Abstract
Continuous increases in anthropogenic nitrogen (N) deposition are likely to change soil microbial properties, and ultimately to affect soil carbon (C) storage. Temperate plantation forests play key roles in C sequestration, yet mechanisms underlying the influences of N deposition on soil organic matter accumulation are poorly understood. This study assessed the effect of N addition on soil microbial properties and soil organic matter distribution in a larch (Larix gmelinii) plantation. In a 9-year experiment in the plantation, N was applied at 100 kg N ha−1 a−1 to study the effects on soil C and N mineralization, microbial biomass, enzyme activity, and C and N in soil organic matter density fractions, and organic matter chemistry. The results showed that N addition had no influence on C and N contents in whole soil. However, soil C in different fractions responded to N addition differently. Soil C in light fractions did not change with N addition, while soil C in heavy fractions increased significantly. These results suggested that more soil C in heavy fractions was stabilized in the N-treated soils. However, microbial biomass C and N and phenol oxidase activity decreased in the N-treated soils and thus soil C increased in heavy fractions. Although N addition reduced microbial biomass and phenol oxidase activity, it had little effect on soil C mineralization, hydrolytic enzyme activities, δ13C value in soil and C–H stretch, carboxylates and amides, and C–O stretch in soil organic matter chemistry measured by Fourier transform infrared spectra. We conclude that N addition (1) altered microbial biomass and activity without affecting soil C in light fractions and (2) resulted in an increase in soil C in heavy fractions and that this increase was controlled by phenol oxidase activity and soil N availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last two centuries, biologically available nitrogen (N) has been increasing in forest ecosystems via N deposition from fossil fuel combustion, and N deposition is predicted to continue increasing in the future (Galloway et al. 2004). Soils contain the largest reservoir of terrestrial carbon (C), and greater available N can influence the cycling and storage of soil C by influencing terrestrial ecosystem processes including plant growth, soil organic matter quantity and quality (Dijkstra et al. 2004; Vries et al. 2006; Cusack et al. 2011b). Although studies have explored forest soil C responses to elevated N availability worldwide, why N deposition can lead to an increase, decrease, or no change on soil C pools in different forest ecosystems remains unclear (Neff et al. 2002; Magnani et al. 2007; Allison et al. 2010). Changes in microbial biomass and microbial activities in response to N addition can lead to changes in decomposition of soil organic matter fractions, then alter soil C storage (Henry et al. 2005; Grandy et al. 2008). Therefore, a better understanding of how N addition affects microbial properties is needed to explain changes in soil organic matter fractions and storage in forest ecosystems.
Nitrogen addition tends to cause a decrease in soil microbial biomass in temperate forest ecosystems (DeForest et al. 2004; Frey et al. 2004; Demoling et al. 2008), but it can increase soil microbial biomass in tropical forests (Cusack et al. 2011a). These inconsistent responses of soil microbial biomass to N additions might be ascribed to either of two potential mechanisms. First, microbial biomass is limited by N availability in some forest soils. Here, N additions could directly increase microbial growth. Second, N addition could indirectly affect soil microbial biomass by changing soil pH and C availability (Treseder 2008). Specifically, N addition can mobilize soil aluminum by decreasing soil pH, which can inhibit microbial growth (Vitousek et al. 1997).
From a functional perspective, soil enzyme activities are important indicators to gain insights into the mechanisms of microbial responses to N addition. In general, soil enzymes are either oxidative or hydrolytic in function. Oxidative enzymes degrade recalcitrant compounds such as lignin, while hydrolytic enzymes degrade simple compounds such as cellulose (Sinsabaugh and Moorhead 1994). Generally, N addition can stimulate cellulose decomposition, but inhibits the decomposition of recalcitrant, lignified organic matter (Fog 1988). Such changes in enzyme activities with N addition have been reported to influence soil C pools (Waldrop et al. 2004). Furthermore, N addition can influence soil organic matter chemistry by changing enzyme activities that depolymerize organic matter compounds (Gallo et al. 2005). Studies of enzyme activity can provide important insight into the effect of increasing N addition on the functioning of soil microbial communities. This, in turn, might link to variation in soil organic C fractions and soil organic matter chemical composition.
N deposition occurs on a global scale, and northern China receives high N deposition contents, ranging from 28.5 to 100.4 kg N ha−1 a−1 at 10 tested sites (Pan et al. 2012). In northern China, Larix spp. species are key commercial tree species and play important roles in the C sequestration (Wang et al. 2006; Jia et al. 2010). We added N in trials in a larch plantation to determine how microbial properties respond to increased N availability, and we related these responses to soil organic matter density fractions. Previous work at this study area investigated the effects of N addition to larch plantation; no change was found in total soil C or total soil N concentrations but soil microbial biomass C and N declined (Hu et al. 2010; Yang et al. 2015). The labile light fraction is thought to include microbial biomass and easily decomposed plant residues. On the basis of these known responses to addition of N, the decay-resistant litter components of larch trees (Liu et al. 1998), and results from meta-analyses (Knorr et al. 2005; Treseder 2008), we hypothesized that N addition would (1) increase the quantity of the heavy fraction in soil organic matter and reduce the quantity of the light fraction in soil organic matter and (2) increase hydrolytic enzyme activities but reduce oxidative enzyme activity because larch trees produce decay-resistant litter. These working hypotheses were tested in this study by quantifying soil C and N mineralization, microbial biomass and activity, and their relations with soil C in light and heavy fractions in a larch plantation after 9 years of continuous N addition. Since soil organic matter chemistry and C and N isotopes can provide relevant evidence on soil microbial properties, we also analyzed soil organic matter chemistry using Fourier transform infrared spectra and δ13C and δ15 N values in the soil.
Materials and methods
Study site and experimental design
Our study located at the Maoershan Experimental Station of Northeast Forestry University, Heilongjiang Province, China (45°21′–45°25′ N, 127°30′–127°34′ E). The regional climate is a continental monsoon type with mean annual temperature of 2.8 °C and mean annual rainfall of 700 mm (Zhou 1994). The soil classified as Hap Boric Luvisol (Gong et al. 1999).
Our subject larch (Larix gmelinii) plantation was established in 1986. We demarcated six 20 m × 30 m sampling plots at the study site. Three plots were fertilized with ammonium nitrate (100 kg N ha−1 a−1) in five separate applications of NH4NO3 each year from 2003 to 2011, and the control plots received no additions. See Yang et al. (2015) for more details on N applications.
Soil sampling
Samples of mineral soil were collected in August 2011. Soil cores (total 15) in 0–10 cm and 10–20 cm depths were collected randomly from each plot, then combined into one composite sample for each soil depth. Soils was sieved through a 2-mm mesh to analyze soil C and N mineralization, microbial biomass C and N, and enzyme activity. Subsamples were air-dried and ground to <2 mm diameter particles for measurement of the soil organic matter fraction. A second subset of samples was oven-dried and passed through <0.25 mm sieve for analysis of soil C and N contents, soil organic matter chemistry, and δ13C and δ15N values in the soil.
Analysis of selected soil chemical properties
Soil was separated into light and heavy fractions using a method of Strickland and Sollins (1987). Soil C and N concentrations were determined using a Vario EL III elemental analyzer. Whole soil and its light and heavy fractions were then analyzed for natural abundances of 13C and 15N using an isotope ratio mass spectrometer. The chemical composition of whole soil and its light and heavy fractions were analyzed using a Nicolet 6700 FT-IR spectrophotometer (Thermo, USA). The four functional groups of soil organic matter (SOM) expressed as peak areas were determined in response to N addition (Johnston and Aochi 1996).
Soil microbial properties
Soil C and N mineralization were quantified by a 28-d laboratory incubation (Zibilske 1994). Inorganic N (i.e., NH4 + and NO3 −) was determined with an Auto Analyzer III (Germany). Soil net N mineralization was calculated by inorganic N in incubated samples minus inorganic N in nonincubated samples (Hart et al. 1994).
Microbial biomass C and N were quantified using the chloroform fumigation extraction method (Brookes et al. 1985; Vance et al. 1987). Microbial biomass C and N were estimated as the difference of total extract between fumigated and unfumigated soil samples.
The activities of phenol oxidase, β-glucosidase, exoglucanase and N-acetyl-β-glucosaminidase were spectrophotometrically assayed. The activities of soil phenol oxidase, β-glucosidase, exoglucanase and N-acetyl-β-glucosaminide were quantified using L-3,4-dihydroxy-phenylalanine (L-DOPA), p-nitrophenyl-β-d-glucopyranoside, p-nitrophenyl-β-d-cellobioside and N-acetyl-β-glucosaminide as the substrates, respectively (von Mersi and Schinner 1996; Parham and Deng 2000; Saiya-Cork et al. 2002; Turner et al. 2002). See Yang et al. (Yang et al. 2013) for analysis of enzyme activities in detail.
Statistical analyses
The effects of N addition and soil depth on C and N in soil, light and heavy fractions, soil chemical composition, soil C and N mineralization, microbial biomass, or enzyme activities were analyzed using a split-plot analysis of variance (ANOVA). Natural abundance of δ13C and δ15N in soil, light and heavy fractions were evaluated using a split–split-plot ANOVA. Enzyme activity was normalized to microbial biomass C concentration to obtain specific enzyme activity indices. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Soil C and N amounts
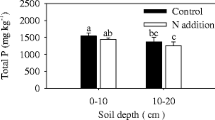
The amounts of C and N in whole soil were statistically similar in control and N addition treatments at 0–10 cm and 10–20 cm depths (Fig. 1). Density fractionation enabled isolation of soil light and heavy fractions with different C and N concentrations. N addition did not affect the amount of C or N in the light soil fraction compared with the control at both two soil depths. N addition increased the amount of C in the heavy soil fraction at the 0–10 cm soil depth. However, the amount of N in the heavy soil fraction did not change with N addition at this soil depth (Fig. 1). Total soil C and N, and C and N in the light and heavy fractions decreased with soil depths for both two treatments.
Mean (±SE) carbon and nitrogen contents in the whole soil and in heavy and light soil fractions in control and N fertilized plots after 9 years in a larch plantation. Data are denoted as means ± stand errors (n = 3). Different letters represent significant differences between the control and the treatment at each soil depth (Tukey’s t-test, P < 0.05), n = 3
Chemical composition of whole soil and its light and heavy fractions
From FTIR spectra of the control and N addition soil samples, we identified four peak areas: (1) O–H = ~ 3400 cm−1; (2) C–H = ~2900 cm−1; (3) C=O = ~1600 cm−1; and (4) C–O = ~1050 cm−1. For whole soil and soil organic matter in the light fraction, peaks associated with O–H stretch decreased significantly in area with N treatment. However, N addition did not affect C–H, C=O, or C–O groups in whole soil or in the light and heavy fractions (Table 1).
Soil stable isotopes (δ13C and δ15N) in whole soil and in light and heavy fractions
Nitrogen addition did not affect δ13C values in whole soil or in the light and heavy fractions at the two soil depths (Table 2). The δ15N values in whole soil and the heavy fraction were significantly lower in the N addition treatment than in the control at 0–10 cm depth. In addition, δ15N values in the light fraction did not change with N addition at either sampling depth. The δ13C and δ15N values for whole soil and the heavy fraction increased with soil depth for both two treatments. Similar to the results for whole soil, δ15N values of the light fraction were also greater at deeper soil depth, but δ13C values of the light fraction did not change with sampling depths.
Soil C and N mineralization, microbial biomass and enzyme activity
Nitrogen addition did not significantly change soil C mineralization at the two soil depths. In contrast, N addition increased soil N mineralization by ~90% over the control at the 0–10 cm depth (Fig. 2). Soil C and N mineralization also decreased with soil depths for two treatments.
At the 0–10 cm depth, soil microbial biomass C and N were reduced by 47 and 44%, respectively, in the N addition compared with the control, but they did not differ significantly between the N treatment and the control at 10–20 cm (Fig. 3). Similar to the microbial biomass C and N, the ratio of microbial biomass C to soil organic C (MBC/SOC) and ratio of microbial biomass N to total N (MBN/TN) after N addition were greater than in the control at 0–10 cm. MBC/SOC and MBN/TN were statistically similar at 0–10 cm and at 10–20 cm for both two treatments.
Mean (±SE) soil microbial biomass C and N, ratio of microbial biomass C to soil organic C (MBC/SOC) and ratio of microbial biomass N to total N (MBN/TN) in control and N-fertilized plots after 9 years in a larch plantation. Different letters represent significant differences between the control and the treatment (Tukey’s t-test, P < 0.05), n = 3
The addition of N reduced the activity of phenol oxidase at the 0–10 cm depth, but the activity of hydrolytic enzymes (exoglucanase, β-glucosidase and N-acetyl-ß-glucosaminidase) did not change significantly (Fig. 4). The effect of N addition on specific enzyme activities (i.e., normalized to soil microbial biomass C) also explained the added N influencing enzyme activities. At 0–10 cm depth, enzymatic activities paralleled changes in microbial biomass C, and phenol oxidase, exoglucanase, β-glucosidase and N-acetyl-ß-glucosaminidase showed a similar trend. Specifically, N addition significantly increased specific oxidative and hydrolytic enzyme activities at the same soil depth (Fig. 4).
Discussion
With N addition at a rate of 100 kg N ha−1 a−1, spread over five applications during the year, for 9 years, total C and N in the soil of the larch plantation did not noticeably change. This result aligns with the results of previous studies at our site (Hu et al. 2010) and at other sites (Scheuner and Makeschin 2005; Chen et al. 2012), but contrasts with the results reported for other sites (Nave et al. 2009; Huang et al. 2011; Frey et al. 2014). Generally, a change in soil C with N addition represents the balance between C inputs of primary production and C outputs from soil respiration. The absence of any change in total C and N in response to addition of N might be explained by the decrease in fine root biomass in the larch plantation (Jia et al. 2010), which can inhibit soil organic matter sequestration. In addition, C or N in light and heavy soil fractions might be affected differently by N addition and might counteract each other, leading to no net change in total C and N in soil across the treatments (Neff et al. 2002).
We recorded soil C in the heavy soil fraction that accounted for ~80% of the total soil C and increased significantly in response to N addition. In contrast, addition of N resulted in no obvious change in soil C in the light fraction. Because the light fraction is formed by newly decomposed plant residues, and therefore is presumed to be more sensitive to N addition, we hypothesized that C in the light fraction would decrease with addition of N because N addition can increase the decomposition rate of the free light fraction (Neff et al. 2002). However, in contrast to our hypothesis, soil C in the light fraction did not change significantly in response to addition of N. One potential explanation is that soil C in the light fraction is closely related to the activity of hydrolytic enzymes, which did not change when N was added. The net increase in soil C content in response to N addition was due to the increase in soil C in the heavy fraction. Neff et al. (2002) also reported that N deposition (100 kg N ha−1 a−1) in an alpine dry meadow increased C content in the heavy fraction, even with no significant increase in total soil C. This increased C content in the heavy soil fraction may be explained as follows. First, N addition could restrain decomposition of humified soil organic matter (Hagedorn et al. 2003) then increase soil C in the heavy fraction. Second, soil oxidative activity is an important factor regulating C in the heavy fraction; in general, declines in oxidative activity with N addition could increase lignin-derived C (Grandy et al. 2008).
Our result showed that addition of N led to a decline in C and N in the microbial biomass. Although this result was based on one sampling date and microbial biomass C and N are temporally sensitive, this finding agrees with that of Hu et al. (2010), who reported that soil microbial biomass C and N were lower in N addition plots at the same study site. In a meta-analysis of 82 published field studies, Treseder (2008) found that addition of N resulted on average in a 15% decrease in microbial biomass. In addition, the extent of decline increased with increased study duration and increased N addition rates. Nevertheless, at our study area, microbial biomass C and N were reduced by ~50% after N addition. The high N dosage and fertilization duration could be responsible for the significant decline in microbial biomass at our study site. Our result is in agreement with the study of Harvard forest with long-term N amendments, which demonstrated that soil microbial biomass decreased more than 50% after ~10 years of fertilization (Compton et al. 2004). Moreover, we found that the soil MBC/SOC and MBN/TN decreased after N addition, indicating N addition inhibited microbial immobilization, then decreased soil C and N availability.
In this study, despite the decline in microbial biomass C, we did not find significant changes in microbial respiration in response to N addition. This lack of effect on microbial respiration after the addition of N coincides with the stability of the hydrolytic enzyme activities per gram of soil and soil C availability. Generally, addition of N may regulate microbial respiration via by controlling oxidative and hydrolytic enzyme activities. First, N addition could suppress phenol oxidase activity (Saiya-Cork et al. 2002; Sinsabaugh et al. 2002), resulting in an increase in soil C storage. In this study, a decrease in phenol oxidase activity after N addition was related to an increase in the quantity of the heavy fraction of soil organic matter. Second, N addition can stimulate hydrolytic enzyme activities and thereby reduce soil C storage (Sinsabaugh et al. 2005). Importantly, N addition affects enzyme activities: their subsequent impacts on organic matter decomposition depends on N availability and lignin and cellulose content (Sinsabaugh et al. 2002). In comparison to the litter of broad-leaf tree species, larch litter decomposes slowly and has more decay-resistant components (Liu et al. 1998). Therefore, it should be expected that N addition might inhibit microbial respiration through its modification of oxidative enzymes activity; however, microbial respiration in bulk soil was unaffected by the addition of N. Neff et al. (2002) reported that N amendment significantly accelerated the light soil C decomposition, but retarded C decomposition in heavier soil, and accordingly, caused no significant change in bulk soil C content; this scenario could explain the case in our study. In this study, microbial biomass declined significantly after N addition, indicating that enzyme activity per microbial biomass increased for oxidative and hydrolytic enzymes (specific enzyme activities). These shifts in specific enzyme activities suggest that microbes were allocating resources toward C acquisition (Sinsabaugh and Moorhead 1994; Allison et al. 2010).
A previous study suggested that N addition increased saturated carbon where organic matter was accreting in northern temperate deciduous forests. The increased abundance of saturated carbon in organic matter with N addition conforms to declines in oxidative enzymes (Gallo et al. 2005). Although we expected that changes in enzyme activities would be linked to variation in soil organic matter chemistry as observed elsewhere (Grandy et al. 2008), there were no significant differences in C–H stretch, carboxylates and amides, or C–O stretch in soil organic matter chemistry measured by FTIR between control and N addition plots. It is possible that shifts in soil organic matter chemistry due to N addition were too minor to detect by FTIR.
Our results indicate that the increase in soil C in the heavy soil fraction of N addition plots was due to the decline in soil phenol oxidase activity. Soil microorganisms discriminate against 13C and preferentially use 12C compounds during metabolism. Thus the control treatment with high microbial biomass should become more enriched in 13C. However, the consistent δ13C levels in the organic matter in both whole soil and the heavy fraction in the N addition and the control plots did not support this supposition. It is important to note that δ13C values in soil organic matter are affected by new C inputs. In general, plant materials contain cellulose and lignin components with different 13C isotopic signatures (Feng 2002). Thus, the δ13C values in organic compounds of plant origin may interfere with microbial isotopic fractionation.
In our study, soil net N mineralization increased after 9 years of N addition. However, the greater rates of N cycling did not lead to greater δ15N values in whole soil. The δ15N value of inorganic N fertilizers approaches 0‰. This result compares with +1 to +9‰ content in natural soil N (Högberg 1997). Therefore, soil δ15N will decline after N addition when there is no microbial processing.
Conclusions
Although total soil C and soil C in the light fraction were not influenced by the addition of N, soil C in the heavy fraction increased when N was added to the larch plantation soil. However, microbial biomass and phenol oxidase activity decreased with N addition, corresponding to an increase of C in the heavy soil fraction. The change in phenol oxidase activity was possibly the mechanism affecting long-term C storage. Our results indicate that soil C mineralization and the chemistry of soil organic matter in this plantation did not change with long-term N addition, despite changes in microbial biomass and soil phenol oxidase activity.
References
Allison SD, Gartner TB, Mack MC, McGuire K, Treseder K (2010) Nitrogen alters carbon dynamics during early succession in boreal forest. Soil Biol Biochem 42:1157–1164
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and release of soil N: a rapid direct extraction method to measure microbial biomass N in soil. Soil Biol Biochem 17:837–842
Chen XM, Liu JX, Deng Q, Yan JH, Zhang DQ (2012) Effect of elevated CO2 and nitrogen addition on soil organic carbon fractions in a subtropical forest. Plant Soil 357:25–34
Compton JE, Watrud LS, Porteous A, DeGrood S (2004) Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. For Ecol Manag 196:143–158
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011a) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92:621–632
Cusack DF, Silver WL, Torn MS, McDowell WH (2011b) Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry 104:203–225
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci Soc Am J 68:132–138
Demoling F, Nilsson LO, Baath E (2008) Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol Biochem 40:370–379
Dijkstra FA, Hobbie SE, Knops JMH, Reich PB (2004) Nitrogen deposition and plant species interact to influence soil carbon stabilization. Ecol Lett 7:1192–1198
Feng XH (2002) A theoretical analysis of carbon isotope evolution of decomposing plant litters and soil organic matter. Glob Biogeochem Cycles 16:1119–1130
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. Forest Ecol Manag 196:159–171
Frey SD, Ollinger S, Nadelhoffer K, Bowden R, Brzostek E, Burton A, Caldwell BA, Crow S, Goodale CL, Grandy AS, Finzi A, Kramer MG, Lajtha K, LeMoine J, Martin M, McDowell WH, Minocha R, Sadowsky JJ, Templer PH, Wickings K (2014) Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–316
Gallo M, Lauber CL, Cabaniss SE, Waldrop MP, Sinsabaugh RL, Zak DR (2005) Soil organic matter and litter chemistry response to experimental N deposition in northern temperate deciduous forest ecosystems. Glob Change Biol 11:1514–1521
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland C, Green P, Holland E, Karl DM, Michaels AF, Porter JH, Townsend A, Vörösmarty C (2004) Nitrogen cycles: past, present and future. Biogeochemistry 70:153–226
Gong ZT, Chen ZC, Luo GB, Zhang GL, Zhao WJ (1999) Soil reference with Chinese soil taxonomic. Soils 31:57–63 (in Chinese)
Grandy AS, Sinsabaugh RL, Neff JC, Stursova M, Zak DR (2008) Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 91:37–49
Hagedorn F, Spinnler D, Siegwolf R (2003) Increased N deposition retards mineralization of old soil organic matter. Soil Biol Biochem 35:1683–1692
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization, and nitrification. In: Weaver RW, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabi A, Wollum A (eds) Methods of soil analysis. Part 2. Microbiological and biochemical properties. Soil Science Society of America, Madison, pp 985–1018
Henry HA, Juarez JD, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob Change Biol 11:1808–1815
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Hu YL, Zeng DH, Liu YX, Zhang YL, Chen ZH, Wang ZQ (2010) Responses of soil chemical and biological properties to nitrogen addition in a Dahurian larch plantation in Northeast China. Plant Soil 333:81–92
Huang ZQ, Clinton PW, Baisden WT, Davis MR (2011) Long-term nitrogen additions increased surface soil carbon concentration in a forest plantation despite elevated decomposition. Soil Biol Biochem 43:302–307
Jia SX, Wang ZQ, Li XP, Sun Y, Zhang XP, Liang AZ (2010) N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 333:325–336
Johnston CT, Aochi YO (1996) Fourier transform infrared and Raman spectroscopy. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner DL (eds) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America and American Society of Agronomy, Madison, pp 269–321
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Liu SR, Li XM, Niu LM (1998) The degradation of soil fertility in pure larch plantation in the northeastern part of China. Ecol Eng 10:75–86
Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzeon S, Grelle A, Hari P, Jarvis PG, Kolari P, Kowalski AS, Lankreijer H, Law BE, Lindroth A, Loustau D, Manca G, Moncrieff JB, Rayment M, Tedeschi V, Valentini R, Grace J (2007) The human footprint in the carbon cycle of temperate and boreal forests. Nature 447:849–851
Nave LE, Vance ED, Swanston CW, Curtis PS (2009) Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 153:231–240
Neff JC, Townsend AR, Gleixner G, Lehman SJ, Turnbull J, Bowman WD (2002) Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–917
Pan YP, Wang YS, Tang GQ, Wu D (2012) Wet and dry deposition of atmospheric nitrogen at ten sites in Northern China. Atmos Chem Phys 12:6515–6535
Parham JA, Deng SP (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32:1183–1190
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Scheuner ET, Makeschin F (2005) Impact of atmospheric nitrogen deposition on carbon dynamics in two Scots pine forest soils of Northern Germany. Plant Soil 275:43–54
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MP, Zak DR (2005) Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Strickland TC, Sollins P (1987) Improved method for separating light and heavy fraction organic material from soil. Soil Sci Soc Am J 51:1390–1393
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Turner BL, Hopkins DW, Haygarth PM, Ostle N (2002) Glucosidase activity in pasture soils. Appl Soil Ecol 20:157–162
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
von Mersi W, Schinner F (1996) Dehydrogenase activity with the substrate INT. In: Schinner F, Õhlinger R, Kandeler E, Margesin R (eds) Methods in Soil Biology. Springer, Berlin, pp 243–245
Vries WD, Reinds GJ, Gundersen P, Sterba H (2006) The impact of nitrogen deposition on carbon sequestration in European forests and forest soils. Glob Change Biol 12:1151–1173
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–1177
Wang ZQ, Guo DL, Wang XR, Gu JC, Mei L (2006) Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 288:155–171
Yang K, Shi W, Zhu JJ (2013) The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 368:535–546
Yang K, Zhu JJ, Gu JC, Yu LZ, Wang ZQ (2015) Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larix gmelinii plantation. Ann For Sci 72:435–442
Zhou XH (1994) Long-term research on China’s forest ecosystems. Northeast Forestry University Press, Harbin, pp 213–221 (in Chinese)
Zibilske LM (1994) Carbon mineralization. In: Weaver RW, Angle S, Bottomly P (eds) Methods of soil analysis. Part 2. Microbiological and biochemical properties. Soil Science Society of America, Madison, pp 835–864
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This work was supported by the National Basic Research Program of China (2012CB416903), and the National Natural Science Foundation of China (31570600).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Rights and permissions
About this article
Cite this article
Yang, K., Zhu, J., Gu, J. et al. Effects of continuous nitrogen addition on microbial properties and soil organic matter in a Larix gmelinii plantation in China. J. For. Res. 29, 85–92 (2018). https://doi.org/10.1007/s11676-017-0430-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-017-0430-7