Abstract
Biochar application to arable soils could be effective for soil C sequestration and mitigation of greenhouse gas (GHG) emissions. Soil microorganisms and fauna are the major contributors to GHG emissions from soil, but their interactions with biochar are poorly understood. We investigated the effects of biochar and its interaction with earthworms on soil microbial activity, abundance, and community composition in an incubation experiment with an arable soil with and without N-rich litter addition. After 37 days of incubation, biochar significantly reduced CO2 (up to 43 %) and N2O (up to 42 %), as well as NH4 +-N and NO3 −-N concentrations, compared to the control soils. Concurrently, in the treatments with litter, biochar increased microbial biomass and the soil microbial community composition shifted to higher fungal-to-bacterial ratios. Without litter, all microbial groups were positively affected by biochar × earthworm interactions suggesting better living conditions for soil microorganisms in biochar-containing cast aggregates after the earthworm gut passage. However, assimilation of biochar-C by earthworms was negligible, indicating no direct benefit for the earthworms from biochar uptake. Biochar strongly reduced the metabolic quotient qCO2 and suppressed the degradation of native SOC, resulting in large negative priming effects (up to 68 %). We conclude that the biochar amendment altered microbial activity, abundance, and community composition, inducing a more efficient microbial community with reduced emissions of CO2 and N2O. Earthworms affected soil microorganisms only in the presence of biochar, highlighting the need for further research on the interactions of biochar with soil fauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The addition of biochar to arable soils has been often shown to increase soil fertility and crop yield (Jeffery et al. 2011; Spokas et al. 2012). Another beneficial effect of biochar could be the reduction of greenhouse gas emissions from soils (Case et al. 2012; Kammann et al. 2012). However, reported effects of biochar on carbon dioxide (CO2) emissions from soil have been variable, ranging from a short-term increase to a decrease in CO2 emissions (Jones et al. 2011; Ameloot et al. 2013a; Kammann et al. 2012). Differences in C mineralization can be explained by different biochar and soil characteristics as well as various underlying processes, such as abiotic C-release from biochar, soil organic carbon (SOC) adsorption, and positive or negative priming effects (Zimmerman 2010; Jones et al. 2011; Bamminger et al. 2014). In addition, the emission of nitrous oxide (N2O), which is 265 times more potent as greenhouse gas than CO2 over a time period of 100 years (IPCC 2013), was found to be significantly reduced by biochar (e.g., Taghizadeh-Toosi et al. 2011; Kammann et al. 2012), while only a few studies have also shown increased N2O emissions (Saarnio et al. 2013). Possible reasons for these inconsistent biochar effects on N2O emissions could be related to different biochar and soil characteristics showing divergent effects of biochar on soil aeration and moisture conditions, nutrient availability, or soil microbial community structure (Clough and Condron 2010).
Biochar-related changes in micro-environmental conditions have been suggested to be responsible for observed modifications in soil microbial community composition (Khodadad et al. 2011) and abundances of different bacterial families (Anderson et al. 2011). Moreover, shifts to bacteria-dominated communities and decreases in fungal abundances have been observed in fields after biochar application (Jones et al. 2012; Chen et al. 2013). This emphasizes that there is a preferential microbial response to biochar addition, which may differ between fungi and bacteria, but the reasons for this are not well understood (Lehmann et al. 2011).
The pyrogenic C in biochar is more recalcitrant than other organic matter pools in soils (Vasilyeva et al. 2011), but it is not inert and can be slowly decomposed by abiotic and biologically mediated oxidation (Zimmerman 2010). Indeed, microbial biomass increased in biochar-amended soil (Jin 2010), but direct microbial consumption of labile fractions of biochar was observed mainly within the first 3 days and declined afterwards (Farrell et al. 2013). This suggests that the major parts of biochar are stable against microbial decomposition and that direct uptake of biochar-C is of minor importance for the activity and abundance of soil microorganisms. Finally, the enhanced soil microbial biomass and reduced CO2 respiration in the presence of biochar indicate a more efficient microbial community (Jin 2010), which may be caused by shifts in the community composition and changed substrate use patterns (Lehmann et al. 2011).
Beside soil microorganisms, which are most responsible for C and N mineralization in soils, earthworms have also been shown to increase emissions of CO2 and N2O (Lubbers et al. 2013) and to affect the mobilization as well as the stabilization of soil C and N (Marhan and Scheu 2005). Burrows and casts of earthworms provide substrates and nutrients for soil microorganisms, enhancing the decomposition and C-mineralization of plant residues. In addition, low oxygen availability in combination with high nutrient content in the gut of earthworms and their cast material provide ideal conditions for denitrifying bacteria and concomitant high N2O emissions (Drake and Horn 2007).
In comparison to the effects of biochar on soil microorganisms, even less is known about biochar effects on earthworms. The few existing studies have detected weight loss and mortality of earthworms after 28 days of incubation (Li et al. 2011), especially in soils with high doses of biochar (67.5 and 90 Mg ha−1) (Liesch et al. 2010). Negative effects on earthworm activity and biomass could arise from physical or chemical effects of biochar amendments, i.e., insufficient soil moisture due to the dry biochar (Li et al. 2011) or toxicity/salinity (Liesch et al. 2010). Furthermore, biochar may interact with earthworms, modifying greenhouse gas emissions from soils. In a pot experiment with endogeic earthworms of the species Aporrectodea icterica, Augustenborg et al. (2012) observed a reduction of the earthworm-induced N2O emissions by 20 to 95 % in the presence of biochar, while biochar-reduced CO2 emissions only in the absence of earthworms. This illustrates the potential of biochar to mitigate the N2O-emission stimulating earthworm effect. However, the stability of biochar against decomposition might be also affected by endogeic earthworms, which have been suspected of increasing the mobilization of old and possibly stable C resources in soils (Marhan et al. 2007).
We performed a factorial incubation experiment based on the following research questions: (1) Are endogeic earthworms able to mobilize and incorporate stable biochar-C, leading to increased decomposition of biochar? and (2) Will there be effects only of the single factors, earthworms, and biochar on C and N turnover, i.e., CO2 and N2O emissions or will there be interactions between both factors? In addition to the second question, we investigated whether the effects and interactions between biochar and earthworms will change when litter, as an additional C and N resource, is present in the soil and to which extent analyses of soil microbial abundance and community composition could help to explain the results? To address these questions, we mixed pyrolysis biochar (Miscanthus) with an arable soil and added specimens of Aporrectodea caliginosa, a common endogeic earthworm in temperate arable soils. Biochar derived from a C4 plant and showing another 13C-signature than the soil made it possible to quantify the earthworm effect on biochar-C mobilization. To one half of the experiment, we added N-rich plant litter, reflecting the incorporation of a green manure into arable soil, which is typically accompanied by high N2O emissions (Baggs et al. 2002). The effects of biochar and earthworms on C and N turnover were investigated by measuring CO2 and N2O emissions, microbial abundance and community composition were quantified by phospholipid fatty acid analyses (PLFA).
Materials and methods
Experimental setup
The experiment was conducted in vessels consisting of airtight Perspex tubes (height 150 mm, Ø 45 mm) fixed on water saturated ceramic plates. The vessels were closed at the top with a lid and a rubber stopper with a three-way stopcock, enabling gas sampling for CO2 and N2O measurements with a syringe from the head space. At the bottom of the lid, a small vial was attached, which was filled with NaOH to trap CO2 for determination of isotopic signature of CO2 produced inside the vessel (Marhan et al. 2007). The following treatments were established: soil only (Ctrl), soil with biochar (BC), soil with one juvenile A. caliginosa (EW), soil with biochar, and one juvenile A. caliginosa (BC + EW). Half of the vessels were set-up without litter (‘no litter’ treatments), the other half with Phacelia litter (‘with litter’ treatments). In total, 46 vessels were established (Ctrl, treatments n = 5; all others, n = 6), all soil mixtures were initially rewetted to 60 % of water holding capacity (WHC) of the control and incubated in darkness in a climate chamber at 20 °C for 37 days.
Materials
Soil
Soil was taken from the Ap-horizon (0–10 cm) of an arable field at the agricultural experimental station ‘Heidfeldhof’ (University Hohenheim, Germany). The soil is a slightly stagnic luvisol with a silty texture of 9 % sand, 69 % silt, and 22 % clay (Table 1). The soil was sieved (<2 mm) to remove stones, plant residues, earthworms, and their cocoons and stored at 4 °C for a few days until the experiment was set up. Each vessel was filled with fresh soil equivalent to 100 g dry weight (DW) and compacted to a bulk density of 1.2 g cm−3.
Biochar
The biochar was produced by slow pyrolysis (approximately 600 °C; production rate up to 40 kg biochar h−1) in a continuous reactor from Miscanthus x giganteus and was provided by Pyreg GmbH (Dörth, Germany) (Table 1). There was a low toxicity potential of PAHs, dioxins, heavy metals, or other persistent organic pollutants in the biochar (see further details in Table S1). The low toxicity of the biochar was revealed in a grassland field experiment showing no negative effects on plant growth (Schimmelpfennig et al. 2014). The biochar was sieved and particles <2 mm were homogenously mixed with soil (2 % w/w) to obtain an application rate of 30 Mg ha−1, assuming biochar incorporation into ploughing depth of 30 cm in the field. This is the typical application rate in several biochar experiments (Augustenborg et al. 2012).
Litter
Litter material was taken from Phacelia tanacetifolia Benth. plants grown in the same soil in a greenhouse for 10 weeks. Aboveground biomass was harvested, fragmented into <10 mm size pieces, and dried at 40 °C to constant weight. The green litter material had a low C/N ratio of 17 (350.2 g C kg−1, 20.6 g N kg−1). Litter was shredded to 5 mm size and homogenously mixed into the soil of the litter treatments at a rate of 1.54 % w/w of soil, which is equivalent to 23.1 Mg ha−1; this represents the amount of Phacelia litter ploughed into the soil as green manure from an arable field at the ‘Heidfeldhof’ field station.
Earthworms
Juvenile endogeic earthworms (A. caliginosa Savigny) were extracted from grassland adjacent to the arable field by hand sorting. The use of juvenile specimens enabled the detection of earthworm biomass decrease as well as increase (Marhan and Scheu 2005). The earthworms were kept in the experimental soil for 6 days until the incubation experiment was set up. Before the earthworms were placed into the vessels, they were kept on wet filter paper for 1 day to void their guts. Afterwards, they were washed with water, dabbed dry, and weighed, giving the initial live weight. The mean body mass of A. caliginosa specimens was 138 mg fresh weight with a range of 90 to 192 mg. Smaller and larger specimens were homogenously distributed over the earthworm containing treatments. After incubation, the soil was carefully removed from the vessels to avoid injuring the earthworms and earthworm body mass was determined in the same way as described above in order to calculate changes in individual body mass.
Analyses
C, δ13C, N analyses, and pH values
Initial soil, litter, and biochar C and N concentrations and their isotopic signatures (δ13C) were measured using an elemental analyzer (EA, Euro EA 3000, Euro Vector, Milan, Italy) coupled with an isotope mass spectrometer (IRMS, DeltaXP Plus, Thermo Finnigan, Waltham, USA). For this analysis, the sieved and dried soil, litter, and biochar were finely ground. Earthworm δ13C signatures were determined by analysing tissue material from the anterior part of specimens, which contained no soil particles. For this, earthworms were killed by freezing and the anterior part of the frozen earthworms was cut off and dried at 60 °C. About 1.3 to 3.98 mg dry tissue material from each individual earthworm was analyzed. The earthworm δ13C signatures were determined for three specimens per treatment. After the incubation, soil pH values were measured in 0.01 M CaCl2 solution (1:4 w/v).
Extractable organic C (EOC), ammonium (NH4 +-N), and nitrate (NO3 −-N)
At the end of the incubation EOC, NH4 +-N and NO3 −-N concentrations in soil were determined by extracting the soil with 0.5 M K2SO4 (1:4 w/v). Soil suspensions were shaken on a horizontal shaker (30 min at 250 rpm) and centrifuged (30 min at 4400×g). Concentrations of EOC in the supernatant were then analyzed with a DOC analyzer (multi N/C 2100 S, Analytik Jena AG, Jena, Germany). NH4 +-N and NO3 −-N concentrations in the supernatant were determined colorimetrically with a continuous flow analyzer (Bran + Luebbe Autoanalyzer 3, SEAL Analytical, Hamburg, Germany).
CO2, N2O, and 13CO2 emission
To measure CO2 and N2O emissions, vessels were tightly closed and 15 ml of the headspace volume was sampled immediately and 60 min after closure. Gas samples were taken with 20 ml syringes via three-way stopcocks and injected into pre-evacuated 5.9 ml exetainers (Labco Ltd., UK). CO2 and N2O concentrations in the headspace samples were determined on an Agilent 7890 gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a methanizer and a FID for CO2 and an ECD for N2O measurements. Three external standards per gas were used for calibration by linear regression (0.304, 1.487, and 2.966 mmol mol−1 CO2; 0.568, 1.099, and 2.056 μmol mol−1 N2O; Westfalengas, Germany). Gas samples were taken at days 1, 2, 3, 6, 8, 10, 13, 16, 21, 27, 30, and 34 of incubation. Cumulative CO2 and N2O fluxes were calculated by linear interpolation between two consecutive measurements.
13C in produced CO2 was determined according to the method of Marhan et al. (2008) by capturing emitted CO2 in NaOH solution (1 M) in the headspace of each vessel and measuring 13C in the precipitated SrCO3 at days 1, 4, 7, 10, 15, 18, 22, 29, 32, and 37 of incubation. Between gas sampling dates, lids or holes were left open to ensure free gas exchange.
Calculation of biochar-derived C in CO2 and priming effects
The biochar derived from the C4 plant Miscanthus has a δ13C value of −13.82‰, different from that of the soil (−27.28‰). Determination of biochar-C and SOC mineralization was possible for the ‘no litter’, but not for the ‘with litter’ treatments, due to the different 13C signature of the litter-C in comparison to soil-C, which served as a third, not quantifiable CO2 source. For the calculation of the relative amounts of biochar-C and native SOC in CO2 at specific dates of the incubation, a simple two-pool mixing model was used (Gregorich et al. 1995).
Priming effects (PEs) were calculated for ‘no litter’ treatments based on the 13C data by determining the difference in the native SOC mineralization between biochar-amended samples (BC and BC + EW) and respective controls (Ctrl and EW) (Bamminger et al. 2014) as shown in Eq. 1:
Phospholipid fatty acid analysis
The PLFAs of 4 g incubated soil (fresh weight) from each vessel were extracted according to Frostegård et al. (1993) with Bligh & Dyer solution (chloroform, methanol, citrate buffer; pH = 4; 1:2:0.8 v/v/v) and separated into glycolipid, neutral lipids, and phospholipid fatty acids with silica acid columns (0.5 g silicic acid, 3 ml; Varian Medical Systems, Palo Alto, California). Only the PLFA-fraction was analyzed. The branched fatty acids i15:0, a15:0, i16:0, and i17:0 were summed as Gram-positive and the cy17:0 and cy19:0 as Gram-negative bacteria (Zelles 1999). In addition to these biomarkers, 16:1ω7 was included for total bacteria calculation (Frostegård and Bååth 1996). The biomarker 18:2ω6,9c was considered as fungal PLFA (Frostegård and Bååth 1996; Kaiser et al. 2010). Total microbial PLFA (PLFAmic) consists of total bacterial and fungal PLFA. Metabolic efficiency (qCO2) of the soil microbial community was calculated by the ratio between CO2-C and microbial PLFA.
Statistical analysis
Data on cumulative CO2-C and N2O-N production, contents of EOC, NH4 +-N, NO3 −-N, soil pH, and microbial PLFA data were analyzed by two-way analysis of variance (ANOVA) separately for the ‘no litter’ and ‘with litter’ treatments due to the strong effect of the litter, which led to inhomogeneity of the variances. Factors for two-way ANOVA were ‘biochar’ (BC; without and with BC) and ‘earthworm’ (EW; without and with EW). For the two-way ANOVA of earthworm biomass changes, the litter treatments were not separated and factors were ‘biochar’ and ‘litter’ (no and with litter). Prior to analysis, data were log-transformed in the case of non-normal distribution and inhomogeneity of variance (Levene test). A statistical probability of P < 0.05 was considered significant. The STATISTICA 6.0 software package (Statsoft, Tulsa, OK, USA) was used for statistical analyses.
Results
Earthworm biomass
All earthworms survived the incubation in the ‘no litter’ treatments and only one earthworm died in the ‘with litter’ treatments. This replicate was excluded from further analyses. Litter significantly affected earthworm biomass; in the ‘no litter’ treatments, earthworms reduced their biomass by 19 and 23 %, whereas in the ‘with litter’ treatments earthworms gained biomass by 42 and 32 % in the absence and presence of biochar, respectively (data not shown). Earthworm biomass showed no significant effects from biochar addition. After 37 days, the δ13C signatures of the earthworms’ biomass seemed to be more depleted in the ‘with litter’ than in the ‘no litter’ treatments (P = 0.08), showing that litter derived-C was assimilated (Fig. S1), but no significant differences in earthworm δ13C signatures were found between treatments with and without biochar.
CO2 emissions
CO2 emission rates declined slightly in the ‘no litter’ treatments, whereas in the ‘with litter’ treatments decomposition of the litter was highest during the first 4 days (Fig. S2a). Basal respiration (RB), which was the CO2 production rate at the end of the experiment, was 36-fold higher in the ‘with litter’ than in the ‘no litter’ treatments. In both litter treatments, RB was significantly reduced by biochar, but not affected by earthworms (Tables 2 and 3). Cumulative CO2 production over the incubation period ranged between 0.05 and 0.11 mg CO2-C g−1 dws in the ‘no litter’ treatments and between 0.91 and 1.30 mg CO2-C g1 dws in the ‘with litter’ treatments (Fig. 1a). Biochar significantly (P < 0.05) reduced cumulative CO2 emissions in the ‘no litter’ and ‘with litter’ treatments by 43 and 27 %, respectively (Fig. 1a, Table 3). Earthworms had no significant effect on the CO2 efflux.
Mineralization of biochar-C and SOC
Calculations of biochar-C derived CO2 were not possible for the first half of the experiment due to strong variations in 13CO2 values, likely caused in part by initial release of inorganic C from the biochar (Bruun et al. 2008). During the second half of the experiment (days 18, 22, 29, and 32), the contribution of mineralized biochar-C to total CO2 emissions was on average 6.3 % in the ‘no litter’ treatment without earthworms (BC) and showed no increasing or decreasing trend (data not shown). In combination with the reduced total CO2 emissions in this treatment, we calculated a negative priming effect of biochar, reducing soil-C mineralization by 56 % on an average. Biochar-C mineralization seemed to be higher, by an average of 9.5 %, in the presence of earthworms in the ‘no litter’ treatment (BC + EW), but this difference was not significant (P = 0.097). However, as total CO2 production was similar to the treatment without earthworms, the negative priming effect of biochar increased due to earthworm activity up to 68 %.
N2O emissions
N2O emission rates were much lower in the ‘no litter’ treatments than in the ‘with litter’ treatments, but were generally highest during the first 2 days of incubation and declined afterwards rapidly until the end of the incubation (Fig. S2b). Cumulative N2O production in the ‘no litter’ treatments ranged from 0.08 to 0.15 μg N2O-N g−1 while in the ‘with litter’ treatments N2O production ranged from 0.68 to 1.35 μg N2O-N g−1 dry soil after 37 days (Fig. 1b). While biochar did not significantly reduce N2O emissions in the ‘no litter’ treatments, cumulative N2O production was significantly reduced by 42 % in the ‘with litter’ treatments (Fig. 1b, Table 3). Earthworms did not exhibit a significant effect on N2O emissions neither in the ‘no litter’ nor in the ‘with litter’ treatments.
pH, EOC, NH4 +-N, and NO3 −-N
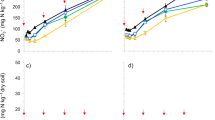
Litter amendment increased pH values consistently above 7. Overall, biochar slightly increased pH values, by 0.1 to 0.3 units (Table 2), which was significant in the ‘no litter’, but not in the ‘with litter’, treatments (Table 3). EOC content in the ‘with litter’ treatments was almost twofold higher than in the ‘no litter’ treatments (Table 2). Neither biochar nor earthworms affected EOC contents significantly (Table 3). NH4 +-N concentrations were between four to sixfold higher in the ‘with litter’ than in the ‘no litter’ treatments (Fig. 2a). In the ‘with litter’ treatments only, both biochar and earthworms reduced NH4 +-N concentrations, but the earthworm effect was less pronounced in the presence of biochar (BC × EW interaction, Table 3). Concentrations of NO3 −-N strongly exceeded those of NH4 +-N and were almost twofold higher in the ‘with litter’ than in the ‘no litter’ treatments (Fig. 2). Biochar significantly (P < 0.05) reduced NO3 −-N by 40 % in the ‘no litter’ and by 16 % in the ‘with litter’ treatments (Fig. 2b, Table 3). Earthworms did not affect the amount of extractable NO3 −-N in the present experiment.
Microbial PLFA content
Total microbial and bacterial PLFA abundances (Table 2) and PLFA abundances of Gram-positive bacteria, Gram-negative bacteria, and fungi were higher in the ‘with litter’ than the ‘no litter’ treatments (Fig. 3a–c). While a two to threefold increase was observed for bacterial PLFAs, fungal PLFA abundance was 8 to 13-fold higher in the ‘with litter’ compared to the ‘no litter’ treatments. In the ‘no litter’ treatments, abundances of total bacteria, Gram-positive, Gram-negative, and fungi were significantly increased by both biochar and earthworms, but generally highest abundances were found in the BC + EW treatment (significant BC × EW interaction, P < 0.05, Tables 2 and 3, Fig. 3a–c). In the ‘with litter’ treatments, biochar significantly (P < 0.05) increased total microbial PLFA abundance (PLFAmic) by 16 % and that of total bacteria by 11 % (Tables 2 and 3). This biochar effect was mostly derived from a significant increase in abundances of Gram-positive bacteria (Fig. 3a, b; Tables 2 and 3). Moreover, biochar significantly (P < 0.05) increased the abundance of fungi by 34 % (Fig. 3c, Tables 2 and 3). The ratio of Gram-positive to Gram-negative bacteria was significantly (P < 0.05) increased by biochar only in the ‘no litter’ treatments (Tables 2 and 3). In the ‘with litter’ treatments, earthworms decreased the ratio of Gram-positive to Gram-negative bacteria only in the presence of biochar (BC × EW interaction). Biochar also significantly (P < 0.05) increased the fungal to bacterial ratio, but only in the ‘with litter’ treatments (Tables 2 and 3). At the end of the experiment, the metabolic quotient (qCO2) was significantly (P < 0.05) decreased by biochar in the ‘no litter’ (86 %) and in the ‘with litter’ (37 %) treatments, but remained unaffected by earthworms (Tables 2 and 3).
Concentrations of phospholipid fatty acids (PLFA) for a) Gram-positive, b) Gram-negative, and c) fungi in the ‘no litter’ and ‘with litter’ treatments at the end of the experiment (after 37 days). Ctrl soil only, BC soil with biochar, EW soil including one endogeic earthworm, BC + EW soil with biochar and one earthworm. Means ± SE
Discussion
The first aim of the present study was to answer the question whether endogeic earthworms are able to mobilize and incorporate stable biochar C, likely leading to increased decomposition of biochar. Biochar had no effect on earthworm biomass and stable isotope technique (13C) revealed that 13C-signatures of earthworm tissues did not change when biochar was present in soil. This indicates that endogeic earthworms did not assimilate biochar-C in relevant amounts. A selective avoidance of biochar by A. caliginosa, as suggested by Tammeorg et al. (2014), can be excluded from the present study as biochar particles were visible in produced earthworm casts. However, earthworms may ingest biochar particles due to its detoxifying and liming effects rather than for nutrient supply (Topoliantz and Ponge 2003).
Although the assimilation of biochar-C by earthworms seems to be negligible, the presence of earthworms led to a slightly higher mineralization of biochar-C in the ‘no litter’ treatments. As stated above, the quantification of biochar-C mineralization was only possible for the ‘no litter’ treatments due to the non-quantifiable litter derived-C contribution to total CO2 emission. Measurements of δ13CO2 revealed that only a small contribution (6.3 %) of the respired CO2 was biochar-derived in the treatment without earthworms. This indicates that the Miscanthus biochar was not inert, but rather stable, resisting decomposition by soil microorganisms. This is comparable to other studies with plant-derived biochars produced at 500–600 °C (Zimmerman 2010; Singh et al. 2012). In the presence of earthworms, the proportion of biochar-C in evolved CO2 was increased slightly (9.5 vs. 6.3 %). However, mineralization of earthworm biomass derived-C could influence 13C-signatures of emitted CO2 from soil (Marhan et al. 2007). During the present incubation, earthworm biomass decreased in the ‘no litter’ treatment and it could be that this earthworm derived-C, which has a δ13C signature between biochar and SOC might have contributed to the produced CO2. This could lead to an overestimation of the earthworm effect on biochar-C mineralization.
The second aim of our study was to determine whether only the single factors, earthworms and biochar, affect C and N turnover, i.e., CO2 and N2O emissions or whether they interact. We found no significant earthworm effect on CO2 and N2O emissions. This is in contrast to a recent meta-analysis by Lubbers et al. (2013), which has shown that earthworms alone often increase CO2 and N2O emissions from soil. The lack of an earthworm effect on CO2 and N2O emission was unexpected because increased CO2 production was found for the same soil and earthworm species by Marhan et al. (2010). In addition, no interactive effect of earthworms and biochar on CO2 and N2O emissions was observed in the present study. This is in contrast to Augustenborg et al. (2012) who found that endogeic earthworms increased GHG emissions and that this was mitigated by biochar.
However, biochar alone strongly reduced CO2 (by 43 %) in the ‘no litter’ and reduced both CO2 (by 27 %) and N2O (by 42 %) emissions in the ‘with litter’ treatments. These strong reductions of GHGs from soil by biochar are comparable to the results from other short-term laboratory studies using biochars from plant feedstock produced at 500–600 °C (Augustenborg et al. 2012; Cayuela et al. 2014). One of the most likely explanations for the reductions of CO2 and N2O emissions by biochar could be a decrease in soil microbial abundance. In contrast, analyses of PLFAs showed an increasing effect of biochar on microbial abundances in the ‘with litter’ treatments and at least no negative effect in the ‘no litter’ treatments in our study. It is still unclear whether biochar could interfere with extraction-based microbial analyses like PLFA, probably stabilizing dead microbial PLFA onto biochar particles, which may result in an overestimation of living microbial abundance (Lehmann et al. 2011). Although we cannot finally exclude these biochar effects on PLFA stabilization in soils, we assume that this could not explain the strong discrepancy between concurrent reduction of CO2 and N2O emissions and increment of PLFA abundances in the ‘with litter’ treatments.
Other potential reasons to explain the reductions of CO2 and N2O emissions by biochar have been also discussed in the literature (Ameloot et al. 2013b; Augustenborg et al. 2012; Cayuela et al. 2013, 2014; Lehmann et al. 2011): (1) Changes of soil moisture conditions, e.g., a biochar induced decrease of soil moisture could lead to water limitation for soil microorganisms, thus decreasing CO2 as well as N2O emissions; (2) Reduced C and N resource availability due to the adsorption onto biochar surfaces, protecting it from microbial decomposition; (3) Immobilization of N by soil microorganisms for building up their biomass thereby reducing mineral-N as substrates for N2O production by nitrification and denitrification; and (4) Changes in the microbial community composition resulting in a modified activity, e.g., due to biochar effects on soil pH. In the following paragraphs, these potential reasons will be discussed in the context of the results of our study:
We analyzed the potential effect of biochar on soil moisture in an additional laboratory analysis (see supplemental text, Fig. S3). This analysis revealed that at 60 % WHC, which was the adjusted water content for the incubation experiment, no changes due to biochar addition were found. We, therefore, excluded different soil moisture conditions as a possible explanation for the observed biochar effect on CO2 and N2O emissions.
Adsorption or diffusion of C and N substrates into biochar micropores could result in reduced SOC mineralization or N2O emission in biochar-amended soil by preventing C and N from being used by microorganisms (Ameloot et al. 2013b). The unavailability of SOC would induce a negative priming effect (Kuzyakov et al. 2009), which was found in the ‘no litter’ treatments, where biochar alone induced a negative priming effect, thus decreasing the mineralization of native soil organic matter by 56 %. Similar negative SOC priming effects of 38 % (Bamminger et al. 2014) and 52 % (Zimmerman et al. 2011) have been reported in other studies with similar biochars. In contrast, the amount of EOC, which is considered as readily available substrate for microorganisms and which serves as electron donor during the denitrification process, was not decreased by biochar in the present study (Table 2). Therefore, we assume that adsorption of C to biochar particles in larger quantities is unlikely. Biochar can also reduce the availability of NH4 + and NO3 − (Clough et al. 2013), which serve as substrates for the N2O producing processes of nitrification and denitrification, respectively. Biochar reduced extractable NO3 −-N in both litter treatments and reduced NH4 +-N in the ‘with litter’ treatments after 37 days (Fig. 2). In both cases, adsorption of inorganic-N on biochar particles could be one explanation for the observed reduction of N2O emissions.
Immobilization of N by soil microorganisms could be another explanation for a reduction of N2O emissions by biochar. Microbial abundance was increased by biochar in the ‘with litter’ treatments, enhancing microbial PLFAs by 16 % (Table 2). Biochar is known to alter physicochemical soil properties and, therefore, the living conditions for soil microorganisms thereby often promoting microbial growth (Ameloot et al. 2013a). In the biochar ‘with litter’ treatments higher PLFAmic contents corresponded to lower extractable NO3 −-N and NH4 +-N (for NH4 +-N only in the treatment without earthworms). This indicates that N-mineralization was decreased in the presence of biochar (Prayogo et al. 2014), i.e., organic N from the litter was assimilated by soil microorganisms and, therefore, immobilized rather than being mineralized. The reduction in the availability of NO3 −-N and NH4 +-N could be responsible for the observed reduction of N2O emissions in the biochar treatments when litter was present. The reductive effect of biochar on N2O emission is lacking in the ‘no litter’ treatments and here PLFAmic is only enhanced in combination with earthworms. The reason for the different effects of biochar might be the lack of C and N resources, which limited microbial growth in the ‘no litter’ treatments.
Soil pH is known to affect soil microorganisms and biochar often enhances pH, thus possibly influencing soil microbial abundance and activity due to a liming effect (Lehmann et al. 2011). In our study, biochar increased pH by on average 0.26 units only in the ‘no litter’ treatments (Table 2). This increase of pH might be the reason for the observed increase of the Gram-positive/Gram-negative ratio. The question is now, whether this significant but rather small shift in bacterial community composition could explain the pronounced reductions of total CO2 emissions by 43 % and the strong decrease of the metabolic quotient (qCO2) by 86 %. In the ‘with litter’ treatments, where no effect of biochar on pH was observed, CO2 emissions (by 27 %) and the metabolic quotient (37 %) were also reduced. The non-existence of a biochar effect on pH in the ‘with litter’ treatments indicates that additional factors influence microbial community composition and activity. Biochar may provide ideal conditions for soil microorganisms by improving nutrient retention and, therefore, higher N bioavailability at the surface of the biochar particles (e.g., Zheng et al. 2013). This biochar-supported co-location of substrates and soil microorganisms on their surfaces could reduce the stress potential leading to a more efficient metabolic C use (Lehmann et al. 2011).
Biochar increased the fungal-to-bacterial ratio and the Gram-positive/Gram-negative ratio (only in the treatments without earthworms) in the ‘with litter’ treatments, and similarly the Gram-positive/gram-negative ratio in the ‘no litter’ treatments. This indicates that biochar selectively influences microbial abundances of some microbial groups, leading to a different microbial community composition (Farrell et al. 2013) with enhanced microbial C use efficiency (Jin 2010). Gram-positive bacteria seem to benefit more from biochar addition than Gram-negative bacteria. Gram-positive bacteria are known to preferentially degrade aromatic C in soil and they may, therefore, better utilize biochar-C, thus profiting more from the presence of the aromatic structure of the biochar (Farrell et al. 2013). Conversely, the relative decrease in the abundance of Gram-negative bacteria could be related to low quantities of easily available substrates in high-temperature biochars (Ameloot et al. 2013a).
In the ‘with litter’ treatments, biochar generally increased fungal abundance more than bacterial abundance, resulting in increased fungal-to-bacterial ratios (Table 2), which was also found in the study of Prayogo et al. (2014). In our study, the presence of N-rich and easily degradable litter could have promoted higher growth rates in fungi, which indicates that the fungal community may serve as major litter decomposers, mobilizing nutrients from dead plant material. In addition, it is possible that fungi are able to grow into biochar pores using additional resources or habitats better than bacteria (Lehmann et al. 2011). Our results stand in contrast to field studies, including litter incorporation into soil, which found decreased fungal abundances and shifts towards more bacterial-dominated soil microbial communities when biochar was present in soil (Chen et al. 2013; Jones et al. 2012). The mechanisms behind the observed suppressed SOC mineralization, improved metabolic efficiency, and change in the soil microbial community composition clearly warrant further investigations to clarify causes and effects.
In addition, the observed shifts in soil microbial community composition after biochar addition probably also influenced nitrification and denitrification processes (Clough and Condron 2010). In the N-rich plant litter containing ‘with litter’ treatments, biochar reduced N2O emissions by 42 %. A mitigating effect of biochar on N2O had been observed in other short-term studies (e.g., Kammann et al. 2012). In addition to the above mentioned possible reason, that reduced availability of N due to adsorption onto biochar surfaces or immobilization by soil microorganisms is responsible for the observed reduction of N2O emissions, other studies found out that biochar promotes the last step of denitrification (Cayuela et al. 2013; Harter et al. 2013). Acceleration of the last step of the denitrification, the reduction of N2O to N2, could decrease N2O emission from soil. Harter et al. (2013) showed that biochar increased the abundance of denitrifying bacteria performing this last step of the denitrification. However, as this was not measured in the present study, we cannot confirm this proposed mechanism as a reason for the biochar induced reduction of N2O emissions for the present experiment.
Overall, our results confirm those of the study of Prayogo et al. (2014), who also showed that the presence of litter influences the effects of biochar on the abundance and activity of soil microorganisms. In addition, earthworms, as an additional factor, partly influenced these effects of biochar in the present study as well. Although earthworms alone and in interaction with biochar showed no effects on greenhouse gas emissions, we found positive interactive effects of earthworms and biochar on Gram-positive, Gram-negative bacteria, and fungi in the ‘no litter’ BC + EW treatment (Fig. 3). As biochar particles were found in earthworm casts, we expect that pure biochar particles were mixed with soil organic matter during passage through the earthworm gut. This would lead to a closer association of soil and earthworm gut microorganisms with biochar (Ameloot et al. 2013b). We conclude that the formation of these was more suitable habitats for soil microorganisms in earthworm worked biochar amended soils will increase microbial abundance. However, the mechanisms behind the increased microbial abundance in the ‘no litter’ treatment with biochar and earthworms cannot yet be identified, as neither pH, available EOC, nor mineral N was similarly affected in the combined treatment.
Conclusion
A major result of this short-term study was that biochar reduced CO2 and N2O emissions, while it simultaneously increased microbial abundance in soil. This resulted in a more efficient metabolic C use, emphasizing the potential beneficial effect of biochar on soil microbial activity. In addition, the effect of reduced N2O emissions was especially pronounced when litter with a low C/N ratio was applied to the soil, suggesting that biochar amendments may mitigate the typical high N2O emissions from arable fields after green manure is ploughed into the soils. The mechanisms for increased respiratory C-use efficiency, negative priming effects, and reduced N2O emissions may be interrelated and are likely connected to the observed changes in the soil microbial community composition, warranting more detailed investigation. Moreover, the observed interactive effects of earthworms with biochar on soil microbial abundance highlight the importance for additional research including the different kinds of soil organisms as well as biochar, under natural field conditions in the medium to long term.
References
Ameloot N, de Neve S, Jegajeevagan K, Yildiz G, Buchan D, Funkuin YN, Prins W, Bouckaert L, Sleutel S (2013a) Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol Biochem 57:401–410
Ameloot N, Graber ER, Verheijen FGA, de Neve S (2013b) Interactions between biochar stability and soil organisms: Review and research needs. Eur J Soil Sci 64:379–390
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54:309–320
Augustenborg CA, Hepp S, Kammann C, Hagan D, Schmidt O, Müller C (2012) Biochar and earthworm effects on soil nitrous oxide and carbon dioxide emissions. J Environ Qual 41:1203–1209
Baggs E, Rees R, Castle K, Scott A, Smith K, Vinten A (2002) Nitrous oxide release from soils receiving N-rich crop residues and paper mill sludge in eastern Scotland. Agric Ecosyst Environ 90:109–123
Bamminger C, Marschner B, Jüschke E (2014) An incubation study on the stability and biological effects of pyrogenic and hydrothermal biochar in two soils. Eur J Soil Sci 65:72–82
Bruun S, Jensen ES, Jensen LS (2008) Microbial mineralization and assimilation of black carbon: Dependency on degree of thermal alteration. Org Geochem 39:839–845
Case SD, McNamara NP, Reay DS, Whitaker J (2012) The effect of biochar addition on N2O and CO2 emissions from a sandy loam soil—the role of soil aeration. Soil Biol Biochem 51:125–134
Cayuela ML, Sánchez-Monedero MA, Roig A, Hanley K, Enders A, Lehmann J (2013) Biochar and denitrification in soils: when, how much and why does biochar reduce N2O emissions? Sci Rep 3:1732
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44
Clough TJ, Condron LM (2010) Biochar and the nitrogen cycle: Introduction. J Environ Qual 39:1218–1223
Clough TJ, Condron LM, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agron J 3:275–293
Drake HL, Horn MA (2007) As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu Rev Microbiol 61:169–189
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465:288–297
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soil 22:59–65
Frostegård Å, Bååth E, Tunlio A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Gregorich EG, Monreal CM, Ellert BH (1995) Turnover of soil organic matter and storage of corn residue carbon estimated from natural 13C abundance. Can J Soil Sci 75:161–167
Harter J, Krause H, Schuettler S, Ruser R, Fromme M, Scholten T, Kappler A, Behrens S (2013) Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J 8:660–674
IPCC (2013) Summary for policymakers. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of working Group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jeffery S, Verheijen F, van der Velde M, Bastos A (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Jin H (2010) Characterization of microbial life colonizing biochar and biochar-amended soils. PhD Dissertation, Cornell University, Ithaca, NY
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Jones DL, Rousk J, Edwards-Jones G, DeLuca T, Murphy D (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124
Kaiser C, Frank A, Wild B, Koranda M, Richter A (2010) Negligible contribution from roots to soil-borne phospholipid fatty acid fungal biomarkers 18:2ω6,9 and 18:1ω9. Soil Biol Biochem 42:1650–1652
Kammann C, Ratering S, Eckhard C, Müller C (2012) Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J Environ Qual 41:1052–1066
Khodadad CL, Zimmerman AR, Green SJ, Uthandi S, Foster JS (2011) Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol Biochem 43:385–392
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Li D, Hockaday WC, Masiello CA, Alvarez PJ (2011) Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem 43:1732–1737
Liesch M, Weyers S, Gaskin J, Das KC (2010) The effects of two different biochars on earthworm survival and microbial carbon levels. Ann Environ Sci 4:1–9
Lubbers IM, van Groenigen KJ, Fonte SJ, Six J, Brussaard L, van Groenigen JW (2013) Greenhouse-gas emissions from soils increased by earthworms. Nat Clim Change 3:187–194
Marhan S, Scheu S (2005) The influence of mineral and organic fertilisers on the growth of the endogeic earthworm Octolasion tyrtaeum (Savigny). Pedobiologia 49:239–249
Marhan S, Langel R, Kandeler E, Scheu S (2007) Use of stable isotopes (13C) for studying the mobilisation of old soil organic carbon by endogeic earthworms (Lumbricidae). Eur J Soil Biol 43:S201–208
Marhan S, Demin D, Erbs M, Kuzyakov Y, Fangmeier A, Kandeler E (2008) Soil organic matter mineralization and residue decomposition of spring wheat grown under elevated CO2 atmosphere. Agric Ecosyt Environ 123:63–68
Marhan S, Rempt F, Högy P, Fangmeier A, Kandeler E (2010) Effects of Aporrectodea caliginosa (Savigny) on nitrogen mobilization and decomposition of elevated-CO2 Charlock mustard litter. J Plant Nutr Soil Sci 173:861–868
Prayogo C, Jones JE, Baeyens J, Bending GD (2014) Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol Fert Soil 50:695–702
Saarnio S, Heimonen K, Kettunen R (2013) Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol Biochem 58:99–106
Schimmelpfennig S, Müller C, Grünhage L, Koch C, Kammann C (2014) Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agric Ecosyst Environ 191:39–52
Singh BP, Cowie AL, Smernik RJ (2012) Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ Sci Technol 46:11770–11778
Spokas KA, Cantrell KB, Novak JM, Archer DW, Ippolito JA, Collins HP, Boateng AA, Lima IM, Lamb MC, McAloon AJ, Lentz RD, Nichols KA (2012) Biochar: a synthesis of its agronomic impact beyond carbon sequestration. J Environ Qual 41:973–989
Taghizadeh-Toosi A, Clough TJ, Condron LM, Sherlock RR, Anderson CR, Craigie RA (2011) Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J Environ Qual 40:468–476
Tammeorg P, Parviainen T, Nuutinen V, Simojoki A, Vaara E, Helenius J (2014) Effects of biochar on earthworms in arable soil: Avoidance test and field trial in boreal loamy sand. Agric Ecosyst Environ 191:150–157
Topoliantz S, Ponge J (2003) Burrowing activity of the geophagous earthworm Pontoscolex corethrurus (Oligochaeta: Glossoscolecidae) in the presence of charcoal. Appl Soil Ecol 23:267–271
Vasilyeva NA, Abiven S, Milanovskiy EY, Hilf M, Rizhkov OV, Schmidt MW (2011) Pyrogenic carbon quantity and quality unchanged after 55 years of organic matter depletion in a Chernozem. Soil Biol Biochem 43:1985–1988
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fert Soil 29:111–129
Zheng H, Wang Z, Deng X, Herbert S, Xing B (2013) Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 206:32–39
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Zimmerman AR, Gao B, Ahn M (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Acknowledgments
We thank Kathleen Regan and Kathleen A. Mackie for English correction and Wolfgang Armbruster for isotopic analyses. In addition, we kindly thank the editor and the two anonymous reviewers for their helpful comments on the manuscript. The first author was funded by a PhD scholarship awarded by the faculty of Agricultural Sciences at the University of Hohenheim.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
δ13C-values of earthworm biomass in soils without (EW) and with biochar (BC+EW) in the ‘no litter’ and ‘with litter’ treatments. (JPEG 946 kb)
Fig. S2
Rates of a) CO2 and b) N2O fluxes in the ‘no litter’ and ‘with litter’ treatments during the 37 days of incubation. Ctrl = only soil, BC = soil with biochar, EW = soil including one endogeic earthworm, BC+EW= soil with biochar and one earthworm. (JPEG 2099 kb)
Fig. S3
Water retention curve (pF curve) of soil without (Ctrl) and with biochar (BC) and without and with litter (+litter). (JPEG 1251 kb)
Table S1
(DOCX 15 kb)
Table S2
(DOCX 13 kb)
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Bamminger, C., Zaiser, N., Zinsser, P. et al. Effects of biochar, earthworms, and litter addition on soil microbial activity and abundance in a temperate agricultural soil. Biol Fertil Soils 50, 1189–1200 (2014). https://doi.org/10.1007/s00374-014-0968-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0968-x