Abstract
Restoring natural forests after field abandonment is a land management objective that fosters the recovery of forest biodiversity. We performed seeding and transplanting of native tree species 40 years after the abandonment of an arable field that became dominated by a dwarf bamboo (Pleioblastus chino (Franch. et Sav.) Makino) and by kudzu (Pueraria lobata (Willd.) Ohwi). By permutation tests, the removal of competing vegetation (gap creation) significantly increased the survival of three seeded species of Fagaceae and of eight transplanted species. In contrast, intact vegetation prevented most individuals of all species from surviving for 1 year after planting. The lack of natural recruitment of Fagaceae in the nonseeded subplots indicated that seed limitation was a cause of the slow afforestation. Although litter accumulation in gaps at the time of seeding slightly increased survival for late-germinating Quercus myrsinifolia Blume and Castanopsis sieboldii (Makino) Hatus. ex T. Yamaz. et Mashiba, the effect was not consistent among plots and was not statistically significant. Our results suggest that for successful afforestation using native trees in abandoned fields, it will be necessary to remove competitive native species to avoid severe limitations on microsite availability and that simultaneous tree establishment by seeding or transplanting should be implemented to accelerate the establishment of native tree species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of vegetation change after farmland abandonment is determined by alterations of biotic and abiotic conditions caused by past agricultural activity (Hobbs and Walker 2007). Previous studies found that deviation of the revegetation trajectory of abandoned fields occurred across a wide geographical range (e.g., Baeten et al. 2010; Cramer et al. 2008; Flinn and Vellend 2005). Many studies of long-term vegetation change and tree seedling ecology from the United States and Europe have provided valuable information to support the management of abandoned fields in temperate regions (Rejmánek and Van Katwyk 2004). However, in temperate Asia, few studies have described afforestation of abandoned fields (Sitzia et al. 2010; Zhang et al. 2010).
In Japan, farmland abandonment has increased for several decades as a result of long-term stagnation of the agricultural industry. Statistics from the Ministry of Agriculture, Forestry and Fisheries (2011) show that 10.6 % of Japan’s total farmland was abandoned in 2010. Under such conditions, afforestation of abandoned fields to produce natural forests has become an important land management goal. Arita and Ohkuro (2007) reported the results of a case study in a temperate region of Japan, and described the dynamics of Salix dominance at former wet rice paddy fields after farmland abandonment. However, knowledge is limited of the vegetation dynamics of abandoned mesic fields after long-term abandonment (Sato and Nakata 2008) and optimal restoration practices for afforestation of these fields has not been examined experimentally.
The dominant plants in abandoned fields can directly interfere with tree establishment as a result of competitive interactions (Löf and Welander 2004) or can facilitate tree establishment (De Steven 1991). In the forest ecosystems of Japan, acorn predation by mice reduced the establishment of Quercus species where site conditions protected foraging mice under dense vegetation dominated by dwarf bamboo (Sasa spp.; Iida 2004; Wada 1993). In abandoned fields in the United States, plant litter decreased the detectability of Quercus acorns by mice (Myster and Pickett 1993). Considering the importance of these factors for tree seedling establishment and the trend of farmland abandonment in Japan, we designed a study to identify how these factors operated at a Japanese site. We chose an abandoned field with no forest canopy that was dominated by both a dwarf bamboo (Pleioblastus chino (Franch. et Sav.) Makino) and by kudzu (Pueraria lobata (Willd.) Ohwi), and performed two types of afforestation study. In the direct seeding experiment, we used three locally dominant species of Fagaceae and tested the influences of two vegetation treatments (periodic removal of vegetation to create gaps versus intact vegetation) and the effects of litter accumulation at the time of seeding (litter added versus bare soil) on seedling survival. In the transplanting experiment, we examined the survival of eight native tree species under the same two vegetation treatments. The survival of the trees after seeding and transplanting was recorded in autumn of the first year and in late spring of the second year. Based on the results of these experiments, we discuss here the importance of vegetation management and tree planting for afforestation when such natives dominate at a site.
Materials and methods
Study site
The study site was located in Omitama city (36°9′N, 140°20′E) in the eastern Kanto plains of Japan. At the study site, wheat had been cultivated for decades until abandonment began around 1968–1969. The northwestern margin of the field was adjacent to a broad-leaved forest mainly composed of late-successional evergreen trees (Castanopsis sieboldii (Makino) Hatus. ex T. Yamaz. et Mashiba and Quercus myrsinifolia Blume) and deciduous trees (Padus grayana (Maxim.) C.K. Schneid. and Quercus serrata Murray). Taxonomic nomenclature for these and other species follows that in the Ylist (BG Plants index: http://bean.bio.chiba-u.jp/bgplants/ylist_main.html), which provides a consistent nomenclatural system for Japan.
According to our vegetation survey, the study site was densely covered by a dwarf bamboo, P. chino, and by a clonal woody vine, P. lobata, during the summer, and no adult trees or saplings had become established within the field. We used the Braun-Blanquet cover-abundance scale (Braun-Blanquet 1932) to quantify the vegetation cover (Table 1). Canada goldenrod (Solidago altissima L.) and other herbaceous vegetation were also found growing among the two codominant plants.
The site is located in a region with a temperate climate, in a lowland rural landscape. Records from 1971 to 2000 at the nearby meteorological station in Tsukuba show a mean annual temperature of 13.5 °C, with the mean monthly temperature reaching a maximum of 25.2 °C in August and a minimum of 2.3 °C in January. Mean annual precipitation was 1235.6 mm. According to the soil classification map produced by the Economic Planning Agency (1973), the soil at the study site was classified as a well-drained volcanic Andosol.
Experimental setup
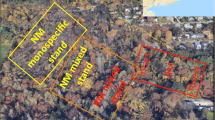
At the center of the field, we established a 12 m × 28 m experimental site. The minimum distance between the site and the adjacent broad-leaved forest margin was about 7 m. Within this area, we created eight plots: four replicates of a 4 m × 4 m plot with intact vegetation and four replicates of a plot with a gap in the vegetation (hereafter, the “gap plots”; details are provided later in this section). These plots were systematically located at the site, with a 4-m buffer of intact vegetation between plots (Fig. 1). At the center of each plot (starting 0.9 m from each edge and representing a 2.2 m × 2.2 m area), we created 16 subplots, each of 0.5 m × 0.5 m. A 0.2-m-wide foot path passed through the center of the plot along both axes, in the shape of a +, and divided 16 subplots into 4 groups of 4 subplots.
Experimental design. At a 12 × 28 m experimental site, eight plots were created: four replicates of a 4 × 4 m plot with intact vegetation (plots 2, 3, 6, and 7) and four replicates of a 4 × 4 m plot with a gap in the vegetation (plots 1, 4, 5, and 8). At the center of each plot, a 2.2 × 2.2 m area was established with a foot path 0.2 m wide passing through the center of the plot along both axes in the shape of a +. Within this design, we created 16 subplots for tree planting, each 0.5 m × 0.5 m. Litter addition and removal (bare soil) were used as the treatments in eight subplots used for the seeding experiment
We selected three native tree species for seeding (Q. serrata, C. sieboldii, and Q. myrsinifolia) and eight species for transplanting (Pinus densiflora Siebold et Zucc., Toxicodendron sylvestre (Siebold et Zucc.) Kuntze., Carpinus tschonoskii Maxim., Celtis sinensis Pers., Aphananthe aspera (Thunb.) Planch., Q. serrata, Q. myrsinifolia, and C. sieboldii). In well-developed forests in the region, Q. myrsinifolia and C. sieboldii are commonly dominant. The other six species mainly occur in early- or mid-successional forests. Except for P. densiflora seeds purchased from a seed supplier, we obtained seeds of the other species from forests located near the site. All seeds were stored in a refrigerator at 4 °C until seeding. To prepare seedlings for transplanting, eight species were seeded on 27 March 2009 in pots filled with a commercial soil medium, Ryousai-Baido Pp (pH 6.0–6.5, electrical conductivity [EC] <1000 mS m−1; Nihon Hiryo Co., Ltd., Tokyo, Japan), in a nursery bed covered by a shade screen that reduced the photosynthetic photon flux density to 69.3 % of the ambient level. Water was provided daily until transplanting.
Removal of the aboveground vegetation in the four gap plots was performed nine times during the experiment between 12 November 2008 and 23 April 2010 at irregular intervals. We used a brush cutter during the first removal (before seeding) and removed the vegetation manually or using scissors thereafter. We divided 16 subplots in each plot as follows: 4 “litter” subplots with litter added (one species per subplot, plus a control subplot with no seeds), 4 “bare” subplots with no litter added (one species per subplot, plus a control subplot with no seeds), and 8 subplots, each with transplanted seedlings from a single species (described later in this section). In the seeded subplots, we used 30 seeds of Q. serrata, 25 seeds of Q. myrsinifolia, or 25 seeds of C. sieboldii. Seeds were planted at a depth of about 2 cm on 10 March 2009. For the litter plots, we collected the litter that was removed from all of the seeding subplots (4 litter subplots and 4 bare subplots) for each of 8 plots. Because the bare subplots were included, this represented the average amount of litter for the litter and bare subplots (0.32 kg per subplot). We then homogenized this sample to produce a single composite sample before applying it to the litter plots. After seeding, we added the prepared litter (at 0.32 kg per subplot) in 4 subplots at one side of the plot, and left the other 4 subplots on the other side bare.
We added netting above the subplots to prevent the added litter from being blown away by the wind from 12 to 26 March 2009. In the transplanting subplots, we did not modify the existing litter.
On 11 and 12 June 2009, we transplanted seedlings of each species into separate subplots (n = 1 subplot per species, for a total of 1 × 8 = 8 subplots) at a spacing of 8 cm. We used 12 seedlings per subplot for all species except Q. myrsinifolia, for which we only used nine seedlings. Survival of the seedlings was investigated in autumn of the same year (15 October 2009) and in late spring of the following year (24 May 2010). We measured the diameter at ground height and the seedling height on 24 May 2010.
To understand the microsite conditions in each plot, we measured the light and soil conditions on 8 July 2009. We obtained a hemispherical photograph at the center of each plot from a height of 50 cm above the ground. The vegetation openness at each plot was then calculated using the Canopon 2 software (http://takenaka-akio.org/etc./canopon2/). Soil pH (the mean value to a depth of 10 cm) was measured at 10 locations using a Soil pH Tester (Takemura Electric Works, Ltd., Tokyo, Japan) and soil EC (mS m−1, the mean value to a depth of 7 cm) was measured at five locations using a Delta-T WET-2 Sensor (Delta-T Devices, Ltd., Cambridge, U.K.), and the values were averaged to produce a single mean for each plot.
Statistical analysis
We used the Welch two-sample t test to identify significant differences in vegetation openness and soil conditions (pH and EC) between the plots with intact vegetation and the gap plots.
We used a permutation test for redundancy analysis (Legendre and Legendre 1998) to test for significant treatment effects on the overall response of seedling survival until the late spring of the next year after seeding and transplanting. We also used binomial generalized linear models (GLMs) and the bias-reduction method of Firth (1993) to examine the response of Q. myrsinifolia and C. sieboldii in the transplanting experiment because of their exceptionally low survival.
In these analyses, we used the vegan package (version 2.0-9) and the brglm package (version 0.5-8) in version 3.0.2 of the R software (R Development Core Team 2013).
We used mean seedling diameter at ground level and mean seedling height to represent the size of the surviving seedlings in each treatment (mean ± SE). For resprouted individuals with two or more stems, we used the maximum diameter and height.
Results
Microsite conditions
Mean vegetation openness was 6.3 ± 0.8 % (mean ± SE) in the plots with intact vegetation and 49.8 ± 0.8 % in the gap plots and differed significantly between these treatments (t = −23.597, df = 4.192, p < 0.05). Mean soil EC was 50.3 ± 7.7 mS m−1 in the plots with intact vegetation and 50.8 ± 6.5 mS m−1 in the gap plots and did not differ significantly between these treatments (t = −0.100, df = 5.833, p = 0.92). Mean soil pH was 5.7 ± 0.3 in the plots with intact vegetation and 5.4 ± 0.2 in the gap plots and did not differ significantly between these treatments (t = 2.115, df = 5.756, p = 0.08).
Survival of seeded and transplanted trees
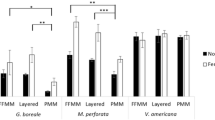
Seed germination and subsequent seedling emergence in the seeding experiment (hereafter, “seedling survival”) and seedling survival in the transplanting experiment were both significantly higher in the gap plots than in the plots with intact vegetation (Figs. 2, 3; Table 2). Litter addition slightly improved survival for Q. myrsinifolia and C. sieboldii (Fig. 2), but this was not consistent among the plots and the overall effect of litter addition on the seedlings was not significant (p > 0.05, Table 2). Binomial GLMs with the bias-reduction method showed that gap creation significantly increased the survival of transplanted Q. myrsinifolia (p = 0.006) but not of C. sieboldii (p = 0.171). No seedlings of the three Fagaceae species were observed in the nonseeded control subplots. “Appendix” section shows that seedling size of six species found in early or mid-successional forests was larger than that of two late-successional evergreen species, Q. myrsinifolia and C. sieboldii.
Although it was not feasible for us to quantify seed damage caused by predation, debris of seeds from the three seeded species (an indication of predation) were commonly observed around the gap plots before germination. Both in autumn of the first year and in late spring of the next year, predation damage was commonly observed in the form of damage to the stems of seedlings of most species, and feces of the Japanese hare were observed around the gap plots.
Discussion
Our experiments suggested that the existence of severe microsite limitations under the intact vegetation cover hindered seedling establishment by the local tree species (Table 2; Figs. 2, 3). In a Japanese temperate mixed forest ecosystem, dominance by dwarf bamboo Sasa nipponica (Makino) Makino et Shibata directly reduced tree seedling establishment as a result of shading (Itô and Hino 2005). Pueraria lobata is a competitive woody vine and is well known to be a problematic weed in forestry (Mitich 2000). Therefore, significantly lower vegetation openness in the plots with intact vegetation than in the gap plots at our site during late spring suggests that the light environment is a possible mechanism that limited tree seedling establishment. Dense coverage by species of Sasa also provided safer foraging sites for mice under their dense evergreen culms (Iida 2004; Itô and Hino 2007; Wada 1993). Gnawing damage on tree seedlings by mice under Sasa palmata (Marliac) Nakai cover in a temperate grassland reduced the establishment of Fagaceae species (Ida and Nakagoshi 1996). Signs of seed predation (perhaps by mice) right after acorn seeding and of browsing damage by hares were observed at our gap plots. This suggests that predation damage might have confounded our results, which thus might not reflect only the effects of the vegetation conditions. However, the creation of gaps in the vegetation without the use of animal exclosures effectively increased survival of most of the transplanted species by late spring of the next year. Therefore, gap creation appears to increase the success of transplanting using the tree species we studied and may be an effective afforestation measure that avoids the limitations caused by decreased microsite availability in plots with intact vegetation.
Although the study site had been abandoned for 40 years and was adjacent to broad-leaved forest composed mainly of adult trees of species in the Fagaceae, such as Q. myrsinifolia and C. sieboldii, no seedlings were observed in plots with intact vegetation in 2008 (Tokuoka et al. 2011). Our results agree with those in our previous study (Tokuoka et al. 2011): we observed no seedling recruitment in nonseeded control subplots. These results suggest that severe seed limitation contributed to the inhibition of natural afforestation at the site.
A previous study indicated that the addition of Quercus litter and Solidago litter reduced the loss of Quercus rubra seeds (Myster and Pickett 1993). Our experiments also showed a slightly beneficial influence of litter addition for Q. myrsinifolia and C. sieboldii in the gap plots (Fig. 2), but the effect of the litter was not consistent among the plots and the difference between the treatments was not significant (Table 2). Among the eight planted species, these two species showed the slowest germination in the nursery (data not shown) and the smallest size under our experimental setup (“Appendix” section). In particular, C. sieboldii seedlings had not opened their first adult leaf on the transplanting date. This would mean that the cotyledon remained similar to its state in the ungerminated seed and would be attractive to mice. Therefore, raising seedlings for a longer period before transplanting them would help to avoid predation damage during the early establishment stages. Future research should determine the optimal seedling age or size for transplanting of each species.
In the agricultural landscape of the Kanto plains where our experiment was conducted, forest patches are highly fragmented and forest-agricultural field ecotones are a common landscape feature. Therefore, vines such as P. lobata that prefer forest edges and dominant species such as P. chino that prefer a forest floor or a mowed field can easily invade these sites as a result of their clonal growth habit. In fact, even in cultivated fields adjacent to secondary forests, farmers in this region usually need to prevent P. chino invasion by digging a ditch around the edges of their fields as a barrier to its rhizomes or annually managing the aboveground culms of these species. Considering the weediness of these two competitive species and their persistence in the agricultural landscape of our study area, our results suggest that simultaneous implementation of vegetation removal and the planting of seeds or seedlings will be required to effectively overcome the effects of competition combined with severe microsite limitations during the initial afforestation process. In addition, it may be necessary to install animal exclosures to decrease seed predation, or to transplant older seedlings to decrease herbivore damage, although the optimal strategy for each tree species must be determined in future research.
References
Arita H, Ohkuro T (2007) A maintenance system aimed to control wood vegetation of abandoned paddy field—study based on a survey of Ohshima-are Jouetsu-shi Niigata, Japan. Trans Jpn Soc Irrig Drain Rural Eng 249:255–260 (In Japanese with English summary)
Baeten L, Hermy M, Daele SV, Verheyen K (2010) Unexpected understorey community development after 30 years in ancient and post-agricultural forests. J Ecol 98:1447–1453
Braun-Blanquet J (1932) Plant sociology (Transl G.D. Fuller and H.S Conrad). McGraw-Hill, New York
Cramer VA, Hobbs RJ, Standish RJ (2008) What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol Evol 23:104–112
De Steven D (1991) Experiments on mechanisms of tree establishment in old-field succession: seedling emergence. Ecology 72:1066–1075
Economic Planning Agency. 1973. Ibaraki-Ken Dojou Bunruizu [Soil Classification Map of Ibaraki Prefecture]. Economic Planning Agency, Tokyo. (In Japanese)
Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80:27–38
Flinn KM, Vellend M (2005) Recovery of forest plant communities in post-agricultural landscapes. Front Ecol Environ 3:243–250
Hobbs RJ, Walker LR (2007) Old field succession: development of concepts. In: Cramer VA, Hobbs RJ (eds) Old fields. Island Press, Washington, DC, pp 17–30
Ida H, Nakagoshi N (1996) Gnawing damage by rodents to the seedlings of Fagus crenata and Quercus mongolica var. grosseserrata in a temperate Sasa grassland–deciduous forest series in southwestern Japan. Ecol Res 11:97–103
Iida S (2004) Indirect negative influence of dwarf bamboo on survival of Quercus acorn by hoarding behavior of wood mice. For Ecol Manag 202:257–263
Itô H, Hino T (2005) How do deer affect tree seedlings on a dwarf bamboo-dominated forest floor? Ecol Res 20:121–128
Itô H, Hino T (2007) Dwarf bamboo as an ecological filter for forest regeneration. Ecol Res 22:706–711
Legendre P, Legendre L (1998) Numerical Ecology, 2nd English edn. Elsevier, Amsterdam
Löf M, Welander NT (2004) Influence of herbaceous competitors on early growth in direct seeded Fagus sylvatica L. and Quercus robur L. Ann For Sci 61:781–788
Ministry of Agriculture, Forestry and Fisheries (2011) Census of agriculture and forestry. Ministry of Agriculture, Forestry and Fisheries, Tokyo. http://www.maff.go.jp/j/nousin/tikei/houkiti/pdf/genjou_1103r.pdf. Accessed 08 Nov 2013 (in Japanese)
Mitich LW (2000) Kudzu [Pueraria lobata (Willd.) Ohwi]. Weed Technol 14:231–235
Myster RW, Pickett STA (1993) Effects of litter, distance, density and vegetation patch type on postdispersal tree seed predation in old fields. Oikos 66:381–388
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rejmánek M, Van Katwyk KP (2004) Old-field succession: a bibliographic review (1901–1991). http://botanika.bf.jcu.cz/suspa/
Sato T, Nakata M (2008) Factors affecting forest formation in abandoned terraced paddy fields in a mountainous region. J Jpn For Soc 90:364–371 (In Japanese with English summary)
Sitzia T, Semenzato P, Trentanovi G (2010) Natural reforestation is changing spatial patterns of rural mountain and hill landscapes: a global overview. For Ecol Manag 259:1354–1362
Tokuoka Y, Ohigashi K, Nakagoshi N (2011) Limitations on tree seedling establishment across ecotones between abandoned fields and adjacent broad-leaved forests in eastern Japan. Plant Ecol 212:923–944
Wada N (1993) Dwarf bamboos affect the regeneration of zoochorous trees by providing habitats to acorn-feeding rodents. Oecologia 94:403–407
Zhang KR, Dang HS, Tan SD, Wang ZX, Zhang QF (2010) Vegetation community and soil characteristics of abandoned agricultural land and pine plantation in the Qinling Mountains, China. For Ecol Manag 259:2036–2047
Acknowledgments
We thank Mr. Toshio Mihashi and his family for approving our experimental use of their field. We thank Dr. Syuntaro Hiradate, Mr. Koukichi Matsumoto, Mr. Katsuo Abe, Mr. Hiroyuki Iino, Mr. Terushi Kamada, and other members of the experimental farm management division of the National Institute for Agro-Environmental Sciences, who helped to prepare the seedlings and assisted in vegetation management in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
The online version is available at http://www.springerlink.com
Corresponding editor: Yu Lei
Appendix
Appendix
See Table 3.
Rights and permissions
About this article
Cite this article
Tokuoka, Y., Ohigashi, K., Watanabe, K. et al. Removal of competitive native species combined with tree planting can accelerate the initial afforestation process: an experiment in an old field in Japan invaded by dwarf bamboo and kudzu. J. For. Res. 26, 581–588 (2015). https://doi.org/10.1007/s11676-015-0072-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-015-0072-6