Abstract
Key message
Annual stem CO2 efflux increases with stem wood production rates and are inhibited by daily moisture stress.
Abstract
Tropical forests cycle a large amount of CO2 between the land and atmosphere, with a substantial portion of the return flux due tree respiratory processes. However, in situ estimates of woody tissue respiratory fluxes and carbon use efficiencies (CUEW) and their dependencies on physiological processes including stem wood production (Pw) and transpiration in tropical forests remain scarce. Here, we synthesize monthly Pw and daytime stem CO2 efflux (ES) measurements over 1 year from 80 trees with variable biomass accumulation rates in the central Amazon. On average, carbon flux to woody tissues, expressed in the same stem area normalized units as ES, averaged 0.90 ± 1.2 µmol m−2 s−1 for Pw, and 0.55 ± 0.33 µmol m−2 s−1 for daytime ES. A positive linear correlation was found between stem growth rates and stem CO2 efflux, with respiratory carbon loss equivalent to 15 ± 3% of stem carbon accrual. CUEW of stems was non-linearly correlated with growth and was as high as 77–87% for a fast-growing tree. Diurnal measurements of stem CO2 efflux for three individuals showed a daytime reduction of ES by 15–50% during periods of high sap flow and transpiration. The results demonstrate that high daytime ES fluxes are associated with high CUEW during fast tree growth, reaching higher values than previously observed in the Amazon Basin (e.g., maximum CUEW up to 77–87%, versus 30–56%). The observations are consistent with the emerging view that diurnal dynamics of stem water status influences growth processes and associated respiratory metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Amazonian forest fixes more atmospheric CO2 than any other terrestrial ecosystem (Nobre et al. 2016). However, observations have suggested that the majority of tree assimilated carbon (~ 70% in the Central Amazon) is returned to the atmosphere through autotrophic respiration, resulting in low carbon use efficiency (CUE) (Amthor 2000a; Valentini et al. 2000; Chambers and Silver 2004; Rowland et al. 2014). Biologically, autotrophic respiration provides chemical energy, reducing power, and carbon skeletons needed in innumerable physiological processes including growth and development, tissue maintenance including biosynthesis of defensive and signaling compounds during abiotic and biotic stress responses, and reproduction and senescence processes (O’Leary et al. 2019). Mitochondrial respiratory activity is also critical for optimizing photosynthetic metabolism, including during periods of stress which can lead to over-reduction of mitochondria and/or chloroplasts and excessive production of reactive oxygen species (Vanlerberghe et al. 2020). Given that field observations and modeling activities have primarily focused on photosynthesis as a primary control over net primary productivity (NPP), our predictive understanding of autotrophic respiration and its dependencies on biological and environmental factors is less advanced than that of photosynthesis (Amthor 2000b; Atkin and Macherel 2009). Thus, our predictive understanding of tropical forest-atmosphere carbon exchange is incomplete, particularly with respect to tree carbon allocation into both anabolic (biosynthesis of biopolymers and metabolites used in new biomass production such as structural and non-structural carbohydrates and defense compounds) and catabolic (e.g., respiration of stored and recently assimilated substrates) pathways (Chambers and Silver 2004; Clark 2004; Feeley et al. 2007; Lloyd and Farquhar 2008; Körner 2009). However, quantifying sensitivities of key anabolic and catabolic metabolism responses to abiotic and biotic stress conditions as mediated by plant physiological processes (e.g., photosynthesis, transpiration, and growth) in diverse tropical forests, remains a grand challenge.
The importance of autotrophic respiration in the global carbon cycle is highlighted by estimates of global terrestrial autotrophic respiration of 45–55 Pg C/year of CO2 (Luyssaert et al. 2007), which is 4.5–6.2 times the average annual CO2 release from anthropogenic fossil fuel combustion over 2008–2017 (8.9–9.9 Pg C/year of CO2) (Le Quéré et al. 2018). Although highly uncertain in the tropics, autotrophic respiration can be more than 50% of total ecosystem respiration in tropical wet forests. In the central Amazon near the city of Manaus, Brazil for example, total autotrophic respiration was an estimated 68% of total ecosystem respiration (Malhi et al. 2009).
Respired CO2 within tree stems can diffuse to the atmosphere driven by the concentration gradient between the inner bark and ambient air (McGuire and Teskey 2004; Aubrey and Teskey 2009). This mechanism is known as stem CO2 efflux (ES, µmol m−2 s−1) and is estimated to represent a large but uncertain fraction of autotrophic respiration in tropical forest ecosystems (Chambers et al. 2004; Trumbore 2006; Malhi et al. 2009). ES is an important regulator of the internal fluxes of CO2 in plants and has been mathematically described by Eq. 1, where Rstem is stem respiration, ES is net stem CO2 efflux (µmol m−2 s−1), FT is net CO2 transport flux vertically through the xylem (µmol m−2 s−1), and ΔS is change in CO2 storage concentration (ppm s−1) (McGuire and Teskey 2004).
Although limited observations have been reported in the Amazon Basin, stem respiration can represent a major fraction (21.2%) of total autotrophic respiration (Malhi et al. 2009), with mean annual ES fluxes of 0.6 μmol m−2 s−1 reported for trees in the central Amazon (Chambers et al. 2004; Trumbore 2006) and the Tapajos National Forest (Nepstad 2002). Slightly higher mean annual Es fluxes of 1.0 ± 0.1 μmol m−2 s−1 were reported from the Caxiuanã National Forest reserve in the eastern Amazonia (Rowland et al. 2018). Outside of the Basin, studies in other Neotropical forest sites have reported fluxes between 1.0 and 1.5 μmol m−2 s−1 in French Guiana with a large seasonal variation during climatic transition periods (Stahl et al. 2011) and 0.83–1.24 μmol m−2 s−1 for two canopy trees in a Costa Rica forest where ES was highly positively correlated with annual wood production rates (Pw) (Ryan et al. 1994). Although not yet reported in a tropical forest, on a diurnal time scale, a growing number of greenhouse and mid-latitude field studies have shown a suppression of ES efflux during the day associated with high transpiration rates (Levy et al. 1999; Wittmann et al. 2006; Maier and Clinton 2006; Saveyn et al. 2007; Teskey and McGuire 2007; Bowman et al. 2008).
Characterizing the dependencies of ES on biological and environmental variables in diverse tropical forests is central to reducing the high uncertainties surrounding the quantitative importance of stem respiration in the tropics. We hypothesize that a direct linkage between carbon allocation to the stem and Rstem exists such that high stem growth results in an increased demand for both carbon skeletons used in new biomass construction as well as respiratory substrates for energy production to meet the increased biosynthetic demands. Thus, we hypothesize that stem growth is positively correlated stem respiration. This is because a higher stem growth rates will require an increased demand for both carbon skeletons used in new biomass construction as well as respiratory substrates for energy production to meet the increased biosynthetic demands. This hypothesis predicts that if Rstem increases due to increased energy demands associated with rapid cell division and biopolymer biosynthesis, based on Eq. 1 and assuming no change to Ft and ∆S, ES should correspondingly increase. We also hypothesize that there is a suppression of daytime respiration during the noon, despite high rates of canopy photosynthesis in combination with the higher daytime temperatures. This is because daytime xylem tension could potentially suppress the demand for respiratory substrates in the sapwood. We test these hypotheses by analyzing monthly observations of basal stem ES and stem diameter for 80 trees in stands with variable biomass accumulation rates within permanent plots of a long-term forest dynamics experiment (known as BIONTE) in central Amazon forests (Higuchi et al. 1997). We also characterized diurnal patterns of basal ES in three canopy trees at the nearby K34 tower together with diurnal observations of physiological and environmental drivers. Diurnal observations were made of crown temperature (27–31 m), vapor pressure deficit (VPD) between the upper canopy and the atmosphere (28 m), as well as sap velocity and stem ES fluxes at the base of the trees (1.7–2.1 m).
Materials and methods
Monthly observations of stem growth and CO 2 efflux in the BIONTE experiment
The field experiment was carried out at the Experimental Station of Tropical Forestry (EEST/ZF-2) 60 km northwest of Manaus/Brazil, which has 23,000 ha of undisturbed forest. We first characterized potential dependencies of ES on stem growth rates in forests north of Manaus, Brazil, using data from a selective logging experiment (BIONTE). BIONTE (the BIOmass and NuTrient Experiment) (S2º 38′ 17ʺ, W60º 09′ 25ʺ) is a long-running study of forest response to experimental logging carried out in forests north of Manaus along the ZF-2 road, and managed by scientists at Brazil’s National Institute for Amazon Research (Instituto Nacional de Pesquisa da Amazônia–INPA) (Otani et al. 2018). The experiment consists of three blocks of 24 ha forest, with treatment replicated in each block, for a total of three replicates per treatment. Selective logging treatments were conducted in the mid-1980s and comprised three levels of commercial tree removal (not total) based on species-specific basal area (T1 = 32%, T2 = 42%, T3 = 69%), and control plots with no logging (T0). A total of 12 ha (9 treatment, 3 control) were established in the central-most area of each 4-ha replicate (Fig. 1). Tree recruitment, growth and mortality were measured annually following logging in all plots, with the exception of two missing years (1994 and 1998). Tree growth was determined by the mean annual change in tree base diameter (measured at 1.3 m height, or above the buttresses). Wood density was used to estimate mean annual wood production rates, or Pw, for all trees in each replicate plot (12 ha total).
Schematic diagram of the BIONTE logging experiment showing the three experimental blocks, the layout of each 4-ha sub-blocks, and the location of the forest sample plot within each sub-block. Also shown is the location of the K34 tower where diurnal ES flux studies were conducted together with observations of transpiration and its environmental drivers
A stem respiration study was carried out to explore changes in stem CO2 efflux (ES) as a function of plot-level biomass accumulation rates, and with variation in tree growth rates and stem diameter, in 2002. Four trees were randomly selected from 5 tree growth rate classes for each treatment block, for a total of 20 trees per treatment block, or 80 trees total from the BIONTE plots with stem diameters ranging from 10 to 52 cm. All species were previously identified by comparing botanical vouchers to a herbarium reference collection organized by the Biological Dynamics of Forest Fragments Project (BDFFP) at the National Institute for Amazon Research (INPA) and also by consulting specialists for taxonomic verification (Gaui et al. 2019). Botanical identification followed the “Angiosperm Phylogeny Group—APG” (APG III, 2009) classification system. Each of the 80 trees selected for the ES study were outfitted with dendrometer bands (da Silva et al. 2002) to increase the precision and accuracy of diameter growth rate measurements. The dendrometer bands were placed on the trees at least 6 months before initiating monthly measurements from January 2002 to November 2002 (note, stem diameter measurements were not made during October 2002). The dendrometers were measured with digital calipers on the same days as the respiration measurements. Stem ES was measured using the static enclosure method described previously (Chambers et al. 2004). Briefly, an infra-red gas analyzer (LiCor 820) was operated as a closed dynamic chamber with a flow rate of 1.0 L min−1. Polyvinyl chloride (PVC) semi-cylindrical chambers (250–400 mL) were cinched to the tree stem just above the dendrometer bands at 1.3 m height using nylon straps, creating a reasonably air-tight seal. The measurement interval spanned 1–2 min, and the ES from the stem of each tree was quantified from the slope of the increase in [CO2] versus time in the static enclosure and the area of the enclosed stem (µmol CO2 m−2 s−1). Stem ES for each of the 80 trees in the BIONTE plots was determined during May, June, July, August, and September of 2002. At the end of the monthly ES measurement period, a small wooden plug was removed from the base of each tree using a tenon cutter (extracting a dowel of wood) and power drill. The wood plug was used to determine wood density D (g dry weight/mL wet volume), enabling calculation of stem growth rates in the same units as stem respiration (µmol CO2 m−2 s−1). Together with unit conversion, the average annual stem growth rate expressed as CO2 capture (Stem_growth_CO2) was calculated according to Eq. 2 where DBH_increment is the average annual diameter increment (µm day−1). By plotting the average annual ES flux versus the average annual stem growth rate expressed as a CO2 flux in µmol CO2 m−2 s−1 for each of the 80 individuals in the BIONTE plots during 2002, the slope of the regression line represents the net respiratory carbon loss to the atmosphere normalized to stem carbon accrual, while the intercept equals the maintenance respiration (RM) (McDowell et al. 1999). Finally, the average annual carbon use efficiency of woody tissue (CUEw) for each individual was estimated using Eq. 3. CUEw was estimated for each individual using both the observed average daytime ES fluxes, as well as nighttime ES fluxes which were assumed to be two times higher during the night than during the day based on results from the diurnal ES studies described in Sect. 2.2.
Continuous observations of crown temperature, sap velocity, and E s during the night and day for three trees near the K-34 tower
While the observations in the BIONTE experiment focused on average annual relationships between stem growth and ES fluxes during the day, a second study was carried out at the nearby K34 tower within the ZF2 forest preserve to evaluate potential diurnal patterns in ES, and potential correlations with temperature and transpiration. These observations took advantage of both continuous line power for real-time sensors (sap velocity, high precision dual channel CO2 gas analyzer, as well as the tower structure for collecting crown temperature and VPD from above canopy sensors mounted on the tower). In contrast to the BIONTE study (and other previous studies in the tropics) where the buildup of CO2 within a static stem enclosure was used to estimate ES ‘snapshots’ during the day, the K34 tower experiment utilized a dynamic stem enclosure where ambient air continuously entered the stem chamber with CO2 efflux estimated from the CO2 concentration difference between ambient air entering the enclosure and air exiting the stem chamber.
Due to logistical issues of working at the remote tropical rainforest site during rainy conditions (site access challenges, power failures, liquid water inside stem chamber and tubing, etc.), only 1 day and 1 day-night transition ES data set was collected for each tree individual. Trees were selected based on the crown proximity to the remote K34 tower, which enabled sap velocity, canopy temperature, and vapor pressure deficit (VPD) measurements by providing line power for the sensors and a mounting structure for sensors as a part of the Large-Scale Biosphere–Atmosphere Program (LBA). Three canopy trees including Pouteria anomala (Pires) T.D.Penn (35.3 cm of DBH, 31 m of height, and 4 cm of bark thickness), Pouteria erythrochrysa T.D.Penn (36.5 cm DBH, 29.3 m height, and 2 cm bark thickness) and Eschweilera bracteosa (Poepp. ex O.Berg) Miers (29.7 cm of DBH, 27 m of height, and 6 cm bark thickness) were selected for the study on the plateau (S 02° 36′ 32ʺ, W 60° 12′ 32.9ʺ). Each tree was within 15 m of the K34 tower such that their canopy branches were accessible from the tower. Diurnal field experiments occurred between June and October 2017 during the regular dry season. The mean value of rainfall is ~ 2500 mm year−1 with the driest months of the year concentrated from July to September (Araújo 2002).
The dynamic ES gas-exchange system consisted of ¼” O.D. Teflon tubing and a dual channel infrared gas analyzer (IRGA) configured in differential mode (Li-7000, Li-Cor Inc., Lincoln, Nebraska, USA) to determine the difference (Δ) in [CO2] between air entering (reference IRGA) and exiting (sample IRGA) an acrylic semi-cylinder chamber (324 ml in volume, 16.5 cm length and 10 cm width). The stem chamber was connected to the stem of the sample tree at 1.3 m height using a 5 cm thick foam to minimize air leaks and secured to the tree using two adjustable nylon slings. Ambient air at 0.5 m height above the ground was delivered to both the reference IRGA and the stem chamber by pumping (Laboport membrane pump, KNF Neuberger Inc., USA) from a 0.5 m3 gas mixing box, to buffer fast changes in [CO2], to the reference IRGA (100 ml min−1) using a mass flow controller (FMA3704, Omega Engineering, USA). In addition, ambient air was pumped (400 ml min−1) into the chamber using a second mass flow controller (EW-32907–67, Cole Parmer, USA). The internal pump of the Li-7000 was used to draw sample air inside the chamber into the sample IRGA (50–100 ml min−1). The excess flow entering the chamber escaped through the porous foam.
The system was calibrated each day before measurements begin with 0 ppm [CO2] using a zero-air generator (Aadco 737, Aadco Inst., USA) with a downstream soda lime cartridge to scrub any remaining CO2 from the ambient air. The calibration gas was placed into the reference IRGA and manually set to read 0 ppm. Following this, the output flow containing the 0 ppm calibration gas from the reference IRGA was delivered to the inlet of the sample IRGA. Following stabilization of the signals, the sample IRGA was manually set to ‘match’ the [CO2] of the reference IRGA. To complete the two-point calibration procedure, the same process was then repeated using a 400 ppm [CO2] calibration gas standard (Praxair Inc., USA). Validation of the system was provided by placing the chamber inside a plastic bag and verifying that the ΔCO2 was less than 5 ppm. In addition, once installed on the sample tree, validation was also obtained when the ambient air flow entering the enclosure was increased resulting in a decrease in ΔCO2, followed by a decrease in the ambient air flow entering the enclosure resulting in an increase in ΔCO2. Experimental data included [CO2] measurements from ambient air entering the dynamic stem enclosure and air exiting the enclosure were logged on a laptop computer at 1 Hz frequency continuously for up to 12 h, followed by a 1-h drying period of back flushing the tubing and Li-7000 system using dry air produced from the zero-air generator. Following the drying period, which was necessary to remove any condensed water, an additional 12 h of data was logged. ES (µmol CO2 m−2 s−1) was calculated based on Eq. 4.
where ΔCO2: difference between [CO2] in the ambient air interring the chamber and inside the chamber (µL L−1), F: ambient air flow rate entering the stem chamber (L min−1), V: molar volume of an ideal gas (24 L mol−1), t: conversion factor of time from minutes to seconds (1 min/60 s) and S: superficial stem area enclosed by the chamber (0.016 m2).
Sap velocity measurements were made every 15 min using a heat ratio sap flow sensor (SFM1, ICT international) installed at 2.1 m (P. anomala), 2.0 m (P. erythrochrysa), and 1.7 m (E. bracteosa) of height above the ground. The SFM1 sensors have three needles inserted parallel to the stem and include a heating needle that emits a rapid pulse of 20 Joules of thermal energy and two needles that determine sap temperature upstream and downstream of the heating needle at 0.75 cm and 2.25 cm of depth inside the xylem for 5 min 32 s following the heat pulse (Green et al. 2003; Christianson et al. 2017). Sap velocity (cm h−1) was calculated using the Sap Flow Tool software version 1.4.1 (Phyto-IT) from the raw sap temperature ratio data downloaded from the SFM1 sensors in the field programmed to collect data every 15 min.
Tree crown temperature measurements were made with three infrared radiometer sensors (SI-131, Apogee) installed on the K-34 tower and aimed at each tree crown (one IR sensor per tree) with 5-minute averages recorded on a data logger (CR-3000 Campbell Scientific). The IR sensors were positioned at 28.8 m height and 4.25 m distance from the P. anomala crown, 25.3 m height and 6.55 m from the P. erythrochrysa crown and 28.6 m height and 4.4 m from the E. bracteosa crown. To validate the IR measurements of crown temperature, Teflon insulated thermocouples (type T, Omega Engineering) were attached to the lower leaf surface of eight leaves in the crown of the P. anomala individual during the two diurnal experiments. The thermocouple sensors were positioned on leaves in a branch approximately 1 m from the flux tower structure at the same height as the IR sensor and connected to a temperature recorder (OM-CP_OCTTEMP-A, Omega Engineering) that registered average leaf temperatures every 15 s. In addition, atmospheric vapor pressure deficit (VPD) was calculated based on K-34 flux tower data collection of air temperature and relative humidity using a thermohydrometer (HC2S3, Campbell Scientific) measured at 28 m during the period of this study (Ewers and Oren 2000). Air temperature and relative humidity was provided by the Large-Scale Biosphere–Atmosphere (LBA) program at the National Institute for Amazon Research (INPA).
Results
Stem growth and CO 2 efflux in the BIONTE experiment
A total of 80 trees were studied across a broad range of growth rates and tree base diameters in plots exhibiting variable rates of net biomass accumulation following a logging disturbance. The supplementary data file (Tree_Diameter.xlsx) summarizes the collected biophysical properties of the 80 tree individuals including BIONTE treatment plot (T0–T3), tree ID, wood density (g ml−1), and monthly diameter DBH values (cm). While the individuals were not identified at the species level, the common name in Brazilian Portuguese was recorded.
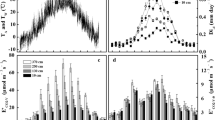
Following the selective logging, all BIONTE plots, including the control plots, experienced a net increase in biomass over time (Fig. 2). Previous studies reported that tree growth rates and biomass accumulation in the BIONTE control plots were greater than other control plots in nearby forests (Chambers et al. 2001), indicating a lack of biomass steady-state in the BIONTE control plots. This allowed for an analysis of the influence of growth on carbon allocation to stem respiration among the high diversity of tree species in the BIONTE plots. For each individual in 2002, monthly average stem diameter increment rates were determined (µm day−1) with values reaching up to 60 µm day−1 for several fast-growing individuals. A clear annual pattern in monthly average stem diameter increment rates was observed with increased rates during the wet season (positive growth rates), and less positive and even negative diameter increments for some individuals during the hot dry season (e.g., July–Sept) (Fig. 3).
Net change in total above-ground tree biomass for the Transect control plots (C) and the BIONTE logging treatment plots (T0–T3). All BIONTE plots including the BIONTE control plots (T0) experienced long-term biomass accumulation, in contrast to the Transect control plots that exhibited biomass steady-state
Using the measured wood density of each stem, the average annual stem growth rate was then calculated for each individual in the same units of stem CO2 efflux according to Eq. 2. By plotting the average annual stem CO2 efflux (ES, µmol CO2 m−2 s−1) against the average annual stem growth rate expressed as a CO2 flux (µmol CO2 m−2 s−1), several key results can be noted (Fig. 4). ES values varied by a factor of 10 from as low as 0.17 to as high as 1.7 µmol CO2 m−2 s−1. Likewise, stem growth rates ranged from near zero to over 40 µm day−1, or 5.0 µmol CO2 m−2 s−1 when expressed as CO2 flux. Despite the high variability in the data, a weak correlation between Es and growth (R2 of 0.3) was observed with ES values tending to increase as a function of stem growth rates. Under zero growth, maintenance respiration (RM) of BIONTE trees is estimated from the y-intercept of Fig. 4 as 0.41 ± 0.04 μmol m−2 s−1 with the slope of the linear fit (0.15 ± 0.03 µmol m−2 s−1) representing the respiratory carbon loss equivalent to 15 ± 3% of stem carbon accrual.
Average ES rates plotted versus stem growth rates expressed as a CO2 flux. Each point represents average annual growth and respiration rate (in µmol m−2 s−1) for individual trees (n = 80 trees) in the BIONTE plots. Note the trend of increased ES fluxes as a function of stem growth rates. Note that slope of the linear fit (0.15 ± 0.03 µmol m−2 s−1) representing the respiratory carbon loss equivalent to 15 ± 3% of stem carbon accrual. Maintenance respiration (RM) is estimated as the y-intercept = 0.41 ± 0.04 mol m−2 s−1
When the average annual carbon use efficiency (CUEW) of wood was calculated for each individual, CUEW was found to increase markedly with the average annual stem growth rate, reaching maximum values of 77–87% for a fast-growing tree (Fig. 5). CUEw estimated using the observed average daytime ES fluxes, were higher by 0.9–17% than CUEw estimated from nighttime ES fluxes, which were assumed to be 2-times higher during the night than during the day based on results from the diurnal ES studies described in Sect. 3.2 below.
Change in estimated CUEw from ES data plotted as a function of tree growth rate across the 80 tree individuals studied in the BIONTE plots. Note, CUEw was estimated using the observed average daytime ES fluxes (orange points). Nighttime CUEw was estimated by assuming nighttime ES fluxes are 2× higher than during the daytime (black points)
Continuous observations of crown temperature, sap velocity, and E s during the night and day for three trees near the K-34 tower
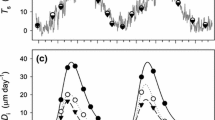
Each of the three individual trees studied near the K34 tower for real-time observations of stem CO2 efflux were coupled together with continuous observations of sap velocity, crown temperature, and vapor pressure deficit (VPD). For each tree studied, crown temperature, VPD, and sap velocity generally tracked each other throughout both the day and night, but showed an apparent inverse relation with ES (Fig. 6). During the day, a reduction of ES by 14–50% relative to the fluxes at night were associated with high transpiration rates when crown temperatures exceeded 24–28.5 °C (Fig. 7). For example, for the P. anomala individual during the day-time, the observed crown temperature range was about seven degrees (27–34 °C), and the ES range between 0.54 and 0.75 µmol m−2 s−1, with the sap velocity between 7.0 and 8.6 cm h−1. Between 10:30 and 11:15, an increase in crown temperature occurred together with elevated sap velocities and this was associated with ES suppression. In contrast, between 11:15 and 12:15, the buildup of mid-day clouds reduced crown temperatures and VPD together with sap velocities while ES increased to maximum values. Nonetheless, throughout the day there was a general trend of increasing crown temperature with VPD and sap velocity in the afternoon (12:45–14:00) and an ES suppression. On the intervals between 10:30–10:35, 11:30–11:35 and 13:15–13:20, transient variations were observed in crown temperature and VPD with corresponding responses in ES. For the P. erythrochrysa individual, the same general day-time pattern could be observed, with the maximum value of crown temperature occurring at the same time as minimum value of ES (12:30). For the E. bracteosa individual, day-time VPD tracked crown temperature and sap velocity with ES generally showing the opposite behavior. For example, between 12:30 and 13:30 when crown temperature and VPD decreased due to the buildup of mid-day clouds, an increase in ES was observed. The apparent inverse relationship between crown temperature and ES that was observed throughout the day for the three individual trees was also observed when daytime data were compared to data during the night. Relative to the day, the crown temperature and VPD reached a minimum at night, whereas the ES reached a maximum (Fig. 6b, d, f).
Example of high temporal dynamics (5 min) of basal ES (µmol m−2 s−1, 1.3 m) and Sap velocity (cm hr−1, 1.5 m) together with crown temperature (°C, 28.8, 25.3 and 28.6 m) measured with an IR radiometer during a 4-h period at the hottest hour of the day for: a P. anomala (21 Jun 2017), c P. erythrochrysa (11 Oct 2017) and e E. bracteosa (22 Jun 2017). Also shown are diurnal patterns of ES (30 min average), crown temperature and sap velocity (15 min averages) for three trees: b P. anomala, d P. erythrochrysa and f E. bracteosa) showing higher ES values during the night-time when crown temperature and sap velocity are low. Shaded areas in parts b, d, and f represent nighttime data
Scatter plots and nonlinear regression analyses of ES versus crown temperature (hourly averages, red points) and ES versus sap velocity (hourly averages, blue points) for three canopy dominant trees in the central Amazon: a and b P. anomala on 21 Jun 2017; c and d P. erythrochrysa on 11 Oct 2017; e and f E. bracteosa 22 Jun 2017). The central line represents the polynomial fit (see included equations) and the two other lines represents ± confidence interval. Regression statistics with 95% probability provided with p value and R-Squared at the top-right corner of the plots
When ES was plotted against crown temperature and sap velocity, for the three individual trees studied near the K34 tower, a negative linear relationship was observed. With increases in crown temperature, VPD, and sap velocity, ES tended to decrease (Fig. 7a, c, e). Good statistical fits between ES and crown temperature were found using the polynomial Es = β0 + β1(Crown Temperature) + β1(Crown Temperature)2, with R2 coefficients of 0.66, 0.15, and 0.65, respectively for P. anomala, P. erythrochrysa, and E. bracteosa. Similarly, by plotting ES against sap velocity, good fits between ES and sap velocity was achieved using the polynomial ES = β0 + β1(sap velocity) + β1(sap velocity)2. This analyses resulted in R2 values of 0.48, 0.13, and 0.23, respectively, for P. anomala, P. erythrochrysa, and E. bracteosa (Fig. 7b, d, f). From this analysis, it was observed that above a threshold range of crown temperature (P. anomala: 24–25 °C, P. erythrochrysa: 27.5–28.5 °C, and E. bracteosa: 25.5–26.5 °C) a suppression in ES occurred and was associated with high sap velocities (> 2–7 cm h−1).
Discussion
Toward the goal of developing a more mechanistic understanding of the biological and environmental factors that influence autotrophic respiration in the tropics, in this study, we evaluated the hypothesis that variations in stem CO2 efflux (ES) are driven by changes in growth rates across both diurnal and annual time while the cales. This hypothesis was evaluated in a highly diverse ‘terra-firme’ tropical forest ecosystem in the central Amazon, by determining relationships between average annual stem growth (wood production, Pw) and stem CO2 efflux (ES) across 80 individuals. We also sought to evaluate the hypothesis across diurnal time scales by characterizing real-time diurnal patterns in ES in connection with observations of sap velocity and estimates of leaf to atmosphere vapor pressure deficits (VPD), the ‘driver’ of plant transpiration. Early work showed a regular pattern in wood formation of many tropical tree species related to a distinct rainfall periodicity (Worbes 1995). During the dry season, changes in water availability together with increased atmospheric demand for water vapor (VPD) can drive higher transpiration rates leading to reductions in plant water content, stem diameter, and new wood production. In contrast, during the wet season when soil moisture is high, tree diameters can increase as a result of both refilling of plant water reservoirs together with new wood production (Dünisch et al. 2003; Schöngart et al. 2017). To minimize the influence of these hydraulic effects on growth rate estimates, we determined the average annual stem diameter increment and ES fluxes for each individual.
Mean annual daytime ES fluxes (0.55 ± 0.33 µmol m−2 s−1), determined here for trees in the BIONTE plot during 2002, compare well with mean annual ES fluxes (0.6 μmol m−2 s−1) previously reported for Manaus and the Tapajos National Forest (Chambers et al. 2004; Trumbore 2006). However, to our knowledge, night time ES fluxes have not yet been reported in the Amazon basin. In general, published measurements of ES in the Amazon Basin have reported highly variable daytime observations from canopy trees (Nepstad 2002; Chambers et al. 2004; Malhi et al. 2009; Rowland et al. 2018). For example, Chambers et al., 2004, values ranged over two orders of magnitude (0.027–3.64 µmol m−2 s−1) with the large variation largely unexplained. In this study across the 80 individuals, daytime ES values also varied substantially by a factor of 10 from as low as 0.17 to as high as 1.7 µmol CO2 m−2 s−1. By observing a statistically significant positive linear relationship between annual average stem growth rates and ES, we show that some of this variability in ES can be attributed to tree growth rates (which ranged from near zero with little net annual growth to over 40 µm day−1). Thus, the fastest growing stems tended to have the highest rates of ES while individuals showing little to no growth tended to have lower rates of ES. In addition, the slope of the linear relationship was determined to be 0.15 ± 0.03, suggesting that between 12 and 18% of total carbon allocated to stems is respired and released to the atmosphere as CO2. These findings are consistent with previous studies in the Tapajos National Forest in the Amazon (Nepstad 2002) and a Costa Rican forest (Ryan et al. 1994) where ES was positively correlated with tree growth rates.
Moreover, when stem growth rates were expressed in the same units as ES, CUEW was found to increase with stem growth rates, with maximum CUEW for a fast-growing tree reaching values between 77 and 87%. CUEW provides a measure of what fraction of total carbon assimilation becomes incorporated into new woody tissues. Previous studies in the ZF2 forest preserve outside of Manaus, Brazil estimated average CUEW of 43% (Chambers et al. 2004) while a second study estimated values ranging from 30 to 56% for Manaus (46%), Tapajós (56%), and Caxiuanã (30%) (Malhi et al. 2009). Our results from the BIONTE experiment near Manaus suggest that high CUEW values (up to 77–87%) are associated with high daytime ES fluxes during fast tree growth. Therefore, trees with higher daytime ES fluxes tend to be faster growing and with higher CUEW values.
Using real-time data from three canopy trees, we also show that ES variability is inversely linked to crown temperature/VPD and sap velocity on diurnal time scales. VPD tracked crown temperature and sap velocity throughout both the day and night. This is consistent with a recent study using a larger set of trees near the K34 tower (including the three that were studied here), which showed that sap velocity, leaf temperature, and leaf to air-VPD were positively correlated during both the day and night with no detectable delay between the variables (< 15 min) (Gimenez et al. 2019). Despite large height differences between ES and sap velocity measurements at the base of the stem and crown temperature and VPD observations at 25–29 m within the canopy, ES was inversely related to these variables at minute to diurnal time scales. A strong (15–50%) ES suppression was observed during the daytime relative to the night associated with elevated values of crown temperature/VPD and high transpiration rates. These findings are consistent with a previous study at the same Amazon field site on a single Scleronema micranthum (Ducke) Ducke individual that found higher ES fluxes at night relative to the day with ES fluxes decreasing with the commencement of xylem sap velocity and elevating air temperature in the early morning (Kunert 2018). These patterns are alternate to what would be expected simply due to changes in stem temperature that increase during the day, which stimulate respiration due to its Q10 thermal dependence. Thus, the mechanism of daytime ES suppression cannot be explained by the impact of temperature on respiration.
Due to the use of sealed static stem enclosures which report only an averaged ES flux over the measurement period, previous studies of ES fluxes from Neotropical rainforests reported little information on potential diurnal patterns including studies in French Guiana (Stahl et al. 2011), the central Amazon (Chambers et al. 2004), and the eastern Amazon (Rowland et al. 2018). However, diurnal studies in subtropical China reported ES fluxes increasing during the daytime following closely the diurnal increases in temperature, enabling an estimate of ES Q10 values (Yang et al. 2012). Similarly, diurnal ES studies from woody stems of eudicots and gymnosperms in Guam, Thailand, and the Philippines showed diurnal ES fluxes that were 36–40% greater than nighttime ES (Marler and Lindström 2020). Based on these and other field measurements, a previous statistical global model predicted that ES increases with temperature in the tropics (Yang et al. 2016). For example, on a global annual basis, ES was suggested to increase with temperature with annual ES values in the Amazon Basin estimated three-to-five times greater than ES fluxes for temperate and Boreal forests.
Although additional research is needed to resolve why some studies have reported positive daytime increases in ES together with transpiration and temperature while others have observed a clear daytime suppression in ES, one possibility may be the variable influence of root/soil respiratory sources of CO2. ES observations at height of 1.3 m, as performed here in the central Amazon, may be sufficiently high to avoid a significant impact of soil/root derived CO2 on observed stem Es fluxes. In contrast, the southeast Asia study in Guam, Thailand, and the Philippines quantified diurnal stem Es fluxes at a much lower stem height of 0.3–0.4 m, and did not observe daytime suppression relative to the night (Marler and Lindström 2020). Stem diameters were a similar range in the southeast Asia study (29–92 cm) and central Amazon (BIONTE: 10–52 cm, K34 tower: 30–37 cm) studies reported here. Thus, we assume stem Es fluxes observations in the central Amazon are mainly influenced by respiratory processes in the local sap wood at the height of measurement (1.3 m), rather than roots/soils. However, additional research is needed to characterize the relative importance of autotrophic and heterotrophic sources of stem Es flux as a function of height in tropical trees.
Nonetheless, the suppression of ES associated with high daytime temperatures and transpiration, as observed here in the central Amazon, are consistent with a similar finding reported at the same Amazon field site on a single Scleronema micranthum (Ducke) Ducke individual (Kunert 2018), as well as numerous greenhouse and field studies outside the tropics. Although mitochondrial respiration is known to increase with temperature (Atkin and Tjoelker 2003; Noctor et al. 2007), a growing number of studies outside of the tropics have shown that daytime ES can be suppressed during the day relative to the night. For example, results from an experimental forest in Georgia showed both reduced transpiration rates and enhanced ES at night relative to the day, despite substantially higher temperatures during the day compared to the night (Maier and Clinton 2006). Mechanistic studies suggested that daytime suppression of ES is strongly related to stem water potential decreases that inhibit growth and its associated respiratory fluxes (Saveyn et al. 2007). Indeed, stem growth rates of several tree species have been documented to be higher at night than the day (Nozue et al. 2007). Although early studies reporting daytime ES suppression mainly discussed a possible role of transport of CO2 in the transpiration stream, an alternative mechanism was proposed (Saveyn et al. 2007) based on the daily dynamics of turgor pressure. The daytime decrease in stem water potential was hypothesized to be a key determinant of ES through its direct negative influences on the rates of growth and maintenance processes in the living tissues of the stem.
Numerous other studies have shown a tendency of a suppression in ES during periods of high transpiration (Levy et al. 1999; Teskey and McGuire 2007; Bowman et al. 2008). These and other studies suggested that a suppression of ES during day-time periods of high sap flow may be a result of numerous processes including enhanced CO2 storage (Bowman et al. 2005; Teskey and McGuire 2007; Teskey et al. 2008), increased respiratory CO2 transport via the transpiration stream (Katayama et al. 2014), a suppression of mitochondrial respiration and growth by reduced daytime xylem water potential (Saveyn et al. 2007), enhanced re-assimilation of respiratory CO2 through both light-dependent photosynthetic green-tissue assimilation (Wittmann et al. 2006), and light-independent bicarbonate fixation via phosphoenol pyruvate carboxylase activity involved in the biosynthesis of dicarboxylic acids like malate that are used as respiratory substrates (Berveiller and Damesin 2008). Thus, an ES and growth suppression could have major implications for stress coping mechanisms during high temperature and droughts such as those experienced during ENSO events (Longo et al. 2018). As has been previously discussed based on CO2 re-assimilation studies (Bloemen et al. 2013a, b), an increased transport of respired CO2 could result in enhancing internal CO2 re-assimilation within stems and leaves and consequently contribute to protective mechanisms during climate extremes. Moreover, a downregulation of growth and respiratory processes during climate warming and drought may act to increase survivability through conservation of valuable respiratory substrates such as non-structural carbohydrates whose exhaustion could lead to carbon starvation and mortality (McDowell and Sevanto 2010).
While the proposed mechanisms of daytime ES suppression are not mutually exclusive, they are reportedly difficult to disentangle. However, more recent studies have attempted to discriminate between internal transport/re-assimilation versus attenuated respiratory activity due to lower turgor pressure. For example, when manipulative greenhouse studies were performed by defoliation and drought treatments, only turgor pressure was a robust predictor of daytime suppression of temperature-normalized ES fluxes (Salomón et al. 2018). Regardless of the mechanisms of ES suppression, we confirm that strong daytime stem ES suppression can occur in Amazon trees during warm periods associated with high rates of transpiration. Despite similar findings outside of the tropics, we acknowledge however, that our findings are restricted to a limited number of trees with measurements capturing only one diurnal period. While verification of a daytime suppression of ES associated with high transpiration rates among highly diverse canopy dominant trees in the Amazon and other tropical forests requires additional research, the observations are consistent with the emerging view that diurnal dynamics of stem water fluxes influence CO2 transport, metabolism, and ES as well as respiratory processes associated with stem growth.
Author contribution statement
KJ, LT, LO, and BG collected all field data with JC, and NH supervising the projects. ER facilitated archiving of the datasets on the NGEE-Tropics archive. All authors discussed the results and contributed to the development of the final manuscript.
Data and materials availability
All data presented in the manuscript, including raw (diameter and wood density) and derived (ES, growth rates, and growth rates as CO2 efflux) datasets from the 80 trees in the BIONTE central Amazon field site are available for public download and use from the NGEE Tropics data archive (NGT0168, Stem CO2 Efflux and growth rates in a selectively logged experiment in the central Amazon 2001–2002, http://dx.doi.org/10.15486/ngt/1767825). In addition to the raw and derived data, important metadata is also available including sampling date and location, tree ID, genus, species, family, tree number, research site, data measurement variables and units. A second data set is also available which includes real time ES from three canopy dominant trees together with canopy temperature and sap flow during the day and night (NGT0149, Stem CO2 Efflux measurements from Manaus, Brazil 2017, http://dx.doi.org/10.15486/ngt/1804760). Data users can view the public datasets and all related metadata through the NGEE Tropics data archive. Once users register with a FluxNet ID, which only requires an email to sign up, the datasets are free to download and use in future experimental and modeling studies focused on understanding the roles of autotrophic respiration and growth in ecosystem carbon storage and cycling.
References
Amthor JS (2000a) Direct effect of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiol 20:139–144. https://doi.org/10.1093/treephys/20.2.139
Amthor J (2000b) The mccree–de wit–penning de vries–thornley respiration paradigms: 30 years later. Ann Bot 86:1–20. https://doi.org/10.1006/anbo.2000.1175
Araújo AC (2002) Comparative measurements of carbon dioxide fluxes from two nearby towers in a central Amazonian rainforest: the Manaus LBA site. J Geophys Res 107:8090. https://doi.org/10.1029/2001JD000676
Atkin OK, Macherel D (2009) The crucial role of plant mitochondria in orchestrating drought tolerance. Ann Bot 103:581–597. https://doi.org/10.1093/aob/mcn094
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351. https://doi.org/10.1016/S1360-1385(03)00136-5
Aubrey DP, Teskey RO (2009) Root-derived CO2 efflux via xylem stream rivals soil CO2 efflux. New Phytol 184:35–40. https://doi.org/10.1111/j.1469-8137.2009.02971.x
Berveiller D, Damesin C (2008) Carbon assimilation by tree stems: potential involvement of phosphoenolpyruvate carboxylase. Trees 22:149–157. https://doi.org/10.1007/s00468-007-0193-4
Bloemen J, McGuire MA, Aubrey DP et al (2013a) Transport of root-respired CO2 via the transpiration stream affects aboveground carbon assimilation and CO2 efflux in trees. New Phytol 197:555–565. https://doi.org/10.1111/j.1469-8137.2012.04366.x
Bloemen J, McGuire MA, Aubrey DP et al (2013b) Internal recycling of respired CO2 may be important for plant functioning under changing climate regimes. Plant Signal Behav 8:e27530. https://doi.org/10.4161/psb.27530
Bowman WP, Barbour MM, Turnbull MH et al (2005) Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol 167:815–828. https://doi.org/10.1111/j.1469-8137.2005.01478.x
Bowman WP, Turnbull MH, Tissue DT et al (2008) Sapwood temperature gradients between lower stems and the crown do not influence estimates of stand-level stem CO2 efflux. Tree Physiol 28:1553–1559. https://doi.org/10.1093/treephys/28.10.1553
Chambers JQ, Silver WL (2004) Some aspects of ecophysiological and biogeochemical responses of tropical forests to atmospheric change. Philos Trans R Soc Lond B Biol Sci 359:463–476. https://doi.org/10.1098/rstb.2003.1424
Chambers JQ, dos Santos J, Ribeiro RJ, Higuchi N (2001) Tree damage, allometric relationships, and above-ground net primary production in central Amazon forest. For Ecol Manage 152:73–84. https://doi.org/10.1016/S0378-1127(00)00591-0
Chambers JQ, Tribuzy ES, Toledo LC et al (2004) Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecol Appl 14:72–88. https://doi.org/10.1890/01-6012
Christianson DS, Varadharajan C, Christoffersen B et al (2017) A metadata reporting framework (FRAMES) for synthesis of ecohydrological observations. Ecol Inform. https://doi.org/10.1016/j.ecoinf.2017.06.002
Clark DA (2004) Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos Trans R Soc Lond B Biol Sci 359:477–491. https://doi.org/10.1098/rstb.2003.1426
da Silva RP, dos Santos J, Tribuzy ES et al (2002) Diameter increment and growth patterns for individual tree growing in Central Amazon, Brazil. For Ecol Manage 166:295–301. https://doi.org/10.1016/S0378-1127(01)00678-8
Dünisch O, Montóia VR, Bauch J (2003) Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees 17:244–250. https://doi.org/10.1007/s00468-002-0230-2
Feeley KJ, Joseph Wright S, Nur Supardi MN et al (2007) Decelerating growth in tropical forest trees. Ecol Lett 10:461–469. https://doi.org/10.1111/j.1461-0248.2007.01033.x
Gaui TD, Costa FRC, Coelho de Souza F et al (2019) Long-term effect of selective logging on floristic composition: a 25 year experiment in the Brazilian Amazon. For Ecol Manage 440:258–266. https://doi.org/10.1016/j.foreco.2019.02.033
Gimenez BO, Jardine KJ, Higuchi N et al (2019) Species-specific shifts in diurnal sap velocity dynamics and hysteretic behavior of ecophysiological variables during the 2015–2016 el niño event in the amazon forest. Front Plant Sci 10:830. https://doi.org/10.3389/fpls.2019.00830
Green S, Clothier B, Jardine B (2003) Theory and practical application of heat pulse to measure sap flow. Agron J 95:1371–1379. https://doi.org/10.2134/agronj2003.1371
Higuchi N, Ferraz JBS, Antony L, Luizão FJ (1997) Biomassa e nutrientes florestais. Projeto BIONTE. MCT-INPA, DFID
Katayama A, Kume T, Komatsu H et al (2014) Vertical variations in wood CO2 efflux for live emergent trees in a Bornean tropical rainforest. Tree Physiol 34:503–512. https://doi.org/10.1093/treephys/tpu041
Körner C (2009) Responses of humid tropical trees to rising CO2. Annu Rev Ecol Evol Syst 40:61–79. https://doi.org/10.1146/annurev.ecolsys.110308.120217
Kunert N (2018) A case study on the vertical and diurnal variation of stem CO2 effluxes in an Amazonian forest tree. Trees 32:913–917. https://doi.org/10.1007/s00468-018-1680-5
Le Quéré C, Andrew RM, Friedlingstein P et al (2018) Global carbon budget 2018. Earth Syst Sci Data 10:2141–2194. https://doi.org/10.5194/essd-10-2141-2018
Levy PE, Meir P, Allen SJ, Jarvis PG (1999) The effect of aqueous transport of CO2 in xylem sap on gas exchange in woody plants. Tree Physiol 19:53–58. https://doi.org/10.1093/treephys/19.1.53
Lloyd J, Farquhar GD (2008) Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos Trans R Soc Lond B Biol Sci 363:1811–1817. https://doi.org/10.1098/rstb.2007.0032
Longo M, Knox RG, Levine NM et al (2018) Ecosystem heterogeneity and diversity mitigate Amazon forest resilience to frequent extreme droughts. New Phytol 219:914–931. https://doi.org/10.1111/nph.15185
Luyssaert S, Inglima I, Jung M et al (2007) CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob Chang Biol 13:2509–2537. https://doi.org/10.1111/j.1365-2486.2007.01439.x
Maier CA, Clinton BD (2006) Relationship between stem CO2 efflux, stem sap velocity and xylem CO2 concentration in young loblolly pine trees. Plant Cell Environ 29:1471–1483. https://doi.org/10.1111/j.1365-3040.2006.01511.x
Malhi Y, Aragão LEOC, Metcalfe DB et al (2009) Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Glob Chang Biol 15:1255–1274. https://doi.org/10.1111/j.1365-2486.2008.01780.x
Marler TE, Lindström AJ (2020) Diel patterns of stem CO2 efflux vary among cycads, arborescent monocots, and woody eudicots and gymnosperms. Plant Signal Behav 15:e1732661. https://doi.org/10.1080/15592324.2020.1732661
McDowell NG, Sevanto S (2010) The mechanisms of carbon starvation: how, when, or does it even occur at all? New Phytol 186:264–266. https://doi.org/10.1111/j.1469-8137.2010.03232.x
McDowell NG, Marshall JD, Qi J, Mattson K (1999) Direct inhibition of maintenance respiration in western hemlock roots exposed to ambient soil carbon dioxide concentrations. Tree Physiol 19:599–605. https://doi.org/10.1093/treephys/19.9.599
McGuire MA, Teskey RO (2004) Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol 24:571–578. https://doi.org/10.1093/treephys/24.5.571
Nepstad DC (2002) The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J Geophys Res. https://doi.org/10.1029/2001JD000360
Nobre CA, Sampaio G, Borma LS et al (2016) Land-use and climate change risks in the Amazon and the need of a novel sustainable development paradigm. Proc Natl Acad Sci USA 113:10759–10768. https://doi.org/10.1073/pnas.1605516113
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134. https://doi.org/10.1016/j.tplants.2007.01.005
Nozue K, Covington MF, Duek PD et al (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448:358–361. https://doi.org/10.1038/nature05946
O’Leary BM, Asao S, Millar AH, Atkin OK (2019) Core principles which explain variation in respiration across biological scales. New Phytol 222:670–686. https://doi.org/10.1111/nph.15576
Otani T, Lima AJ, Suwa R et al (2018) Recovery of above-ground tree biomass after moderate selective logging in a central Amazonian forest. iForest 11:352–359. https://doi.org/10.3832/ifor2534-011
Rowland L, Hill TC, Stahl C et al (2014) Evidence for strong seasonality in the carbon storage and carbon use efficiency of an Amazonian forest. Glob Chang Biol 20:979–991. https://doi.org/10.1111/gcb.12375
Rowland L, da Costa ACL, Oliveira AAR et al (2018) Drought stress and tree size determine stem CO2 efflux in a tropical forest. New Phytol 218:1393–1405. https://doi.org/10.1111/nph.15024
Ryan MG, Hubbard RM, Clark DA, Sanford RL (1994) Woody-tissue respiration for Simarouba amara and Minquartia guianensis, two tropical wet forest trees with different growth habits. Oecologia 100:213–220. https://doi.org/10.1007/BF00316947
Salomón RL, De Schepper V, Valbuena-Carabaña M et al (2018) Daytime depression in temperature-normalised stem CO2 efflux in young poplar trees is dominated by low turgor pressure rather than by internal transport of respired CO2. New Phytol 217:586–598. https://doi.org/10.1111/nph.14831
Saveyn A, Steppe K, Lemeur R (2007) Daytime depression in tree stem CO2 efflux rates: is it caused by low stem turgor pressure? Ann Bot 99:477–485. https://doi.org/10.1093/aob/mcl268
Schöngart J, Bräuning A, Barbosa ACMC et al (2017) Dendroecological studies in the neotropics: history, status and future challenges. In: Amoroso MM, Daniels LD, Baker PJ, Camarero JJ (eds) Dendroecology: tree-ring analyses applied to ecological studies. Springer International Publishing, Cham, pp 35–73
Stahl C, Burban B, Goret J-Y, Bonal D (2011) Seasonal variations in stem CO2 efflux in the Neotropical rainforest of French Guiana. Ann for Sci 68:771–782. https://doi.org/10.1007/s13595-011-0074-2
Teskey RO, McGuire MA (2007) Measurement of stem respiration of sycamore (Platanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ 30:570–579. https://doi.org/10.1111/j.1365-3040.2007.01649.x
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32. https://doi.org/10.1111/j.1469-8137.2007.02286.x
Trumbore S (2006) Carbon respired by terrestrial ecosystems—recent progress and challenges. Global Change Biol 12:141–153. https://doi.org/10.1111/j.1365-2486.2006.01067.x
Valentini R, Matteucci G, Dolman AJ et al (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865. https://doi.org/10.1038/35009084
Vanlerberghe GC, Dahal K, Alber NA, Chadee A (2020) Photosynthesis, respiration and growth: a carbon and energy balancing act for alternative oxidase. Mitochondrion 52:197–211. https://doi.org/10.1016/j.mito.2020.04.001
Wittmann C, Pfanz H, Loreto F et al (2006) Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant Cell Environ 29:1149–1158. https://doi.org/10.1111/j.1365-3040.2006.01495.x
Worbes M (1995) How to measure growth dynamics in tropical trees a review. IAWA J 16:337–351. https://doi.org/10.1163/22941932-90001424
Yang Q, Xu M, Chi Y et al (2012) Temporal and spatial variations of stem CO2 efflux of three species in subtropical China. J Plant Ecol 5:229–237. https://doi.org/10.1093/jpe/rtr023
Yang J, He Y, Aubrey DP et al (2016) Global patterns and predictors of stem CO2 efflux in forest ecosystems. Glob Chang Biol 22:1433–1444. https://doi.org/10.1111/gcb.13188
Acknowledgements
This material is based upon work supported as part of the Next Generation Ecosystem Experiments-Tropics (NGEE-Tropics) funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research through contract No. DE-AC02-05CH11231 to LBNL, as part of DOE’s Terrestrial Ecosystem Science Program. This work was also supported in part by the US Department of Energy, Office of Science, Office of Workforce Development for Teachers and Scientists (WDTS) under the Science Undergraduate Laboratory Internship (SULI) Program and the Brazilian fund: “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES). We would like to kindly acknowledge the field logistics support by the forest management department at the National Institute for Amazon Research (INPA) in Manaus, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Communicated by V. Resco de Dios.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jardine, K.J., Cobello, L.O., Teixeira, L.M. et al. Stem respiration and growth in a central Amazon rainforest. Trees 36, 991–1004 (2022). https://doi.org/10.1007/s00468-022-02265-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-022-02265-5