Abstract
To investigate the effects of biochar addition (1 or 3%) to the soil of a China fir plantation with or without litter, we conducted a 90-day incubation experiment. We also studied the C and N dynamics and the microbial community structure of the soil. In soil without litter, the application of biochar at a rate of 3% significantly decreased CO2 emissions, while addition of 1% biochar had no effect. Biochar application did not affect the net N mineralization rate but significantly reduced the NH4+ concentration after 90 days. In litter-enriched soil, biochar application had no significant effect on total CO2 emissions; however, application of 3% biochar significantly reduced the net N mineralization rate. Biochar application to soil with or without litter immediately reduced the dissolved organic carbon (DOC) concentration independent of the application rate, which was primarily due to sorption of DOC by the biochar. Phospholipid fatty acid analysis demonstrated that both concentrations of added biochar to soil (with or without litter) altered the soil microbial community structure at the end of incubation, although the effect of biochar was not as strong as the effect of time or litter application. The effect of biochar addition alone on microbial community structure was inconsistent over time. Litter added to soil significantly increased fungi and reduced Gram-positive bacteria. In the presence of litter, biochar applied at both 1% and 3% significantly increased (p < 0.05) the proportion of actinomycete only at day 90. Our results indicate biochar as a potentially effective measure for C sequestration in the test soil of a China fir plantation, even in the presence of litter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biochar has been proposed as an effective means to sequester C in the soil, and thus to mitigate atmospheric carbon dioxide (CO2) emissions while enhancing carbon storage (Lehmann 2007). Biochar is produced through the pyrolysis of biomass under limited oxygen and is predominantly composed of aromatic compounds (Hansen et al. 2016) that are largely resistant to microbial decay (Baldock and Smernik 2002; Lu et al. 2014). Biochar addition to soil affects the mineralization of soil organic matter (SOM) and added material, which is crucial for the assessment of the carbon sequestration potential of biochar application. Currently, contradicting reports exist regarding the effects of biochar addition on the mineralization of SOM, both in field and laboratory experiments, reporting either positive effects (Maestrini et al. 2014), negative effects (Lu et al. 2014), or no effects (Santos et al. 2012). Nitrogen (N) is one of the most important elements for plant growth and productivity (Bai et al. 2017). Both suppression and stimulation of the conversion of organic nitrogen to inorganic nitrogen (N mineralization) by biochar have been reported (Bruun et al. 2013; Dempster et al. 2012; Xu et al. 2015). These inconsistent results might be due to differences in biochar feedstock, pyrolytic condition, soil properties, or various other underlying processes as reported previously.
Microorganisms are responsible for both C and N mineralization in soils (Ng et al. 2014). The use by soil microbes of labile matter from biochar or the change of soil physicochemical properties due to biochar addition, can lead to changes of soil microbial abundance and composition (Bruun et al. 2013; Pan et al. 2016). Decomposition of soil organic carbon (SOC) is promoted due to biochar addition, which is accompanied by increased microbial activity (Wardle et al. 2008) or by changes in the microbial community (Farrell et al. 2013). In contrast, biochar addition has been reported to suppress SOC decomposition and increase Gram-positive bacteria 30 days after biochar addition (Lu et al. 2014). Novak et al. (2010) reported that the observed increase in microbial biomass after biochar addition was accompanied by N immobilization. The presence of ethylene from biochar inhibited nitrifying bacteria and consequently changed N cycling (Spokas et al. 2009). To date however, due to the varying properties of biochar and soils, few studies have clarified the relationships between SOC decomposition and microbial community structure after biochar treatment.
China fir (Cunninghamia lanceolata) is one of the most important tree species for plantations in south China due to its wide planting area, high productivity, and good timber quality. The soil types in south China are acidic aluminum-rich red loams that are phosphorus-limited, a condition thought to impede the primary productivity of the China fir plantation (Ma et al. 2007). Consequently, successive plantings lead to reduced soil fertility. Timber harvest and slash burning after tree harvest can cause substantial loss and redistribution of OM and N (Ma et al. 2007). Biochar addition offers an attractive strategy for increasing the soil refractory organic C pool and for enhancing soil fertility (Lehmann 2007). The structure of biochar depends on the feedstock species, plant part, soil type, climate conditions, and the time of feedstock harvest (Heitkötter and Marschner 2015). Few studies on soil improvement via biochar application have been conducted in China fir plantations. The effects of biochar on the decomposition of SOC and its mechanisms are not well understood.
Inputs of litter to China fir plantations are non-periodic. Thus, the effects of litter must be investigated in controlled laboratory experiments to evaluate the biochar impact on SOM decomposition. The presence of litter might influence the response of SOM and microorganisms to biochar addition because litter contains more labile matter (Jiang et al. 2014). At the same time, decomposition of SOC and litter, due to their different chemical characteristics, might be affected by biochar (Cui et al. 2017). Previously, a range of added materials (e.g. glucose, straw, and forest litter) have been used to study the responses of two different OM decompositions to biochar addition (Bamminger et al. 2014; Lu et al. 2014; Prayogo et al. 2014). However, the mechanisms underlying the effect of biochar on native SOC decomposition in the presence of added materials are complicated and still remain unclear.
A 90-day laboratory incubation study was performed to assess the effects of various concentrations of China fir biochar in soil in a China fir plantation on mineralization of C and N, and to quantify changes in the microbial community in the presence or absence of China fir leaf litter. The primary goals of these analyses were as follows:
-
(1)
To evaluate the effect of different quantities of China fir biochar application to soils from China fir plantations with regard to net mineralization or SOM decomposition in both presence and absence of leaf litter;
-
(2)
To determine the effect of biochar supplementation with or without China fir litter on N mineralization based on changes in NH4+ and NO3−;
-
(3)
To analyze the change of phospholipid fatty acids (PLFAs), to investigate the effects of biochar on the composition and biomass of microbial communities, and to improve our understanding of the mechanisms that mediate the effects of biochar on mineralizing C and N from the perspectives of substrate availability and microbial community.
Materials and methods
Site selection and sampling
Soil was collected from the top 0–15 cm of the topsoil layer of a 17-year-old China fir plantation in October 2015. The site was located at the Xinkou forest farm (117°27′E–118°14′E, 26°07′N–27°13′N) in northwest Fujian Province, southeastern China. The soil was classified as mountain acidic red loam soil, based on the Chinese soil classification system, which is equivalent to humic planosols in the FAO system. The entire soil layer was homogenized to form a composite soil for the incubation study. One part of the fresh soil was sieved to be less than 2 mm and all roots and visible residues were manually removed. The other part was air dried for subsequent analysis of soil properties.
Biochar feedstock and production conditions
Fresh China fir surface litter was collected from the same forest soil. We collected fresh litter leaves that were complete, had not yet been decomposed by microorganisms, were not defective, had yellow brown color, and were hard in texture. Leaves were dried at 80 °C after cleaning with deionized water and were then ground to pass through a 1-mm sieve before determining their chemical properties.
Biochar was produced from branches of China fir that were collected from the same middle-aged plantation of China fir. The feedstock was air dried at room temperature and ground to pass through a 1-mm sieve. The ground samples were then placed in ceramic crucibles and then covered and pyrolyzed under limited oxygen supplied in a muffle furnace. The pyrolysis temperature was increased to 550 °C at a rate of approximately 20 °C min−1 and held constant for 4 h (Yuan et al. 2011). Thereafter, biochar samples were left to cool to room temperature before their chemical properties were analyzed.
Experimental design
We conducted an incubation experiment for 90 days, using six treatments of soil and different rates of biochar and litter: Soil was either incubated without biochar or with biochar at 1 and 3% of soil mass (0.01 and 0.03 g g−1soil, respectively). Leaf litter was added to the soil at 1% of the soil mass (0.01 g g−1soil): (1) natural soil (CK); (2) soil + 1% biochar (1B); (3) soil + 1% biochar + 1% litter (1BL); (4) soil + 3% biochar (3B); (5) soil + 3% biochar + 1% litter (3BL); and (6) soil + 1% litter (L). With a soil bulk density of 1.33 g cm−3, rates of biochar that were applied to soil were equivalent to 20 and 60 t ha−1, respectively.
A series of 500 mL glass Mason jars (8 cm diameter; 11.2 cm height) with 50 g (oven-dried basis) of fresh soil sample were placed in an incubator at 25 °C to acclimate the soil microbes to the new environmental conditions. Seven days later, appropriate amounts of biochar and/or litter were added to each jar, while using a sterilized large spoon to thoroughly mix the samples. Control soil samples were identically mixed. A small plastic measuring glass containing 10 mL of NaOH solution was put into each Mason jar to capture evolved CO2. Jars were then sealed. Soil moisture was periodically monitored and maintained at 70% of the maximum water holding capacity throughout the incubation period. Jars were left open to the ambient atmosphere for 20 min once per week to allow oxygen to diffuse into the soil, thus maintaining aerobic conditions. For each treatment, gas analyses were conducted with four replicates per treatment (n = 24 samples), while destructive soil sampling was conducted with three replicates per treatment at each measurement time. This was used to determine C and N fluxes and microbial biomass and community structure (n: 18 × 7 = 126 samples).
Analysis of basic properties of forest soil, biochar, and litter
Prior to setup of the incubation experiment, the texture of the forest soil and chemical properties of forest soil, biochar, and litter were determined (Table 1). The soil texture was obtained via the pipette method after sodium hydroxide (NaOH) treatment to disperse soil particles. Soil water holding capacity and bulk density were measured via ring sampling (98.22 cm3, D = 5 cm, H = 5 cm) (Institute of Soil Science Chinese Academia Sinica 1978). The pH of forest soil in water solution was measured at 1:2.5 (w/v) and that of biochar and litter in water solution was measured at 1:10 w/v using a pH meter (model PB-20, Sartorius, Germany) (Yuan et al. 2011). Total C, total N, and total H concentrations in the soil, biochar, and litter were separately determined via the dry combustion method using an Elemental Analyzer (Thermo Flash 2000, Elemental, USA). Soil total P and K were determined according to the agriculture industry standard of China and total P was determined according to NY/T 88–1988. The total K contents were determined according to NY/T 87–1988. Biochar and litter total P and K were determined after biochar digestion with HNO3 via inductively coupled plasma spectrometry (ICP, NexIon300X, USA), (Zhang et al. 2017). The amount of inorganic carbon (carbonate) in biochar was determined via volumetric analysis of the CO2 that was liberated through addition of HCl solution to the biochar samples, as described in Yuan et al. (2011). Dissolved organic carbon (DOC) of biochar and litter were extracted by shaking the biochar or litter with 2 M KCl (1:25, w/v) for 1 h, then filtering through a 0.45 μm membrane filter, and measuring the DOC concentration with a TOC-VCPH (Shimadzu Corp. Kyoto, Japan) (Heitkötter and Marschner 2015).
Analysis of soil C and N mineralization and microbial community structure
CO2 flux in each treatment was estimated using a previously described method for alkali absorption (Winkler et al. 1996). A small plastic measuring glass, containing 0.5 M NaOH solution of 10 mL was planted into each Mason jar to catch evolved CO2; background CO2 concentrations were assessed by placing vials of NaOH in incubation jars without soil. Soil respiration data were reported as mg of CO2 respired per gram of soil. CO2 released from the soil samples was monitored daily for 20 days and thereafter, at intervals of 6 days until the end of the incubation period.
Destructive sampling of soil samples was conducted at 0, 15, 30, 45, 60, 75, and 90 days for measurements of soil inorganic N (NO3− and NH4+) and DOC. 5 g (oven-dried basis) of fresh soil samples were extracted in 50 mL of 2 M KCl after sampling and shaking for 1 h, the supernatant was filtered through a No. 41 Whatman filter and a 0.45 μm micro-filter, and the extracts were analyzed for soil NH4+ and NO3− concentration with an Auto Analyzer 3 (AA3 Bran and Luebbe, Norderstedt, Germany) and for DOC concentration with a TOC-VCPH (Shimadzu Corp. Kyoto, Japan).
Phospholipid fatty acids (PLFA) were extracted to determine the soil microbial community composition at 0, 30, 60, and 90 days. First, fresh soil samples (equivalent to 8 g dry weight) were extracted with 20 mL of solvent (consisting of chloroform in a 1:2:0.8 mixture with methanol, and phosphate buffer) in a shaker for 2 h, and were centrifuged at 4000 rpm for 10 min, removing the supernatant, and continuously following the above extraction step twice. Both extracts were combined and maintained for 12 h after they were added to 10 mL chloroform under dark conditions for stratification in a separating funnel, and the lower solution was evaporated under N2. Phospholipids from the concentrated extracts were separated on silicic acid columns by sequentially eluting them with organic solvents of increasing polarity and amending them with a non-adecanoic acid standard of 100 μL. Subsequently, they were saponified and methylated, to investigate the formation of fatty acid methyl esters (FAMEs). Individual FAMEs were identified via gas chromatography with a Hewlett Packard 5890 GC, equipped with a 6890 series injector, a flame ionization detector, and an Ultra 2 capillary column of 25 m × 200 µm with 0.33-μm film thickness according to retention times, and combined with the MIDI Sherlock Microbial Identification System (MIDI Inc., Newark, DE, USA). Total microbial PLFAs were determined in the range of C12 to C20 PLFAs. The PLFAs i14:0, i15:0, a15:0, i16:0, i17:0, and 17:0 were used as biomarkers for Gram-positive bacteria, with 16:1ω9, 16:1ω7, cy17:0, 18:1ω7, 18:1ω5, and cy19:0 used for Gram-negative bacteria, 12:00, 14:00, 16:00, 17:00, 18:00, and 20:00 for general bacteria, 10 Me 16:0, 10 Me 17:0, and 10 Me 18:0 for actinomycetes, 18:1ω9 and 18:2ω6 for fungi, and 16:1ω5 to indicate arbuscular mycorrhizal fungi (Swallow et al. 2009).
Statistical analysis
Arithmetic means of the evolved CO2 were calculated from each consecutive measurement date. Net NO3−, NH4+, and N mineralization rates were calculated as changes in the sizes of the NH4 pools, NO3− pools, and inorganic N (NO3− + NH4+) of two sampling times divided by the time interval between the sampling periods. Three-way analysis of variance (ANOVA) was used to examine differences in N mineralization, DOC, and PLFA among treatments and incubation periods. Significantly different means were compared via least significant difference (LSD) at a 5% probability level. Principal component analysis (PCA) on the relative abundances of PLFAs (all PLFAs present at more than 1% of the total PLFAs) was used to assess differences in the microbial community structure among all treatments. All statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA), Origin 9.2 (Origin Lab, Northampton, MA, USA), and CANOCO 5.0 (Microcomputer Power, Inc., Ithaca, NY, USA).
Results
Carbon mineralization
The rate of CO2 emissions increased rapidly during the first 16 days and gradually decreased thereafter for all treatments (Fig. 1). Application of 1% biochar alone had no effect on total CO2 emissions compared to the control soil (where emissions increased by 0.3%). Application of 3% biochar alone significantly reduced total CO2 emissions over a 90-day period, amounting to a 14% reduction in CO2 emissions.
Litter addition significantly increased soil CO2 emissions and total CO2 emissions over the 90-day incubation period were similar for all three litter treatments. Total CO2 emissions from the L treatment were only slightly higher than from 1BL and 3BL treatments (0.6 and 4.0%, respectively).
Biochar contained carbonate of 7.2 g kg−1 yet carbonate was not detected in any treatment at the end of incubation. We therefore assumed that all carbonate-C had been converted to CO2 during incubation. This would equate to 0.072 and 0.216 mg CO2–C g−1 soil in the 1 and 3% biochar applications, respectively.
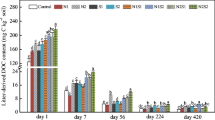
DOC concentration
Soil DOC concentration was significantly affected (p ≤ 0.001) by biochar, incubation time, and interactions between both factors (Table 2). In soil without litter, addition of 3% biochar resulted in a significantly lower (p ≤ 0.001) DOC concentration compared to the control treatment and the 1% biochar treatment throughout the study (Fig. 2). Although the DOC concentration in the 1% biochar treatment was lower than in control soil, significant differences were recorded only at days 30, 45, and 75. DOC concentrations were similar in control and litter-only treatment throughout incubation. In soil with litter, 3% biochar significantly reduced (p < 0.01) DOC concentrations over the 90-day period compared to the 1% biochar treatment and the litter only treatment. DOC concentrations were significantly lower in the 1% biochar + litter treatment than in the litter alone treatment throughout the incubation period except on days 0 and 60.
N mineralization
Soil NH4+ concentrations in all treatments generally showed declining trends over the incubation duration (Fig. 3a) and were significantly affected by biochar, litter addition, incubation duration, and all possible interactions between these factors (p ≤ 0.001) (Table 2). Addition of biochar alone significantly reduced NH4+ concentration compared to the control after 45 days of incubation. When more biochar was added, NH4+ reduction was more pronounced. The addition of litter significantly reduced NH4+ concentration and NH4+ mineralization rate (Table 3). In litter treatments, biochar applications of 1 or 3% had no significant effect on the NH4+ concentration during the entire incubation except at day 0. The addition of biochar to soil with or without litter had no significant effect on NH4+ mineralization rate at the end of the incubation period (Table 3).
NO3− concentrations increased over time in all treatments (Fig. 3b). NO3− concentration, was affected by addition of litter, by incubation duration, and by the interaction between these factors (p ≤ 0.001) (Table 2). In soils without litter, NO3− concentrations were not affected by addition of biochar at either 1 or 3% by the end of incubation. Addition of biochar alone had little effect on NO3− mineralization rate and net N mineralization rate. Addition of litter significantly reduced NO3− concentration except at days 0 and 15, and significantly reduced both NO3− mineralization rate and net N mineralization rate (Table 3). In soils with litter, NO3− concentrations were not significantly altered by 1 or 3% biochar application. Biochar application did, however, significantly reduce net N mineralization rate and NO3− mineralization rate, and the effect on both rates was greater at 3% application of biochar (Table 3).
Soil microbial community structure
Total PLFA concentrations in all treatments were measured in soils that were destructively sampled after incubation for 0, 30, 60, and 90 days and PLFAs were assigned to five main groups of microorganisms: Fungi, Actinobacteria, Gram-positive bacteria, Gram-negative bacteria, and bacteria in general. The proportions of these groups in the microbial community were determined for all treatments and for all sampling times (Table 4).
Total microbial biomass (represented by total PLFA concentration) changed significantly over time (F = 80.561, p < 0.001) (Fig. 4). Addition of 3% biochar alone significantly decreased microbial biomass at day 30 but 1% biochar had little effect. Microbial biomass in 3% biochar treatment progressively increased and resulted in higher PLFA concentrations compared to 1% biochar treatment and control soil. Litter treatments exhibited higher microbial biomass than corresponding treatments without litter for the entire incubation time. Biochar addition (1 or 3%) with litter had no significant effect on microbial biomass compared to the litter alone treatment prior to day 60. However, at the end of the incubation, and in both presence and absence of litter, a rate of 3% biochar significantly increased total PLFA concentration relative to the corresponding 1% biochar treatments and control treatment.
Dynamic variation in phospholipid fatty acid (PLFA) concentrations in soils amended with or without biochar and litter during the 90-day incubation period. Different letters denote significant differences between treatments at the same incubation time at p < 0.05. Values represent means (n = 3) ± S.E. (error bars)
The first two principal components (PC1 and PC2) explained 60.94% of the total variation of the data (PC1 and PC2 explained 40.27 and 20.67%, respectively) (Fig. 5). The plot of PC2 vs. PC1 indicates a successional shift in PC1 in the microbial community structure at different incubation times. Evidence was found of a difference in community structure between soils with and without litter, which were separated along PC2. Incubation time and litter affected microbial community structure. PCA was also used to identify changes in microbial communities at different sampling times (Fig. S1). The PLFA profiles of litter treatments were all well separated from treatments without litter along PC1 at all sampling times, explaining most of total variation. Litter application significantly affected microbial community structure but the effect of biochar was not as strong as the effect of time or litter application. Biochar applied at both 1 and 3% altered the microbial community structure at the end of the incubation period both in the absence and presence of litter (Fig. S1). However, in the absence of litter, a change of microbial community structure at 3% biochar-only treatment was recorded at day 30 compared to control soil. For the 1% biochar-only treatment, a similar change was recorded at day 60. In the presence of litter, the influence of the addition of biochar on microbial community structure was more complex.
Principal component analysis (PCA) ordination based on the relative abundances of individual phospholipid fatty acids (PLFAs) in soil amended with biochar and/or litter during the 90-day incubation period. Each point is labeled with the treatment code (CK, 1B, 1BL, 3B, 3BL, or L) followed by a number that indicates the incubation time (0, 30, 60, or 90 days)
At day 30, in soil without litter and compared to the control soil, treatment with biochar (1 and 3%) significantly increased the proportions of Gram-positive bacteria and Actinomycetes (Table 4). At day 60, the addition of biochar significantly reduced the proportion of fungi and Gram-positive bacteria. After 90 days, the addition of biochar alone (both 1 and 3%) significantly increased the proportion of fungi and total bacteria, but reduced the proportion of total bacteria compared to the control soil.
Relative to the control soil, all litter treatments showed a significantly higher proportion of fungi and total bacteria at all sampling times and a significantly lower proportion of Gram-positive bacteria after 60 days. In soils with litter, biochar applied at 1 or 3% significantly increased the proportion of Actinomycetes only at day 90.
Discussion
Effect of biochar addition on soil CO2 emissions
Zimmerman (2010) reported that biochar can be partially mineralized by both abiotic and biotic mechanisms. Abiotic degradation mainly involves the release of inorganic carbon (particularly in the form of carbonate), which might also contribute to overall CO2 emissions, particularly when biochar is added to acidic soil. Our results showed that addition of 3% biochar alone caused a significant decline in CO2 emissions compared to control soil, while addition of 1% biochar had no effect. After all carbonate-derived CO2 from biochar was subtracted from total CO2 emissions, CO2 emissions at the end of incubation were similar between 1% biochar-only treatment and control treatment. But the decomposition of SOC declined in biochar-only soil at the 3% rate of biochar addition. Similar results were reported by others although the extent of the reported decreases varied widely (Prayogo et al. 2014; Lu et al. 2014). In contrast, in most other studies, the addition of biochar did not promote decomposition of SOM (Maestrini et al. 2014; Cui et al. 2017). Zimmerman et al. (2011) reported that the effect of biochar on SOC mineralization decreased and changed from promotion to suppression in response to an increase in pyrolytic temperature from 250 to 650 °C. The extent of SOM decomposition in biochar-amended soil is strongly affected by the physical and chemical properties of both soil and biochar (Zimmerman et al. 2011; Jiang et al. 2016). Pyrolytic temperature is an important factor that affects the properties of biochar. Reduced SOC mineralization under biochar-only treatment was documented by other short-term laboratory experiments that used biochar from plant feedstock mainly produced at 450–600 °C (Bamminger et al. 2014; Lu et al. 2014; Prayogo et al. 2014).
In the presence of litter, total CO2 emissions declined only slightly with biochar addition compared to the litter-only treatment. Total CO2 emissions from SOM + litter after treatment with biochar decreased during 90 days in response to increasing rates of biochar addition. It is possible that the mineralization of soil plus litter was reduced. Similarly, Jiang et al. (2016) reported that 10% biochar application significantly reduced the CO2 emissions from soil plus low molecular weight C (LMW-C). But the mineralization of biochar was promoted in the presence of LMW-C. More complex interactions might take place when biochar is added to soil with other added materials. Some studies reported that the effect of biochar differs for the mineralization rate of SOM and added materials. Keith et al. (2011) reported that the application of wood biochar significantly reduced total CO2 emissions and mineralization of added materials (sugar cane residue) and they suggested that this might be due to biochar stabilizing added materials-OC by tapping into organo-mineral fractions. However, Novak et al. (2010) reported that biochar addition to switchgrass-soil mixtures stimulated the decomposition of switchgrass litter due to preferential microbial utilization of litter over SOM in the presence of biochar without causing an increase in CO2 evolution. Similarly, Cui et al. (2017) reported that biochar addition to soil did not change the total CO2 release in straw litter mixtures, but increased litter decomposition and reduced the CO2 release by SOM. The reasons for these different results may be linked to the disparity of biochar, soil, and the characteristics and degradability of added materials. The quality of the added material is likely a central factor for the determination of the trend of the interactive effect (Zimmerman et al. 2011).
Effect of biochar addition for DOC concentration and microbial community
Substrate availability and microbial activity are important factors for controlling SOM decomposition. DOC can be an indicator of total available carbon. We found a decrease in DOC concentration in soils after biochar treatment (with or without litter), with a negatively related magnitude to biochar concentration. This result could be partly attributed to sorption of DOC on either the pores or surfaces of biochar. Sorption of DOC can prevent soil-C movement to microbial consumers, thus resulting in the reduction of SOC decomposition (Lu et al. 2014). This offers a plausible explanation for the observed reduction of CO2 emissions and SOM mineralization in biochar-only amended soils in other studies (Lu et al. 2014; Kuzyakov et al. 2009). However, these studies were commonly conducted at low biochar application rates. In contrast, Jiang et al. (2016) found that addition of 10% biochar significantly increased soil DOC concentration while decreasing SOC mineralization. The authors suggested that this would be due to significantly increased pH resulting from biochar addition that led to higher DOC solubility.
Analyses of PLFA showed that application of 3% biochar-only significantly reduced total PLFA concentration compared to control soils at day 30 but that 1% biochar-only treatment had little effect. We infer that a significant reduction of DOC concentration due to the sorption by biochar likely limited the growth of microorganisms during the initial stage. Therefore, microbes would be compelled to gradually switch their food source from the labile fraction of SOM to the recalcitrant fraction of SOM, or to new input (biochar) following the addition of 3% biochar. The observed change in the relative abundance of the microbial community indirectly confirmed this. Addition of biochar alone (1 or 3%) increased the abundance of Gram-positive bacteria and Actinomycetes relative to control soil at day 30, especially when only 3% biochar was applied (p < 0.05). Gram-positive bacteria prevail in nutrient limited soil and are known to preferentially degrade aromatic C in soil (Santos et al. 2012). Actinomycetes are effective in the decomposition of complex aromatic materials via the production of a series of extracellular enzymes (Mccarthy and Williams 1992). These microbial groups can better utilize biochar-C. The total PLFA concentration subsequently increased in the 3% biochar-only treatment, which probably indicates the adaptation of microorganisms. However, this could also be explained by a gradual reduction in biochar adsorption capacity for nutrients and DOC after saturation. After this, the microbial community relative abundance changed and the response varied over time. Previous studies have shown large disparities in the shifts of microbial community composition treated with biochar (Chen et al. 2014; Lu et al. 2014). Some researchers highlighted the importance of the type of biochar that led to differential responses of fungi and bacteria with respect to their preferred energy sources (Lehmann et al. 2011; Jiang et al. 2016). In addition, different sampling times could be responsible for differences in the results reported for previous studies. In our study, the soil microbial community structure was significantly affected by biochar addition after 90 days; however, the change in response to 3% biochar-only treatment was observed earlier than in the 1% biochar-only treatment. This result is consistent with the results of Jiang et al. (2016), who reported that a higher biochar application rate altered the microbial community structure more rapidly than did a lower application rate.
In our study, litter addition significantly increased the PLFA concentration compared to control soil. This could be a result of the litter-C source availability for microbial growth. Higher PLFA concentration corresponds to higher cumulative CO2 emissions and N immobilization in the litter treatments. In addition, litter added to soil significantly increased fungi and reduced Gram-positive bacteria relative to the control soil both with and without biochar, thereby resulting in increased fungal to bacterial ratios during the incubation period. C availability is key to boosting fungal and bacteria growth rates, and the fungal community is primarily responsible for litter decomposition due to its ability to degrade the lignin within litter (Lehmann et al. 2011). Our result was consistent with previous studies (Bamminger et al. 2014; Lu et al. 2014; Prayogo et al. 2014). In addition, we found that the proportion of actinomycetes increased when biochar (1 and 3%) was applied to soil with litter, which was observed only at day 90 with increasing rate of biochar application. The relative abundance of the microbial community at other sampling times did not vary between litter treatments. Actinomycetes are involved in the breakdown of complex C forms (Jiang et al. 2016). It is possible that in the presence of litter, microbes preferentially mineralized easily decomposable substrates in the litter rather than the more recalcitrant OC in both SOC and biochar (Jiang et al. 2016; Cui et al. 2017).
A similar trend of DOC concentration was observed in litter treatments following different rates of biochar application. However, in the presence of litter, decreasing DOC concentration with biochar addition seemingly did not affect microbial biomass or microbial community composition within 60 days, even at a rate of 3% biochar addition. One possible explanation for this result is that the decreasing DOC concentration was not sufficient to limit the growth of microorganisms in the short term due to the presence of litter. At the end of the incubation period, increasing proportions of actinomycetes in biochar-plus-litter treatments might signal the gradual depletion of litter-C. However, this speculation requires verification.
Our results also suggest that biochar addition to soils with or without litter increased the total PLFA concentration compared to corresponding control treatments (CK and L) at the end of the incubation period. Similar results have been reported for previous studies (Prayogo et al. 2014, Bamminger et al. 2014). It is possible that the addition of biochar ultimately altered the usage of C sources by microorganisms and enhanced microbial C use efficiency (CUE), thus preventing soil-C loss respired CO2.
Soil nitrogen mobilization and immobilization
N mineralization is closely linked with C mineralization. Our results showed that addition of biochar reduced NH4+ concentration with increasing application rate of biochar. This might be partly attributed to biochar absorption of NH4+ due to its negatively charged surface (Dempster et al. 2012). In addition, we also found that biochar-only treatment (1 or 3%) had lowered the NO3− concentration compared to the control soil in the middle of incubation (Fig. 3b). We suggest that biochar can adsorb NH4+ ions, which consequently become unavailable and constrain nitrification through substrate limitation, leading to a reduction of the NO3− concentration (Nelissen et al. 2012). The process also reduced mineralization of SOC. At the same time, labile fractions in biochar would contribute to N immobilization via microbial uptake, which would be accompanied by an increase in microbial N demand (NH4+ and NO3−) that could be a short-term phenomenon due to depletion of the labile fraction (Bruun et al. 2013). Changes of soil microbial community structure under biochar addition will affect N dynamics.
All litter treatments had significantly lower inorganic N concentrations (Fig. S2) due to inorganic N immobilization. Litter had a wide range of C: N ratios, which causes soil microbes to immobilize more N to meet OC assimilation. In the presence of litter, and despite the small effect of biochar amendment on NH4+ (except at day 0) and NO3− concentration, biochar significantly reduced the rate of NO3− mineralization and net N mineralization with increasing application rate of biochar (Table 3). Hence, biochar added to soil with litter caused N immobilization. This implies enhanced N uptake and/or retention by microbes in soil-litter mixtures due to biochar addition. This finding accords with results reported by Cui et al. (2017), who also reported that biochar addition to soil-maize litter mixtures resulted in the immobilization of mineral N. Jmdl and Knicker (2011) reported that biochar-contained N can be utilized by biomass. The biochar used in our experiment contained higher inorganic N than soil (Table 1). Hence, biochar added to soil with litter could enhance soil inorganic N concentration and alleviate microbial N limitations. This might be conducive to the decomposition of litter as well as N immobilization. The process might also lessen microbial N requirements from soil, thus decreasing SOM decomposition (Cui et al. 2017). The labile C component of biochar may have provided additional C sources for microbial N immobilization.
Conclusion
Our results showed that application of biochar alone reduced the mineralization of SOM with an increasing rate of biochar application at 90 days, but did not change N mineralization. Adsorption effects of biochar on DOC play an important role by affecting the microbial community in its stabilization of SOC and reduction of CO2 emissions. The addition of litter increased total CO2 emissions, thereby promoting N immobilization and significantly changing the microbial community structure compared to control soil. In the presence of litter, addition of biochar reduced total CO2 emissions from SOM + litter. Decreasing DOC concentration due to biochar adsorption in litter treatments did not significantly affect the microbial community composition in the short term, but significant differences in relative abundances of the microbial community composition between litter treatments were observed at day 90. It is possible that in the presence of litter, microorganisms typically prefer to utilize litter-C over biochar or SOM. Furthermore, biochar addition promoted N immobilization in the presence of litter. Therefore, we suggest that biochar addition would promote the decomposition of litter and decrease SOM mineralization. These findings have the important implication that incorporating biochar into management of logging residues together with applying biochar to China fir plantation soil would offer a good option to mitigate CO2 emission, control leaching loss, and favor microbial communities that play key functional roles in the forest ecosystem. Further work is required to partition the sources of CO2 emissions via isotopic labeling to further clarify the underlying mechanisms. Field experiments are required to fully investigate and verify the long-term behavior of biochar with regards to SOC cycling and microbial communities.
References
Bai SHR, Dempsey F, Reverchon TJ, Blumfield S, Ryan LA, Cernusak LA (2017) Effects of forest thinning on soil-plant carbon and nitrogen dynamics. Plant Soil 411:1–13
Baldock JA, Smernik RJ (2002) Chemical composition and bioavailability of thermally, altered Pinus resinosa (Red Pine) wood. Org Geochem 33:1093–1109
Bamminger C, Zaiser N, Zinsser P, Lamers M, Kammann C, Marhan S (2014) Effects of biochar, earthworms, and litter addition on soil microbial activity and abundance in a temperate agricultural soil. Biol Fertil Soils 50(8):1189–1200
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2013) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46(1):73–79
Chen R, Mehmet S, Sergey B, Olga M, Klaus D, Lin X, Evgenia B, Yakov K (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Global Change Biol 20(7):2356–2367
Cui J, Ge T, Kuzyakov Y, Nie M, Fang C, Tang B, Zhou C (2017) Interactions between biochar and litter priming: a three-source 14C and δ13C partitioning study. Soil Biol Biochem 104:49–58
Dempster DN, Gleeson DB, Solaiman ZM, Jones DL, Murphy DV (2012) Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 354(1):311–324
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465(6):288–297
Hansen V, Müllerstöver D, Munkholm LJ, Peltre C, Hauggaardnielsen H, Jensen LS (2016) The effect of straw and wood gasification biochar on carbon sequestration, selected soil fertility indicators and functional groups in soil: an incubation study. Geoderma 269:99–107
Heitkötter J, Marschner B (2015) Interactive effects of biochar ageing in soils related to feedstock, pyrolysis temperature, and historic charcoal production. Geoderma 17:56–64
Institute of Soil Science, Academia Sinica (1978) Physical and chemical analysis methods of soil. Shanghai Science Technology Press, Shanghai, pp 157–161, 466–532
Jiang X, Cao L, Zhang R (2014) Effects of addition of nitrogen on soil fungal and bacterial biomass and carbon utilisation efficiency in a city lawn soil. Soil Res 52(1):97
Jiang X, Denef K, Stewart CE, Cotrufo MF (2016) Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol Fertil Soils 52(1):1–14
Jmdl R, Knicker H (2011) Bioavailability of N released from N-rich pyrogenic organic matter: an incubation study. Soil Biol Biochem 43(12):2368–2373
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ Sci Technol 45(22):9611–9618
Kuzyakov Y, Subbotina I, Chen HQ, Bogomolova I, Xu XL (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41(2):210–219
Lehmann J (2007) A handful of carbon. Nature 447(7141):143–144
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43(9):1812–1836
Lu W, Ding W, Zhang J, Li Y, Luo J, Bolan N, Xie Z (2014) Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76(1):12–21
Ma X, Heal KV, Liu A, Jarvis PG (2007) Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. For Ecol Manag 243(1):61–74
Maestrini B, Herrmann AM, Nannipieri P, Schmidt MWI, Abiven S (2014) Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol Biochem 69(69):291–301
Mccarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment–a review. Gene 115(1–2):189–192
Nelissen V, Rütting T, Huygens D, Staelens J, Ruysschaert G, Boeckx P (2012) Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol Biochem 55:20–27
Ng EL, Patti AF, Rose MT, Schefe CR, Wilkinson K, Smernik RJ, Cavagnaro TR (2014) Does the chemical nature of soil carbon drive the structure and functioning of soil microbial communities? Soil Biol Biochem 70(2):54–61
Novak JM, Busscher WJ, Watts DW, Laird DA, Ahmedna MA, Niandou MAS (2010) Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 154(3):281–288
Pan F, Li Y, Chapman SJ, Khan S, Yao H (2016) Microbial utilization of rice straw and its derived biochar in a paddy soil. Sci Total Environ 559(559):15–23
Prayogo C, Jones JE, Baeyens J, Bending GD (2014) Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol Fertil Soils 50(4):695–702
Santos F, Torn MS, Bird JA (2012) Biological degradation of pyrogenic organic matter in temperate forest soils. Soil Biol Biochem 51(3):115–124
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77(4):574–581
Swallow M, Quideau SA, MacKenzie MD, Kishchuk BE (2009) Microbial community structure and function: the effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol Biochem 41(4):770–777
Wardle DA, Nilsson MC, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320(5876):629
Winkler JP, Cherry RS, Schlesinger WH (1996) The Q10 relationship of microbial respiration in a temperate forest soil. Soil Biol Biochem 28(8):1067–1072
Xu CY, Hosseini-Bai S, Hao Y, Rachaputi RC, Wang H, Xu Z, Wallace H (2015) Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ Sci Pollut Res Int 22(8):6112–6125
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102(3):3488–3497
Zhang G, Guo X, Zhu Y, Han Z, He Q, Zhang F (2017) Effect of biochar on the presence of nutrients and ryegrass growth in the soil from an abandoned indigenous coking site: the potential role of biochar in the revegetation of contaminated site. Sci Total Environ 601–602:469–477
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44(4):1295–1301
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43(6):1169–1179
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding
This work was supported by the National Science and Technology Support Program (2015BAD09B010102) and International Science and Technology Cooperation Program of Fujian Agriculture and Forestry University (KXB16008A).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Zhou, C., Qiu, Y. et al. Effects of biochar and litter on carbon and nitrogen mineralization and soil microbial community structure in a China fir plantation. J. For. Res. 30, 1913–1923 (2019). https://doi.org/10.1007/s11676-018-0731-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0731-5