rubiaceae | 38 |
Dracaena steudneri Engl | Dracaenaceae | 38 |
Eucalyptus camaldulensis Dehnh | Myrtaceae | 38 |
Diospyros abyssinica (Hiern) F.White | Ebenaceae | 33 |
Vernonia amygdalina Del | Asteraceae | 33 |
Cupressus lusitanica Mill | Cupressaceae | 29 |
Entada abyssinica Steud.ex.A.Rich | Fabaceae | 29 |
Ficus vasta Forssk | Moraceae | 29 |
Ricinus communis L | Euphorbiaceae | 29 |
Ritchiea albersii Gilg | Capparidaceae | 29 |
Bersama abyssinica Fresen | Melianthaceae | 25 |
Euclea racemosa Murr | Ebenaceae | 25 |
Grevillea robusta R.Br | Proteaceae | 25 |
Rhus quartiniana A.Rich | Anacardiaceae | 25 |
Dovyalis abyssinica (A.Rich.) Warb | Flacourtiaceae | 21 |
Flueggea virosa (Willd.) Voigt | Euphorbiaceae | 21 |
Osyris quadripartita Decn | Santalaceae | 21 |
Podocarpus falcatus (Thunb.) R. B. ex. Mirb | Podocarpaceae | 21 |
Rhus vulgaris Meikle | Anacardiaceae | 21 |
Rothmannia urcelliformis (Hiern) Robyns | Rubiaceae | 21 |
Schrebera alata (Hochst.) Welw | Oleaceae | 21 |
Senna singueana (Del.) Lock | Fabaceae | 21 |
Acokanthera schimperi (A. DC.) Schweinf | Apocynaceae | 17 |
Arundo donax L | Poaceae | 17 |
Ekebergia capensis Sparrm | Meliaceae | 17 |
Euphorbia tirucalli L | Euphorbiaceae | 17 |
Ilex mitis (L.) Radlk | Aquifoliaceae | 17 |
Acanthus pubescens (Oliv.) Engl | Acanthaceae | 13 |
Brucea antidysenterica J.F.Mill | Simaroubaceae | 13 |
Casuarina equisetifolia L | Casuarinaceae | 13 |
Clerodendrum myricoides (Hochst.) Vatka | Lamiaceae | 13 |
Dodonaea angustifolia L.f | Sapindaceae | 13 |
Ehretia cymosa Thonn | Boraginaceae | 13 |
Erythrina abyssinica Lam.ex DC | Fabaceae | 13 |
Flacourtia indica (Burm.f) Merr | Flacourtiaceae | 13 |
Gardenia fiorii Chiov | Rubiaceae | 13 |
Jasminum abyssinicum Hochst. ex DC | Oleaceae | 13 |
Jasminum grandiflorum L | Oleaceae | 13 |
Phytolacca dodecandra L'H'erit | Phytolaccaceae | 13 |
Pittosporum viridiflorum Sims | Pittosporaceae | 13 |
Prunus africana (Hook. f.) kalkm | Rosaceae | 13 |
Apodytes dimidiata E. Mey ex. Arn | Icacinaceae | 8 |
Azadirachta indica A.Juss | Meliaceae | 8 |
Citrus aurantifolia (Christm.) | Rutaceae | 8 |
Citrus aurantium L | Rutaceae | 8 |
Combretum molle R.Br.ex G.Don | Combretaceae | 8 |
Dombeya torrida (J.F.Gmel.) P.Bamps | Sterculiaceae | 8 |
Euphorbia abyssinica Gmel | Euphorbiaceae | 8 |
Galiniera saxifraga (Hochst.) Bridson | Rubiaceae | 8 |
Olea capensis L | Oleaceae | 8 |
Opuntia ficus-indica (L.) Miller | Cactaceae | 8 |
Piliostigma thonningii (Schumach.) Milne-Redh | Fabaceae | 8 |
Rhamnus prinoides L’ Herit | Rhamnaceae | 8 |
Rhus glutinosa A.Rich | Anacardiaceae | 8 |
Schefflera abyssinica (Hochst. ex A. Rich.) Harms | Araliaceae | 8 |
Senna didymobotrya (Fresen.) Irwin and Barneby | Fabaceae | 8 |
Vachellia abyssinica (Hochst. ex. Benth.) Kyal. and Boatwr. | Fabaceae | 8 |
Abutilon figarianum Webb | Malvaceae | 4 |
Albizia anthelmintica (A. Rich.) Brogn | Fabaceae | 4 |
Bridelia micrantha (Hochst.) Baill | Euphorbiaceae | 4 |
Buddleja polystachya Fresen | Loganiaceae | 4 |
Cassipourea malosana (Baker) Alston | Rhizophoraceae | 4 |
Clematis hirsuta Perr. and Guill | Ranunculaceae | 4 |
Croton dichogamus Pax | Euphorbiaceae | 4 |
Delonix regia (Boj.ex Hook.) Raf | Fabaceae | 4 |
Dichrostachys cinerea (L.) Wight and Arn | Fabaceae | 4 |
Dolichos sericeus E. Mey | Fabaceae | 4 |
Eucalyptus saligna Smith | Myrtaceae | 4 |
Ficus ingens (Miq.) Miq | Moraceae | 4 |
Ficus sycomorus L | Moraceae | 4 |
Gladiolus psittacinus Hook. F | Iridaceae | 4 |
Gossypium arboreum L | Malvaceae | 4 |
Hippocratea africana (Willd.) Loes | Celastraceae | 4 |
Indigofera arrecta Hochst. ex A. Rich | Fabaceae | 4 |
Lepidotrichilia volkensii (Giirke) Leroy | Meliaceae | 4 |
Mangifera indica L | Anacardiaceae | 4 |
Myrica salicifolia A. Rich | Myricaceae | 4 |
Oxyanthus speciosus Dc. | Rubiaceae | 4 |
Persea americana Mill | Lauraceae | 4 |
Phoenix reclinata Jacq | Arecaceae | 4 |
Psidium guajava L | Myrtaceae | 4 |
Sapium ellipticum (krauss) pax | Euphorbiaceae | 4 |
Senna petersiana (Bolle) Lock | Fabaceae | 4 |
Sesbania sesban (L.) Merr | Fabaceae | 4 |
Solanecio gigas (Vatke) C. Jeffrey | Asteraceae | 4 |
Solanum giganteum Jacq | Solanaceae | 4 |
Stereospermum kunthianum Cham | Bignoniaceae | 4 |
Syzygium guineense (Willd.) DC | Myrtaceae | 4 |
Urera hypselodendron (A. Rich.) Wedd | Urticaceae | 4 |
Vachellia lahai (Steud. and Hochst. ex. Benth.) Kyal. and Boatwr. | Fabaceae | 4 |
Vangueria apiculata K. Schum | Rubiaceae | 4 |
Vangueria madagascariensis Gmel | Rubiaceae | 4 |
Ximenia americana L | Olacaceae | 4 |
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Mequanint, F. et al. (2022). Woody Vegetation Composition and Structure of Church Forests in Southeast of Lake Tana, Northwest Ethiopia. In: Kindu, M., Schneider, T., Wassie, A., Lemenih, M., Teketay, D., Knoke, T. (eds) State of the Art in Ethiopian Church Forests and Restoration Options. Springer, Cham. https://doi.org/10.1007/978-3-030-86626-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-86626-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86625-9
Online ISBN: 978-3-030-86626-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)