Abstract
Although most Sinojackia species are endangered, they contribute greatly to the biodiversity of local ecosystems. Sinojackia microcarpa, an endangered species, is distributed only in three provinces of eastern China. Determining the genetic diversity of S. microcarpa provides key information for germplasm evaluation and species conservation. Here we used simple sequence repeat (SSR) markers to investigate the genetic diversity of eight natural populations of S. microcarpa. Leaf samples were collected from 144 individuals in 8 wild populations. The 156 bands were generated from 14 pairs of informative SSR primers, with an average percentage of polymorphic bands of 45.67%. The average values of Nei’s genetic diversity (He) and Shannon’s diversity index (I) were 0.1007 and 0.1658, respectively. The total genetic variation of S. microcarpa existed mainly within the eight populations, rather than among populations, and reached 86.41%. A cluster analysis showed that the eight wild populations of S. microcarpa could be classified into four groups, at a threshold of 4.0, based on an analysis of the SSR genotypes. Furthermore, there was a significant association between the phylogenetic relationships and the geographic locations of the S. microcarpa populations. In particular, populations from Fuyang, Jiande, and Lin’an in Zhejiang Province had close phylogenetic relationships and geographic distances. In addition, these three populations had the highest genetic diversity and the most individuals, suggesting that these three locations may be the S. microcarpa distribution center. This study serves as a model for studying the genetic diversity of endangered plant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Sinojackia comprises numerous species, including Sinojackia xylocarpa Hu, Sinojackia dolichocarpa C.J.Qi, Sinojackia rehderiana Hu, Sinojackia huangmeiensis J. W. Ge and X .H. Yao, and Sinojackia henryi (Dummer) Merr. (Zhang et al. 2012). They are all endangered species that are threatened by habitat loss and human activities (Guo and Zang 2013). Sinojackia microcarpa C. T. Chen and G. Y. Li, an important medicinal and garden-greening tree species (Liu et al. 2011), is distributed only in an extremely narrow range, including Zhejiang, Anhui, and Jiangxi provinces and classified as a second-class endangered plant in China (Su et al. 2009; Guo and Zang 2013).
Most studies of Sinojackia Hu have focused on its morphological characteristics, physiological mechanisms, and chromosome karyotypes (Cao et al. 2006; Fritsch et al. 2001; Li et al. 2013; Shi et al. 1999; Wang et al. 2003; Xie et al. 2012; Yao et al. 2006). Few reports have investigated the genetic diversity of S. microcarpa (Luo and Luo 2011; Yao et al. 2008; Zhang et al. 2010; Zhong et al. 2014, 2017).
Molecular markers have been used widely to study species diversity (Motalebipour et al. 2016; Sakar et al. 2016; Yilmaza et al. 2012). Ideal molecular markers should have the characteristics of no deviation, co-dominance, high detection accuracy, and no direct influence by the environment. Simple sequence repeats (SSRs) are distributed widely in the genome, and they exhibit abundant polymorphisms and co-dominance, which has the advantage of providing a large amount of information. SSRs have been used as markers in many studies of plant genetic relationships, genetic diversity analyses, phylogenetic evolution, types of fingerprint identification, and quantitative trait locus mapping (Morgante and Olivieri 1993; Wang et al. 2014; Zhao et al. 2016; Zietkiewicz et al. 1994). The genetic diversity analyses of Yilmaza et al. (2012) on Turkish apricot germplasms using SSRs, inter simple sequence repeats (ISSRs), and random amplified polymorphic DNA (RAPD) and of Wang et al. (2014) on Cynodon dactylon using SSRs and ISSRs clearly showed that SSRs are more suitable than ISSRs and RAPD for studies of the genetic diversity of plant species.
Here we used 14 pairs of SSRs to investigate the genetic diversity, genetic differentiation, and population clusters of 8 wild populations of S. microcarpa distributed in Zhejiang, Anhui, and Jiangxi Province, China. This study provided a theoretical basis for germplasm resource protection, diversity evaluation, and gene bank development, as well as essential information for developing a system to examine the biological diversity of Styracaceae. This study will increase our understanding of the genetic characteristics of Sinojackia species and will serve as a useful reference for other endangered plants.
Materials and methods
Plant materials
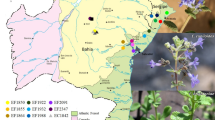
To analyze the genetic diversity of S. microcarpa, we selected sampling sites in Zhejiang, Anhui, and Jiangxi Provinces of China, which are the main S. microcarpa distribution areas according to our investigation and the studies of Yao et al. (2011) and Ruan et al. (2012). We collected 144 leaf samples of S. microcarpa from eight populations in Zhejiang Province (n = 6), Anhui Province (n = 1) and Jiangxi Province (n = 1) (Fig. 1). For accurately sampling the diversity of the populations, the sampling sites were more than 50 m apart. The leaf samples were stored at −80 °C until DNA isolation.
Map showing the geographical distribution of the eight S. microcarpa populations and the sampling sites in China. ZJLA: Qingshanhu, Lin’an, Hangzhou, Zhejiang; ZJJD: Dakeng and Fujiawu, Jiande, Hangzhou, Zhejiang; ZJFY: Longmen, Yaowu, Shushi and Huanshan, Fuyang, Hangzhou, Zhejiang; ZJTL: Mount Gao, Tonglu, Hangzhou, Zhejiang; ZJJH: Changgeng, Jindong, Jinhua, Zhejiang; ZJYW: Shimenkeng, Yiwu, Jinhua, Zhejiang; AHJX: Dingdu, Jing County, Xuancheng, Anhui; JXPZ: Taohongling, Pengze, Jiujiang, Jiangxi
Experimental methods
DNA extraction
Genomic DNA was extracted using a modified cetyltrimethyl ammonium bromide method (Zhong et al. 2017). DNA integrity was checked on 0.8% agarose gels, and DNA quantification was determined using a NanoDrop 1000 micro-spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The DNA samples were diluted to 30 ng/µL and stored at − 20 °C for the SSR analysis.
SSR-polymerase chain reaction (PCR) amplification
A set of 14 SSR primer pairs (Table 1), which yielded clear and stable amplicons as reported for Sinojackia Hu by Yao et al. (2006), Zhang et al. (2010), and Zhao et al. (2016), were used to genotype the 144 S. microcarpa samples. SSR amplification reactions were performed in a volume of 25 µL, which contained 1.0 µL of (30 ng µL−1) genomic DNA, 12.5 µL of KAPA2G Fast Multiplex Mix, 1.0 µL (10 µM) of each primer, and 9.5 µL of double-distilled H2O. PCRs were performed with an initial denaturation at 94 °C for 5 min; followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at the optimal temperature of 55 °C for 30 s, and extension at 72 °C for 60 s; followed by a final extension at 72 °C for 5 min. The primers were labeled with a fluorescent dye (5-FAM, HEX, or TAMRA) and amplified by PCR using previously described protocols (Wang et al. 2014). PCR products were separated by size on an ABI3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Alleles were scored manually with the aid of GeneMarker software version 2.2 (Applied Biosystems) and manually rechecked. Each polymorphic fragment was scored as 1 or 0 for the presence or absence of amplicons, respectively (Gomes et al. 2013; Motta et al. 2014; Xing et al. 2015).
Statistical analysis
Genetic parameters, such as the number of alleles per locus and the polymorphism information content (PIC) of the SSR data, were analyzed. The percentage of polymorphic bands (PPBs), Shannon’s diversity index (I), Nei’s genetic diversity (He), and the number of effective alleles per locus (Ne) of the S. microcarpa populations were calculated using POPGEN32 software. Additionally, the total genetic diversity (Ht), the within-population genetic diversity (Hs), and the between-population genetic diversity (Gst) were calculated. Using Jaccard’s coefficient, a cluster analysis was performed using the similarity matrix to construct a dendrogram and the unweighted pair-group method with arithmetic averages (UPGMA) in NTSYS-pc software (version 2.1) (Rohlf 2000). To infer the population structure and assign individuals to populations, we used the program Structure 2.2 (Wang et al. 2014) to analyze the SSRs. The analyses were run using the admixture model with independent allele frequencies. The number of K was set to vary from 1 to 6. For each value of K, we performed six runs with a burn-in of 100,000 and a run length of 1,000,000 iterations. The posterior probability of the data (ln PD) for a given K was computed using the runs with the highest probability for each K.
Results
SSR amplification
A total of 156 alleles, ranging from 5 to 18, were generated by the 14 SSR primers, with an average of 11.14 alleles per locus. Among the SSR loci, SX149 generated the highest number of alleles (18), while locus SX70 had the lowest (5 alleles; Table 1). Within the 144 S. microcarpa samples, 14 primers generated 156 polymorphic bands, and all 14 primers yielded polymorphic markers. After analyzing all 144 samples, the PIC values for individual loci ranged from 0.8426 (SX8) to 0.9490 (SX112), with an average of 0.9118 (Table 1).
Genetic diversity analysis of S. microcarpa
Fourteen polymorphic microsatellite loci were used to evaluate the genetic diversity of S. microcarpa. The results, shown in Table 2, indicate that the PPBs of the eight populations ranged from 22.44 to 83.33%, and the average number of polymorphic loci ratio was 45.67%. The observed number of alleles (Na) was in the range of 1.2431–1.8333, and the average number of alleles was 1.4619. Ne ranged from 1.1143 to 1.1684, with an average of 1.1519. He was in the range of 0.0704–0.1217, and the average was 0.1007. I ranged from 0.1096 to 0.2153, with an average of 0.1658. Compared with the genetic diversity parameters of the different S. microcarpa populations, the genetic diversity of the ZJJD region was the highest, with a He of 0.1217, followed by the ZJFY region with a He of 0.1187. The ZJYW region had the lowest diversity, with a He of 0.0704. At the species level, the PPBs was 100.00%, and Na was 2.0000. Ne was 1.1698, and He was 0.1257, while I was 0.2301.
Genetic differentiation analysis of the S. microcarpa populations
There was a certain genetic differentiation among the S. microcarpa populations (Table 3). The Ht of the eight populations of S. microcarpa was 0.1148, for which the Hs was 0.0992. The genetic diversity among the populations (Dst = Ht − Hs) was 0.0156, and Nei’s gene differentiation index (Gst = Dst/Ht) was 0.1359, indicating that the among-population variation accounted for 13.59% of the total genetic variation of S. microcarpa, while the within-population genetic variation accounted for 86.41%. Thus, the genetic differentiation within populations was greater than that among populations. The population gene flow coefficient [Nm = 0.5(1 − Gst)/Gst] was 3.1791.
Cluster analysis
The ΔK peak at K = 4 in the structure analysis indicated that the genetic structure of the eight populations could be divided into four groups (Fig. 2). The populations of ZJJH, ZJLA and ZJJD, ZJTL and ZJYW were similar. However, the populations of Zhejiang, Anhui, and Jiangxi Provinces mixed together, and did not differ significantly.
Analysis of the Bayesian gene clustering method by SSRs (K = 4). ZJLA: Qingshanhu, Lin’an, Hangzhou, Zhejiang; ZJJD: Dakeng and Fujiawu, Jiande, Hangzhou, Zhejiang; ZJFY: Longmen, Yaowu, Shushi and Huanshan, Fuyang, Hangzhou, Zhejiang; ZJTL: Mount Gao, Tonglu, Hangzhou, Zhejiang; ZJJH: Changgeng, Jindong, Jinhua, Zhejiang; ZJYW: Shimenkeng, Yiwu, Jinhua, Zhejiang; AHJX: Dingdu, Jing County, Xuancheng, Anhui; JXPZ: Taohongling, Pengze, Jiujiang, Jiangxi
UPGMA clustering based on Nei’s genetic diversity was analyzed by NTYSY (Fig. 3). The results showed that the eight S. microcarpa populations were divided into two subgroups according to the coefficient of 0.90. The Fuyang Zhejiang, Jiande Zhejiang, Jinhua Zhejiang, Lin’an Zhejiang, Jingxian Anhui, and Pengze Jiangxi populations were classified as one subgroup (subgroup A), while those from Tonglu Zhejiang and Zhejiang Yiwu belonged to another subgroup (subgroup B). In subgroup A, the Fuyang, Jiande, Jinhua, Lin’an, Zhejiang and Jingxian Anhui populations were classified into one group (A1 subgroup), and the Pengze Jiangxi population was classified as another group (A2 subgroup). The A1 subgroup could be divided into three subgroups: the Fuyang Zhejiang and Jiande Zhejiang populations, the Jinhua Zhejiang population, and the Lin’an Zhejiang and Jingxian Anhui populations.
UPGMA cluster analysis of the genetic relationships among the S. microcarpa populations using SSRs. ZJLA: Qingshanhu, Lin’an, Hangzhou, Zhejiang; ZJJD: Dakeng and Fujiawu, Jiande, Hangzhou, Zhejiang; ZJFY: Longmen, Yaowu, Shushi and Huanshan, Fuyang, Hangzhou, Zhejiang; ZJTL: Mount Gao, Tonglu, Hangzhou, Zhejiang; ZJJH: Changgeng, Jindong, Jinhua, Zhejiang; ZJYW: Shimenkeng, Yiwu, Jinhua, Zhejiang; AHJX: Dingdu, Jing County, Xuancheng, Anhui; JXPZ: Taohongling, Pengze, Jiujiang, Jiangxi
Discussion
Most endangered plant species, such as Camellia sinensis and Calocedrus macrolepis, have low genetic variation (Taniguchi et al. 2014; Wang et al. 2004; Zawko et al. 2001), and a poor gene pool threatens their survival (Gitzendanner and Soltis 2000). The present study investigated the genotype diversity of S. microcarpa from eight sites using SSRs. Our results clearly demonstrate that S. microcarpa has a surprisingly high level of genotype diversity. In total, 156 polymorphic SSR loci were detected from eight paired primers (on average 11.14 loci for each), and the PIC value of each paired primer was 0.9118 (Table 1). Table 2 also shows that the PPBs and Na values were 100 and 2.0000, respectively. Many studies showed that the destruction of habitat and the extremely low germination rate have been responsible for S. microcarpa becoming one of the most critically endangered plant species in central and southeastern China (Su et al. 2009), which raises the question of how S. microcarpa maintains a high level of genetic variation. We speculate that the special biological characteristics of S. microcarpa may contribute to its high genetic variation. S. microcarpa has seeds with a hard shell, which is advantageous for long-distance and long-term seed transfers, compared with other endangered species. Moreover, S. microcarpa did not suffer from a mass extinction during the glacial period (Yao et al. 2006; Zhang et al. 2012). These factors may have allowed S. microcarpa to retain a high level of genetic variability. Our results are consistent with findings in S. dolichocarpa and S. xylocarpa (Ruan et al. 2012; Yao et al. 2006, 2011), reflecting that the Sinojackia Hu species have a special ability to retain their high genetic biodiversity.
Furthermore, Tables 2 and 3 show that the average PPBs and Ne values were 45.67% and 1.1519, respectively, while the Hs and Dst values were 0.0156 and 0.1359, respectively, reflecting that among-population diversity accounted for 13.59% of the total genetic variability of S. microcarpa, whereas the within-population variability accounted for 86.41%. Thus, it is particularly interesting why S. microcarpa has such a high within-population level of genomic diversity (Zhang et al. 2012). Factors such as recent speciation from a more widespread species, recent changes in distribution or habitat, somatic mutations, breeding systems, multiple founder events, or Pleistocene refugia have been invoked to explain high levels of genetic diversity (Gomes et al. 2013; Rumbou et al. 2016).
The high outcrossing rate observed here in S. microcarpa provides strong population genetic evidence that this species is predominantly outcrossing, which is consistent with our field observations. In forests containing S. microcarpa, natural pollinators were observed frequently to aid pollination. Insects, especially Bombus atripes (Triconibombus) and Apis cerana (Chinese honey bees), are the primary pollinators of S. microcarpa, and honey bees are a long-distance pollinator (Ruan et al. 2012). Bee-foraging behavior has been investigated at the site located in Lin’an, Zhejiang. They prefer to pollinate S. microcarpa, and they have a high specificity when pollinating this species. Consequently, it is possible that pollinators contribute to the high outcrossing rate of S. microcarpa. In addition, seeds from outcrossing fall into the soil, thereby producing more trees with high genomic diversity, which also contributes to the high genetic diversity.
While comparing differences of genetic variability among these eight populations, the ZJJD and ZJFY samples had the highest levels of genomic diversity, with He values of 0.1217 and 0.1187, respectively, whereas the ZJJH and ZJYW samples had the lowest (0.0809 and 0.0704, respectively) (Table 2). ZJJD and ZJFY are mountainous areas, while ZJJH and ZJYW are flatlands (Fig. 1). Humans are a threat to biodiversity and genetic diversity, especially to endangered species (Kiziroglu et al. 2013); therefore, we hypothesize that the urbanization and human activities in the S. microcarpa habitats in these two flatlands (ZJJH and ZJYW) may be the main reasons for the low genomic variation. Conversely, better habitats and lower population densities in ZJJD and ZJFY result in higher genetic variability and larger S. microcarpa population. Furthermore, Fig. 3 shows that samples from AHJX and ZJLA were much closer in terms of their genetic variability and clustered into a group, which differed from that in JXPZ, ZJTL and ZJYW. Figures 2 and 3 show that there the genetic distance was closer between ZJJD and ZJJH and between ZJTL and ZJYW, perhaps because they were geographically. The ZJTL and ZJYW samples clustered into another group, and the JXPZ samples clustered into a single group perhaps because geographical factors such as mountain chains, rivers, and spatial distances cause geographical isolation, which subsequently affects the genetic diversity of S. microcarpa.
In the previous study, Zhang et al. (2012) collected S. rehderiana from JXPZ. We also sampled a population from JXPZ, but we cannot be sure whether our sample is same as Zhang et al. (2012). However, we found that the fruit size of our sample in Pengze was shorter than that of S. rehderiana and bigger than that of S. microcarpa. We speculated that our sample may be a transitional type between S. rehderiana and S. microcarpa. Our clustering diagram showed that our sample was between S. rehderiana and S. microcarpa, and more likely S. microcarpa, which provides further evidence to our speculation.
In addition, S. microcarpa is an endemic species in China and has only several isolated populations in the Middle Eastern provinces, so a high priority should be given to the conservation of this species. The ultimate objectives of conservation are to perpetuate the sustainable survival of populations and to preserve their evolutionary potential. Knowledge of the levels and distribution of genetic diversity is important for designing conservation strategies for threatened and endangered species (Cao et al. 2006; Zhang et al. 2012). For a long time, the most suitable strategy for the conservation of S. microcarpa was the protection of its habitat. However, other uses of S. microcarpa could serve as effective preservation strategies. S. microcarpa is likely to be a good ornamental plant (Zhong et al. 2014), and the successful artificial propagation of this species could not only guarantee its ex situ conservation and sustainable survival, but also enhance its in situ conservation.
In summary, we investigated the genetic diversity of eight S. microcarpa populations. Among these populations, populations from Fuyang, Jiande, and Lin’an in Zhejiang had close phylogenetic relationships and geographic distances. Furthermore, these three populations had the most individuals and highest genetic diversity, inferring that these three locations may be the S. microcarpa distribution center. Additionally, our results demonstrate that this endangered plant species has a high level of genetic variation, which may be determined by its biological characteristics, human activities, and geographical factors. This study is a good model for studying endangered plant species with high genetic diversity and demonstrates that SSRs are good tools for related studies. Further investigations should focus on the mechanism underlying the high genetic diversity of S. microcarpa, and the conservation of this endangered species.
Conclusion
In our study, 156 bands were generated from 14 pairs of informative SSR primers, with an average PPB of 45.67%. The average He and I values were 0.1007 and 0.1658, respectively. The within-population genetic variation accounted for 86.41% of the total genetic variation of S. microcarpa, while the among population diversity accounted for only 13.59%. A cluster analysis showed that the eight wild populations of S. microcarpa could be classified into four groups, at the threshold of 4.0, based on SSR genotypes using a structure analysis. Moreover, there was a significant association between the phylogenetic relationships and the geographic locations of the S. microcarpa populations. In particular, populations from Fuyang, Jiande, and Lin’an in Zhejiang Province had close phylogenetic relationships and geographic distances, and highest genetic diversity and the most individuals, suggesting that these three locations may be the S. microcarpa distribution center.
References
Cao PJ, Yao QF, Ding BY, Zeng HY, Zhong YX, Fu CX, Jin XF (2006) Genetic diversity of Sinojackia dolichocarpa (Syrtacaeeae), a species endangered and endemic to China, detected by inter-simple sequence repeat (ISSR). Biochem Syst Ecol 34:231–239
Fritsch PW, Morton CM, Chen T, Meldrum C (2001) Phylogeny and biogeography of the Styracaceae. Int J Plant Sci 162:S95–S116
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Gomes F, Costa R, Ribeiro MM, Figueiredo E, Canhoto JM (2013) Analysis of genetic relationship among Arbutus unedo L. genotypes using RAPD and SSR markers. J For Res 24:227–236
Guo Z, Zang RG (2013) Evaluation index system of endangered levels of the wild plants with tiny population in China. Sci Silva Sin 49:10–17 (in Chinese)
Kiziroglu I, Erdogan A, Turan L (2013) Biological diversity and its threats in Turkey. Fresen Environ Bull 22:772
Li JJ, Huang JH, Ge JW, Huang XY, Xie SC (2013) Chemotaxonomic significance of n-alkane distributions from leaf wax in genus of Sinojackia species (Styracaceae). Biochem Syst Ecol 49:30–36
Liu SJ, Cao M, Li DX, Zhang J, Feng S, Nie YY, Yan L, Zhang D, Zhang Y, Yang ZR, Zhao J (2011) Purification and anticancer activity investigation of pentacyclic triterpenoids from the leaves of Sinojackia sarcocarpa LQ Luo by high-speed counter-current chromatography. Nat Prod Res 25:1600–1606
Luo LQ, Luo C (2011) Taxonomic circumscription of Sinojackia xylocarpa (Styracaceae). J Syst Evol 49:163–164
Morgante M, Olivieri AM (1993) PCR-amplified microsatellites as markers in plant genetics. Plant J 3:175–182
Motalebipour EZ, Kafkas S, Khodaeiaminjan M, Coban N, Gozel H (2016) Genome survey of pistachio (Pistacia vera L.) by next generation sequencing: development of novel SSR markers and genetic diversity in Pistacia species. BMC Genom 17:998
Motta LB, Soares TCB, Ferrão MAG, Caixeta ET, Lorenzoni RM, Neto JDD (2014) Molecular characterization of Arabica and Conilon coffee plants genotypes by SSR and ISSR markers. Braz Arch Biol Technol 57:728–735
Rohlf FJ (2000) NTSYS-pc: numerical taxonomy and multivariate analysis system, version 2.1. Exeter software. Setauket, New York
Ruan YM, Zhang JJ, Yao XH, Ye QG (2012) Genetic diversity and fine-scale spatial genetic structure of different lifehistory stages in a small, isolated population of Sinojackia huangmeiensis (Styracaceae). Biodivers Sci 20:460–469 (in Chinese with English Abstract)
Rumbou A, von Bargen S, Demiral R, Langer J, Rott M, Jalkanen R, Buttner C (2016) High genetic diversity at the inter-/intra-host level of Cherry leaf roll virus population associated with the birch leaf-roll disease in Fennoscandia. Scand J For Res 31:546–560
Sakar E, Unver H, Ercisli S (2016) Genetic diversity among historical olive (Olea europaea L.) Genotypes from Southern Anatolia based on SSR markers. Biochem Genet 54:842–853
Shi XH, Li NL, Jin L, Zhu QG, Xu BM (1999) Seed dormancy and germination of Sinojackia xylocarpa. J Zhejiang Forest Coll 16:228–233 (in Chinese with English Abstract)
Su XL, Ma DD, Li GY, Liu X (2009) Population quantity surveillance of the rare and endangered plant in Zhejiang Province: Sinojackia microcarpa. J Zhejiang Forest Coll 26:142–144 (in Chinese with English Abstract)
Taniguchi F, Kimura K, Saba T, Ogino A, Yamaguchi S, Tanaka J (2014) Worldwide core collections of tea (Camellia sinensis) based on SSR markers. Tree Genet Genomes 10:1555–1565
Wang HC, He ZC, Li JQ, Meng AP (2003) Studies on karyotype of Sinojackia xylocarpa Hu and observations on its meiosis of pollen mother cell. J Wuhan Bot Res 21:198–202 (in Chinese with English Abstract)
Wang DL, Li ZC, Hao G, Chiang TY, Ge XJ (2004) Genetic diversity of Calocedrus macrolepis (Cupressaceae) in southwestern China. Biochem Syst Ecol 32:797–807
Wang DY, Chen YJ, Zhu HM, Lv GS, Zhang XP, Shao JW (2014) Highly differentiated populations of the narrow endemic and endangered species Primula cicutariifoliain China, revealed by ISSR and SSR. Biochem Syst Ecol 53:59–68
Xie GW, Wang WR, He JX, Li RB, Guo HY, Lin F (2012) Floristic characteristics of a community with endangered plant Sinojackia rehderiana in the north of Jiangxi. J Guangzhou Univ (Nat Sci Edn) 11:18–24 (in Chinese with English Abstract)
Xing C, Tian Y, Guan FC, Meng FJ (2015) Evaluation of genetic diversity in Amygdalus mira (Koehne) ricker using SSR and ISSR markers. Plant Syst Evol 301:1055–1064
Yao XH, Ye QG, Kang M, Zhou JF, Xu YQ, Wang Y, Huang HW (2006) Characterization of microsatellite markers in the endangered Sinojackia xylocarpa (Styracaceae) and cross-species amplification in closely related taxa. Mol Ecol Notes 6:133–136
Yao XH, Ye QG, Fritsch PW, Cruz BC, Huang HW (2008) Phylogeny of Sinojackia (Styracaceae) based on DNA sequence and microsatellite data: implications for taxonomy and conservation. Ann Bot 101:651–659
Yao XH, Zhang JJ, Ye QG, Huang HW (2011) Fine-scale spatial genetic structure and gene flow in a small, fragmented population of Sinojackia rehderiana (Styracaceae), an endangered tree species endemic to China. Plant Biol 13:401–410
Yilmaza KU, Paydas-Kargib S, Doganb Y, Kafkas S (2012) Genetic diversity analysis based on ISSR, RAPD and SSR among Turkish Apricot Germplasms in Iran Caucasian eco-geographical group. Sci Hortic 138:138–143
Zawko G, Krauss SL, Dixon KW, Sivasithamparam K (2001) Conservation genetics of the rare and endangered Leucopogon obtectus (Ericaceae). Mol Ecol 10:2389–2396
Zhang JJ, Ye QG, Yao XH, Huang HW (2010) Microsatellite diversity and mating system of Sinojackia xylocarpa (Styracaceae), a species extinct in the wild. Biochem Syst Ecol 38:154–159
Zhang JJ, Ye QG, Gao PX, Yao XH (2012) Genetic footprints of habitat fragmentation in the extant populations of Sinojackia (Styracaceae): implications for conservation. Bot J Linn Soc 170:232–242
Zhao J, Tong YQ, Ge TM, Ge JW (2016) Genetic diversity estimation and core collection construction of Sinojackia huangmeiensis based on novel microsatellite markers. Biochem Syst Ecol 64:74–80
Zhong TL, Zhao GW, Chu JM, Guo XM, Li GY (2014) A study on photosynthetic physiological characteristics of six rare and endangered species. Agr Sci Technol 15:1265–1270
Zhong TL, Liang LY, Zhu LF, Du MY, Guo XM, Li GY (2017) Optimization of PCR-ISSR reaction system of the rare and endangered plant Sinojackia microcarpa. China Bot Gard 20:219–226 (in Chinese with English Abstract)
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgements
We thank Professor Li Genyou of Zhejiang Agriculture and Forestry University and Zhu Longfei, Du Yong, Liang Liuying, Du Menyang, Dai Yingchao, Zhang Qin, Fu Xiaoqiang, Tong Liang, Zheng Zhigang, Li Lumeng, Shao Saifen, Gong Jiameng, Zhu Jiaming, Shi Xiaodeng, Ma Dandan of the Nurturing Station for State Key Laboratory of Subtropical Silviculture, Zhejiang Agriculture and Forestry University. We also thank He Xujiang, Chen Zhenghai, Xie Wenyuan, Zhang Fenyao, Shao Jianwen, Zhang Xiaoping, Zhu guangquan, Liu Wuhua, Wu Yefei, Zhang Chu, Wu Di and Liu Danna et al. for their assistance during plant collection and experiment.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project funding: This study was supported by the Natural Science Foundation of Zhejiang Province, China (LY13C160007), the Graduate Special Fund Innovative Projects of Jiangxi Province (YC2014-B035), Talent start research projects of Jiyang college of Zhejiang Agriculture and Forestry University (JY2018RC0X), the University Teachers’ Professional Development Project of Zhejiang Province (FX2015050), and the Experiment Program of Forest Resources Monitoring Center of Zhejiang Province (2014088).
The online version is available at http://www.springerlink.com
Corresponding editor: Yu Lei.
Rights and permissions
About this article
Cite this article
Zhong, T., Zhao, G., Lou, Y. et al. Genetic diversity analysis of Sinojackia microcarpa, a rare tree species endemic in China, based on simple sequence repeat markers. J. For. Res. 30, 847–854 (2019). https://doi.org/10.1007/s11676-018-0660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0660-3