Abstract
Soil chemistry influences plant health and carbon storage in forest ecosystems. Increasing nitrogen (N) deposition has potential effect on soil chemistry. We studied N deposition effects on soil chemistry in subtropical Pleioblastus amarus bamboo forest ecosystems. An experiment with four N treatment levels (0, 50, 150, and 300 kg N ha−1 a−1, applied monthly, expressed as CK, LN, MN, HN, respectively) in three replicates. After 6 years of N additions, soil base cations, acid-forming cations, exchangeable acidity (EA), organic carbon fractions and nitrogen components were measured in all four seasons. The mean soil pH values in CK, LN, MN and HN were 4.71, 4.62, 4.71, and 4.40, respectively, with a significant difference between CK and HN. Nitrogen additions significantly increased soil exchangeable Al3+, EA, and Al/Ca, and exchangeable Al3+ in HN increased by 70% compared to CK. Soil base cations (Ca2+, Mg2+, K+, and Na+) did not respond to N additions. Nitrogen treatments significantly increased soil NO3−–N but had little effect on soil total nitrogen, particulate organic nitrogen, or NH4+–N. Nitrogen additions did not affect soil total organic carbon, extractable dissolved organic carbon, incorporated organic carbon, or particulate organic carbon. This study suggests that increasing N deposition could increase soil NO3−–N, reduce soil pH, and increase mobilization of Al3+. These changes induced by N deposition can impede root grow and function, further may influence soil carbon storage and nutrient cycles in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic nitrogen (N) deposition has significantly increased worldwide since the beginning of the 20th century (Vitousek et al. 1997; Gruber and Galloway 2008). It has been predicted that annual N deposition will increase considerably in the 21st century, given the growing N demands of global agriculture and industries, as well as the generally existing phenomenon of inefficient N use (Galloway et al. 2008). China has the greatest emissions of NOx and NH3 around the world (Vet et al. 2014), and among the most intensive N deposition rates. In the next several decades, the largest increases in N deposition are expected to occur in China and South Asia (Dentener et al. 2006).
Increased N deposition has profound effects on forest ecosystems (Fenn et al. 2003; Michopoulos et al. 2004; Manning et al. 2006). Plant growth generally responds to N deposition positively, since primary productivity in most forests is N-limited (Lebauer and Treseder 2008). However, the effects of N deposition on soil biochemistry are complex (Magill et al. 1997). Under the global climate change background, the effect of N deposition on forest soil carbon (C) storage gets more attention, since forest soil are the most important carbon sinks in terrestrial ecosystems (Lal 2005). However, the effects of N deposition on soil organic carbon pools remain controversial. Some studies have indicated that N additions significantly increase soil C sequestration in some ecosystems (Cusack et al. 2011; Huang et al. 2011; Du et al. 2014), but other studies have indicated substantial C loss in other ecosystems (Cleveland and Townsend 2006). The soil carbon pool is comprised of many different carbon compounds and N additions have varying impacts on these carbon fractions (Song et al. 2014). Thus further research is warranted on how soil C fractions respond to N additions.
Nitrogen deposition has the most direct effect on the soil N cycle and soil N status is important for the ecosystem response direction and intensity to N deposition (Homyak et al. 2014). N deposition usually increases soil NO3−–N concentrations and can lead to soil acidification and cation depletion (Tian and Niu 2015). This has been verified in grassland ecosystems and many boreal forests (Lucas et al. 2011; Tian and Niu 2015). However, there are few reports on soil cation responses to N deposition in subtropical or tropical forest. Subtropical or tropical forest soil cations might respond to N deposition in ways similar to those of boreal forests. Whereas, most results show that N deposition has little effect or increased cation concentrations in subtropical or tropical forest soil (Lu et al. 2009; Lucas et al. 2011). Base cations are essential elements for plant growth and the lack of base cations limits ecosystem productivity (Lucas et al. 2011). Understanding the impact of N deposition on soil base cations is therefore of concern.
Bamboo forests represent one of the most important forest types in the world (FAO 2010). But to date, most studies regarding the effects of N addition on soil chemical properties have been conducted in coniferous and broadleaf forests (Tu et al. 2014). Bamboo is widely distributed in the southern provinces of China, contributing about 10% of the C stocks of living biomass of forests in China (Chen et al. 2009). Bamboo forests in China are currently experiencing high rates of N deposition (Du et al. 2014; Zhan et al. 2015), but the potential consequences remain unclear.
We conducted a monthly N addition experiment in a bamboo forest (Pleioblastus amarus) in southwest China beginning in 2007 to examine variation in the concentrations of soil base cations, acid-causing cations, exchangeable acidity, C fractions (including total organic carbon, TOC) and N fractions under different levels of N input. We tested the hypotheses that: (1) six years of N addition will induced loss of base cations and soil acidity will increase; (2) active components of soil C fractions might increase; and (3) N additions will alter soil N status in bamboo forest ecosystems.
Materials and methods
Site area
The study was conducted in a 10-ha P. amarus plantation at Liujiang (29°42′N, 103°14′E), Sichuan, China. This area has a mid-subtropical, humid, mountainous climate. Annual mean relative humidity is 86%, and monthly temperatures range from 6.6 °C in January to 25.7 °C in July. Mean annual precipitation (1980–2000) is 1490 mm. The annual frost-free period ranges from 352 to 360 days. The study site (10 ha) was converted from cropland to a P. amarus plantation in 2000 as part of the National Project of Converting Farmland to Forests (NPCFF). There was very little shrub or herb in the understory. Pleioblastus amarus mean density on the study stand was 52,200 stems ha−1, and the mean diameter at breast height was 2.3 cm. The aboveground dry biomass was 25.4 kg m−2 in November 2007. The soil at the site is classified as a Lithic Dystrudepts according to USDA Soil Taxonomy, derived from purple sandstone and shale. The average soil depth to bedrock was approximately 1 m, and the thickness of the surface organic layer was approximately 1 cm before the experimental treatments began.
Experimental design
In October 2007, twelve plots (3 m × 3 m) were established within the study site, at about 5 m intervals. The plots were randomly allocated to four treatments depending on the amount of annual N addition: control (CK, no N added), low-N (LN, 50 kg N ha−1 a−1), medium-N (MN, 150 kg N ha−1 a−1), and high-N (HN, 300 kg N ha−1 a−1), with three replicates for each treatment. Nitrogen addition began in November 2007, in the form of ammonium nitrate (NH4NO3) applied monthly (12 uniform applications per year). Before each application, the NH4NO3 was weighed, dissolved in 1 L of water, and applied to each plot using a portable sprayer. The control plot received 1 L of water without fertilizer.
Soil sample collection
Soil samples were collected in November 2013, and in January, April, and July 2014, after more than 6 years of N additions. Five subsamples of soil (0–20 cm) were taken from each plot using a soil auger (27 mm in diameter). The subsamples of each plot were composited and visible roots were removed with tweezers. Soil samples were homogenized, passed through a 2-mm sieve and stored at 4 °C for analysis, which occurred within one week. The soil water content was determined gravimetrically by drying a 10-g subsample from each plot at 105 °C. To measure soil C and N concentration, 5 g soil samples were air-dried then ground and sieved through a 0.25-mm mesh.
Chemical analysis
Soil pH was determined by a glass electrode in aqueous extracts. Exchangeable base cations (Ca2+, Mg2+, K+, and Na+) were extracted with 1 M ammonium acetate (Tamm et al. 1999), and quantified by an atomic absorption spectrophotometer (TAS-986, PGENERAL, Beijing, China). For measurement of exchangeable acidity (EA), 5 g of air-dried soil was eluted with 1 M potassium chloride solution. One part of this solution was used with a sodium hydroxide standard solution titration for measurement of EA. The other part we added sodium fluoride solution and then titrated by hydroxide standard solution to determine exchangeable H+. Exchangeable Al3+ was calculated as the difference between EA and exchangeable H+.
Soil total organic carbon (TOC) was measured by the dichromate digestion method (Kalembasa and Jenkinson 1973). Soil total nitrogen (TN) was determined through acid digestion, using the Kjeldahl method (Grimshaw et al. 1989). The soil extractable dissolved organic carbon (EDOC) was analyzed by a total CN analyzer (Shimadzu model TOC-VcpH+TNM-1, Kyoto, Japan) after extract using a 0.5 M K2SO4 solution. NH4+–N and NO3−–N were extracted with a 2 M KCl solution, and their concentrations were estimated using a colorimeter. Particulate organic carbon (POC) and particulate organic nitrogen (PON) were measured using the method described by Cambardella and Elliott (1992). Ten grams of air-dried soil was dispersed in 50 ml of 5 g L−1 sodium hexametaphosphate solution and then shaken for 18 h on a reciprocal shaker (90 r min−1). The dispersed soil sample was passed subsequently thorough a 53-μm sieve and soil fractions immersed in water were gently swirled in a beaker to achieve preferential resuspension of light organic particles. The resulting material was dried at 60 °C to a constant weight and weighed, after which C and N contents were measured. Incorporated organic carbon (IOC) was calculated as the difference between TOC and POC (Gong et al. 2008).
Data analysis
Plot mean values were used in all analyses. Repeated measures ANOVA with Fisher’s LSD test were performed to analyze soil pH, EA, and exchangeable base cations. The annual average value for each parameter was determined, after which one-way ANOVA with Fisher’s LSD test and Pearson correlation analysis with a two-tailed test were used to evaluate differences between means. All analyses were conducted using SPSS 20.0 for Windows (IBM SPSS Inc. Chicago, USA). Effects were considered significant at α = 0.05 unless otherwise stated. Values are expressed as mean ± SE.
Base saturation (BS) was calculated by the following equation:
where c(Ca2+), c(Mg2+), c(K+), c(Na+), c(EA) represent the corresponding concentrations (cmol kg−1) of each ion.
Results
Base cations and acid-forming cations
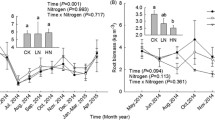
In this P. amarus forest, soil pH was 4.71 and Al3+ was the main acid-forming cation. Exchangeable acidity, exchangeable H+, exchangeable Al3+, BS, and all base cations (Ca2+, Mg2+, K+, and Na+) varied seasonally (Figs. 1, 2). Mean pH in CK, LN, MN and HN were 4.71, 4.62, 4.71, and 4.40, respectively, with pH greater in CK than in HN (Fig. 1a). Similarly, N additions increased EA from 6.12 cmol kg−1 in CK to 10.74 cmol kg−1 in HN (an increase of 75%, p < 0.05) (Fig. 1c). High-N treatment significantly reduced exchangeable H+ and significantly increased exchangeable Al3+. Exchangeable Al3+ in HN (9.8 cmol kg−1) increased by 70% compared to CK (5.74 cmol kg−1) (Fig. 1b, d). Nitrogen additions significantly affected BS only in the HN treatment, with a decrease from 17.04 cmol kg−1 in CK to 9.30 cmol kg−1 in HN (Fig. 2c). Among the four base cations (Ca2+, Mg2+, K+, and Na+), Na+ increased significantly in the MN and HN treatments, but N addition had no effect on other base cations (Fig. 2a, b, d, e). The ratio of exchangeable Al3+ to Ca2+ in HN (22.67) was significantly higher than in CK (9.71) (Fig. 2f). Soil acidity was significantly negatively correlated with EA, Al3+ and NO3−–N, and was significantly positively correlated with BS (Table 1).
Seasonal variations in soil pH, exchangeable acidity (EA), H+ and Al3+ in a Pleioblastus amarus plantation (mean ± SE, n = 3). Results of repeated measures ANOVAs are shown in the text. Different letters indicate significant differences among N treatments (p < 0.05, Fisher’s least significant difference test)
Seasonal variations in soil exchangeable Mg2+, Ca2+, Na+, K+ and base saturation (BS) in a Pleioblastus amarus plantation (mean ± SE, n = 3). Results of repeated measures ANOVAs are shown in the text. Different letters indicate significant differences among N addition treatments (p < 0.05, Fisher’s least significant difference test)
Soil organic carbon fractions
No amount of N addition significantly affected the concentrations of TOC, EDOC, IOC, or POC (Table 2). After 6 years of monthly N additions, EDOC and POC in HN plots increased by 24 and 14%, respectively, when compared to CK plots.
Soil nitrogen fractions
NO3−–N concentration in HN (14.75 mg kg−1) was significantly higher than that in CK (12.33 mg kg−1). N treatments had no significant effect on soil TN, PON, or NH4+–N (Table 3). Soil TN concentration in HN was 8% higher than that in CK.
Discussion
Soil acidification after six years of N additions
Confirming our first hypothesis, N additions significantly reduced soil pH and increased exchangeable Al3+ in HN. The following mechanisms may explain these results. After 6 years of N addition, significantly higher NO3− concentrations were recorded in the HN treatment, indicating that greater nitrification took place when high levels of N were applied. From a related study we reported significantly higher rates of soil N mineralization, nitrification and ammonification at the same study site after 65 months of monthly N additions (Xiao et al. 2015). When the soil inorganic N content exceeds the demand of plants and microorganisms, nitrification is enhanced, and as a result, the concentration of NO3− increases in the soil solution (Aber et al. 1998; Evans et al. 2008). Soil nitrification generally releases H+ into the soil solution (Teklehaimanot and Mmolotsi 2007), and leads to a substitution reaction with base cations (Ca2+, Mg2+, K+, Na+) adsorbed on soil colloids. This results in the loss of base cations, particularly in wet areas (Lu et al. 2009; Lucas et al. 2011). When the H+ absorbed by organic-mineral compounds or aluminum silicate clays exceeds a certain threshold, soil mineral lattices disintegrate and release exchangeable Al3+. In general, soil acidity depends on the balance of acid-forming cations and base cations. In this study, increases in exchangeable Al3+ disrupted this balance and consequently increased soil acidity. Soil acidification induced by Al3+ activation is a common phenomenon in many different ecosystems (Asner et al. 2001; Duan et al. 2004; Huang et al. 2014).
In this study, HN treatment significant reduced soil BS. This was induced by increased exchanged acidity, rather than by base cation depletion. Inconformity with our first hypothesis, N additions did not significant reduce soil base cations. This result confirms reports from studies of most tropical or subtropical forests, i.e. N deposition had little effect or increased soil cation concentrations (Lu et al. 2009; Lucas et al. 2011). This phenomenon might be due to an increase in litterfall and decomposition by N additions (Lucas et al. 2011). For subtropical or tropical soil, many base cations are leached during the soil forming process and plants adapt to environments of low base cations while forming biotic retention mechanisms for base cations (Perakis et al. 2013). N additions increase litterfall and litter decomposition rates in tropical and subtropical forests, and increased cation return to soils. Thus, N additions increased or showed no significant change on base cations. Moreover, large amounts of N might be lost as gases through denitrification. Rosi-Marshall et al. (2016) conducted an acid rain mitigation experiment showed that Ca2+ additions converted a forest watershed from a net N sink to a net N source. This result implies that there is a complex relationship between N and base cation leaching and that this needs more in-depth study.
Soil acidification as a consequence of an increase in exchangeable Al3+ in the soil is a significant ecological and environmental issue. Soil acidification can affect plant communities, bacterial diversity, nitrogen cycling and carbon sequestration, and can even induce forest decline (Koptsik et al. 2001; Wuyts et al. 2013; Zhang et al. 2015). Additionally, active Al3+ has specific toxic effects on plants, such as disruption of nutrient availability and root function, which can result in forest decline (Akaya and Takenaka 2001; Dise et al. 2001). The ratio of exchangeable Al3+ to Ca2+ (Al/Ca) in mineral soils is a predictor of the impact of soil acidification on tree growth (Sverdrup et al. 1992). In this study, the Al/Ca ratio increased greatly under N addition, indicating increased negative effects on plant growth. Chen et al. (2017) reported that after 6 years of N additions, there was a significant decrease in the mean lengths of second and third order fine roots of P. amarus. Our results confirm this result.
Effects of N additions on the soil total organic C pool and C fractions
In support of our second hypothesis, N additions did not significantly affect soil TOC, EDOC, POC or IOC. This result is similar to that reported by Lu et al. (2011), who reviewed 257 studies on this subject and reported that N additions do not significantly change soil carbon pools, irrespective of biome. Soil carbon storage is influenced by many ecosystem processes, including litterfall, soil respiration, litter decomposition, root biomass, and enzymatic activity. Our previous study indicated that N additions significant increased litterfall and soil respiration, while reducing litter decompositions rate (Tu et al. 2011, 2013). Chen et al. (2017) reported that N additions reduced root biomass in P. amarus forest. Although N additions do not significantly affect soil carbon fractions, they do affect many processes related to soil carbon storage. The reason that N addition did not impact soil total carbon content is that different carbon-related processes vary in their responses to N addition. In the future, the response of soil carbon storage depends on these process response magnitudes to N additions.
Soil environments affect soil carbon stability (Schmidt et al. 2011). Nitrogen additions increased soil acidification and aluminum mobilization. This could have long-term and substantial effects on soil carbon pools. We already observed a trend that N additions increased soil EDOC and reduced fine root biomass (Chen et al. 2017). In the future, the changes in soil chemical properties induced by N additions may have substantial negative effects on soil carbon composition and storage.
Effects of N addition on soil N status
N additions increased soil inorganic N content and TN. In our previous study, we estimated the N requirement of P. amarus at 39 kg N ha−1 a−1, which is less than the annual N deposition rate. Nevertheless, N additions increased NPP and had a positive effect on P. amarus growth (Tu et al. 2013). This is because the bamboo stand was initiating a period of rapid growth at the start of the study, and N demand was consequently high. Furthermore, large amounts of N are lost through ammonia volatilization and denitrification. Thus we previously concluded that the P. amarus stand was N-limited. After N additions for 6 years, the increase in the proportion of NO3−–N in soil-available N could be interpreted as a sign of N saturation (Aber et al. 1998, 2003). This confirmed our third hypothesis, that is, N additions alter soil N status. This can cause negative effects on forest ecosystems (Aber et al. 1989; Pardo et al. 2006).
Conclusion
Our study suggested that N additions significantly reduced soil pH and resulted in nitrogen saturation in bamboo forest. However, there were no significant effects on soil carbon or nitrogen fractions, or on soil base cation content after 6 years of N treatment. However, the effects of the changed soil chemistry, as a result of N additions, on the soil organic carbon pool remain unknown. Further long-term studies regarding C storage under different ambient N deposition rates are necessary to supplement our knowledge of how forest C processes respond to N deposition.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM (1989) Nitrogen saturation in norther forest ecosystems. Biosciences 39:378–386
Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNuldty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems. Bioscience 48:921–934
Aber JD, Goodale CL, Ollinger SV, Smith ML, Magill AH, Martin ME, Hallett RA, Stoddard JL (2003) Is nitrogen deposition altering the nitrogen status of northeastern forests? Bioscience 53:375–389
Akaya M, Takenaka C (2001) Effects of aluminum stress on photosynthesis of Quercus glauca Thumb. Plant Soil 237:137–146
Asner GP, Townsend AR, Riley WJ, Matson PA, Neff JC, Cleveland CC (2001) Physical and biogeochemical controls over terrestrial ecosystem responses to nitrogen deposition. Biogeochemistry 54:1–39
Cambardella CA, Elliott ET (1992) Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci Soc Am J 56:777–783
Chen XG, Zhang XQ, Zhang YP, Booth T, He XH (2009) Changes of carbon stocks in bamboo stands in China during 100 years. For Ecol Manag 258:1489–1496
Chen GT, Tu LH, Peng Y, Hu HL, Hu TX, Xu ZF, Liu L, Tang Y (2017) Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 412(1–2):441–451
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci 103:10316–10321
Cusack DF, Silver WL, Torn MS, McDowell WH (2011) Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry 104:203–225
Dentener F, Drevet J, Lamarque JF et al (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cycles 20:1–21
Dise NB, Matzner E, Armbruster M (2001) Aluminum output fluxes from forest ecosystems in Europe: a regional assessment. J Environ Qual 30:1747–1756
Du E, Jiang Y, Fang J, de Vries W (2014) Inorganic nitrogen deposition in China’s forests: status and characteristics. Atmos Environ 98:474–482
Duan L, Huang Y, Hao J, Xie S, Hou M (2004) Vegetation uptake of nitrogen and base cations in China and its role in soil acidification. Sci Total Environ 330:187–198
Evans CD, Norris D, Ostle N, Grant H, Rowe EC, Curtis CJ, Reynolds B (2008) Rapid immobilisation and leaching of wet-deposited nitrate in upland organic soils. Environment Pollution 156:636–643
FAO (2010) Global Forest Resources Assessment 2010: Main report. Food and Agriculture Organization of the United Nations, Rome
Fenn ME, Baron JS, Allen EB, Rueth HM, Nydick KR, Geiser L, Bowman WD, Sickman JO, Meixner T, Johnson DW, Neitlich P (2003) Ecological effects of nitrogen deposition in the Western United States. Bioscience 53:404–420
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends questions and potential solutions. Science 320:889–892
Gong W, Yan XY, Cai ZC, Wang JY, Hu TX, Gong YB, Ran H (2008) Effects of long-term fertilization on soil humus carbon and nitrogen fractions in a wheat-maize cropping system. Chin J Appl Ecol 19:2375–2381 (Chinese with English abstract)
Grimshaw HM, Allen SE, Parkinson JA (1989) Nutrient elements. In: Allen SE (ed) Chemical analysis of ecological material. Blackwell Scientific, Oxford, pp 81–159
Gruber N, Galloway JN (2008) An earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Homyak PM, Sickman JO, Miller AE, Melack JM, Meixner T, Schimel JP (2014) Assessing nitrogen-saturation in a seasonally dry chaparral watershed: limitations of traditional indicators of N-saturation. Ecosystems 17:1286–1305
Huang Z, Clinton PW, Baisden WT, Davis MR (2011) Long-term nitrogen additions increased surface soil carbon concentration in a forest plantation despite elevated decomposition. Soil Biol Biochem 43:302–307
Huang J, Mo JM, Zhang W, Lu XK (2014) Research on acidification in forest soil driven by atmospheric nitrogen deposition. Acta Ecol Sin 34:302–310
Kalembasa SJ, Jenkinson DSA (1973) Comparative study of titrimetric and gravimetric methods for determination of organic carbon in soil. J Sci Food Agric 24:1085–1090
Koptsik S, Berezina N, Livantsova S (2001) Effects of natural soil acidification on biodiversity in boreal forest ecosystems. Water Air Soil Pollut 130:1025–1030
Lal R (2005) Forest soils and carbon sequestration. For Ecol Management 220:242–258
Lebauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Lu XL, Mo JM, Gundersern P, Zhu WX, Zhou GY, Li DJ, Zhang X (2009) Effect of Simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere 19:189–198
Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B (2011) Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agriculture Ecosystems Environment 140:234–244
Lucas RW, Klaminder J, Futter MN, Bishop KH, Egnell G, Laudon H, Högberg P (2011) A meta-analysis of the effects of nitrogen additions on base cations: implications for plants, soils, and streams. For Ecol Manag 262:95–104
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7:402–415
Manning P, Newington JE, Robson HR, Saunders M, Eggers T, Bradford MA, Bardgett RD, Bonkowski M, Ellis RJ, Gange AC, Grayston SJ, Kandeler E, Marhan S, Reid E, Tscherko D, Godfray HCJ, Rees M (2006) Decoupling the direct and indirect effects of nitrogen deposition on ecosystem function. Ecol Lett 9:1015–1024
Michopoulos P, Baloutsos G, Economou A, Nikolis N (2004) Effects of nitrogen deposition on nitrogen cycling in an Aleppo pine stand in Athens Greece. Sci Total Environ 323:211–218
Pardo LH, Templer PH, Goodale CL et al (2006) Regional assessment of N saturation using foliar and root 15N. Biogeochemistry 80:143–171
Perakis SS, Sinkhorn ER, Catricala CE, Bullen TD, Fitzpatrick JA, Hynicka JD Jr, Cromack K (2013) Forest calcium depletion and biotic retention along a soil nitrogen gradient. Ecol Appl 23:1947–1961
Rosi-Marshall EJ, Bernhardt ES, Buso DC, Driscoll CT, Likens GE (2016) Acid rain mitigation experiment shifts a forested watershed from a net sink to a net source of nitrogen. Proc Natl Acad Sci 113:7580–7583
Schmidt MWI, Torn MS, Abiven S et al (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Song B, Niu S, Li L, Zhang L, Yu G (2014) Soil carbon fractions in grasslands respond differently to various levels of nitrogen enrichments. Plant Soil 384:401–412
Sverdrup H, Warfvinge P, Rosén K (1992) A model for the impact of soil solution Ca: al ratio soil moisture and temperature on tree base cation uptake. Water Air Soil Pollut 61:365–383
Tamm CO, Aronsson A, Popovic B, Flower-Ellis J (1999) Optimum nutrition and nitrogen saturation in Scots pine stands. Studia Forestalia Suecica 206:1–126
Teklehaimanot Z, Mmolotsi RM (2007) Contribution of red alder to soil nitrogen input in a silvopastoral system. Biol Fertil Soils 43:843–848
Tian D, Niu S (2015) A global analysis of soil acidification caused by nitrogen addition. Environ Res Lett 10:024019
Tu LH, Hu HL, Hu TX, Zhang J, Liu L, Li RH, Dai HZ, Luo SH (2011) Decomposition of different litter fractions in a subtropical bamboo ecosystem as affected by experimental nitrogen deposition. Pedosphere 21:685–695
Tu LH, Tx Hu, Zhang J, Li XW, Hu HL, Liu L, Xiao YL (2013) Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol Biochem 58:255–264
Tu LH, Chen G, Peng Y, Hu HL, Hu TX, Zhang J, Li XW, Liu L, Tang Y (2014) Soil biochemical responses to nitrogen addition in a bamboo forest. PLoS ONE 9:e102315
Vet R, Artz RS, Carou S, Shaw M, Ro C, Aas W, Baker A, Bowersox VC, Dentener F, Galy-Lacaux C, Hou A, Pienaar JJ, Gillett R, Forti MC, Gromov S, Hara H, Khodzher T, Mahowald NM, Nickovic S, Rao PSP, Reid NW (2014) A global assessment of precipitation chemistry and deposition of sulfur nitrogen sea salt base cations organic acids acidity and pH and phosphorus. Atmos Environ 93:3–100
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Technical report: human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Wuyts K, De Schrijver A, Staelens J, Verheyen K (2013) Edge effects on soil acidification in forests on sandy soils under high deposition load. Water Air Soil Pollut 224:1545–1569
Xiao YL, Tu LH, Chen G, Peng Y, Hu HL, Hu TX, Liu L (2015) Soil-nitrogen net mineralization increased after nearly six years of continuous nitrogen additions in a subtropical bamboo ecosystem. J Forestry Res 26:949–956
Zhan X, Yu G, He N, Jia B, Zhou M, Wang C, Zhang J, Zhao G, Wang S, Liu Y, Yan J (2015) Inorganic nitrogen wet deposition: evidence from the North-South Transect of Eastern China. Environ Pollut 204:1–8
Zhang X, Liu W, Zhang G, Jiang L, Han X (2015) Mechanisms of soil acidification reducing bacterial diversity. Soil Biol Biochem 81:275–281
Acknowledgements
We thank Han Yuan, Xiao-fang Yang, Jia-lei Guo and Yi Cheng for conducting the soil analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This study was financially supported by the Open-end Fund of Ecological Security and Protection Key Laboratory of Sichuan Province. Mianyang Normal University (ESP1507) and the National Natural Science Foundation of China (31300522).
The online version is available at http://www.springerlink.com
Corresponding editor: Hu Yanbo.
Rights and permissions
About this article
Cite this article
Chen, Gt., Tu, Lh., Chen, Gs. et al. Effect of six years of nitrogen additions on soil chemistry in a subtropical Pleioblastus amarus forest, Southwest China. J. For. Res. 29, 1657–1664 (2018). https://doi.org/10.1007/s11676-017-0587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-017-0587-0