Abstract
Quantitative relationships between the aboveground biomass of dwarf bamboo, Sasa nipponica, and the survivorship and emergence of seedlings of Abies homolepis, Fraxinus lanuginosa f. serrata, and Fagus crenata were estimated. We show that dwarf bamboo acts as an ecological filter since the responses of tree species differ according to the biomass of dwarf bamboo. Deer exclusion without management of dwarf bamboo would make it impossible for any tree species to regenerate due to rapid increases in the biomass of dwarf bamboo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
George and Bazzaz (1999a) pointed out that the fern understory modifies the forest floor microenvironment and that it differentially influences tree-seedling species emergence and establishment. They described the function of it as “a selective filter that influences future forest composition through reducing density, altering species composition, and determining spatial distribution of the seedling bank.” These selectivity was due to a differential response of tree-seedling species to the fern understory, which affects not only emergence and establishment, but also seedling growth and survival (George and Bazzaz 1999b). They suggested that the fern understory acts as an “ecological filter.” In addition to fern, Lei et al. (2002) reported on another understory plant, the evergreen understory shrub Rhododendron maximum L., the effects of which differ depending on the degree of shade tolerance of tree seedlings.
Besides the studies described above, it is well-known that some understory plants can inhibit the emergence, establishment, and survival of tree seedlings in forests (Cross 1981; Maguire and Forman 1983; Smith and Vankat 1991). In East Asia, many studies have shown that dwarf bamboos typically act in this way (Nakashizuka and Numata 1982; Nakashizuka 1987, 1988; Taylor and Qin 1988; Hiura et al. 1995; Taylor et al. 2004). However, most previous studies have investigated the responses of tree seedlings to the presence or absence of understory plants, rather than developing quantitative relationships between seedling performance and the abundance of the understory plants.
Sasa nipponica Makino et Shibata, a species of dwarf bamboo that can dominate forest understories, is also known to be an important forage for sika deer (Cervus nippon Temminck) in Japan (Takatsuki 1983, 1986, 1990; Yokoyama and Shibata 1998a). In previous papers, we reported the results from a nine-year field experiment that combined exclusion of browsing by sika deer and the removal of S. nipponica in a mixed forest in an area with large deer populations (Itô and Hino 2004, 2005). These studies revealed that (1) dwarf bamboo quickly recovered its biomass after the exclusion of deer, (2) increasing the biomass of dwarf bamboo decreased the survivorship of tree seedlings, and (3) the magnitude of the negative impact on the seedlings differed among tree species. These findings suggest that S. nipponica works as an ecological filter. However, we have not previously documented the effects of the bamboo biomass on seedling survival, even though the bamboo biomass varied among plots or among years.
Exclosures are widely used to protect vegetation from deer grazing pressure. However, deer can act as a “keystone herbivore” (Waller and Alverson 1997; Rooney 2001), and exclusion of deer may cause other effects on the ecosystem, such as the dominance of dwarf bamboo, which suppresses seedlings of tree species, as described above. Therefore, not only exclusion of deer but also control of dwarf bamboo will be required for smooth tree regeneration. From this perspective, it is important to know the quantitative relationships between the abundance of dwarf bamboo and the emergence/survival of tree seedlings.
In this paper, we report on the results from a study that estimated the effect of aboveground biomass of S. nipponica on the survival rate of seedlings and on the number of emerging seedlings of three major species in the forest: Abies homolepis Sieb. et Zucc., Fraxinus lanuginosa Koidz. f. serrata (Nakai) Murata, and Fagus crenata Blume. Based on these relationships, we discuss quantitatively how dwarf bamboo works as an ecological filter, and the implications of this for forest restoration in areas with excessively large deer populations.
Methods
Study site
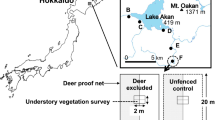
The study site was located in a temperate mixed forest on Mt. Ôdaigahara, in Nara Prefecture of west-central Japan (lat. 34°11′N, long. 136°06′E, 1,540 m a.s.l.). The forest on the site consisted mainly of evergreen coniferous trees such as Abies homolepis and deciduous broadleaved trees such as Fagus. crenata (Itô and Hino 2003). The forest floor was extensively covered with S. nipponica, but its aboveground biomass was kept at relatively low levels by deer browsing (Yokoyama and Shibata 1998b). The population density of the deer around the study site was estimated at 22.3–34.1 individuals/km2 (Maeji et al. 1999).
Species to be studied
Statistical analysis was conducted for the three major regenerating tree species (A. homolepis, F. lanuginosa f. serrata, and F. crenata). These species had a sufficient number of emerging seedlings (Itô and Hino 2005). A. homolepis and F. crenata were the two dominant tree species in the study site (Itô and Hino 2003). It is said that Abies is more shade-tolerant than Fagus at the sapling stage, though F. crenata seedlings can establish on thick litter, while A. homolepis seedlings do not seem to be able to do this (Nakashizuka 1991). F. lanuginosa is a shade-tolerant species (Koike 1986), and Wada (1993) showed that the sapling density of this species was not significantly correlated with the coverage of dwarf bamboo.
Data collection
We established five experimental plots in 1996; each plot was a square of 20 × 20 m. We divided each plot into eight subplots corresponding to eight different experimental treatments based on combinations of the presence or absence of deer, mice, and dwarf bamboo: deer exclusion or control-mice exclusion or control-dwarf bamboo clipping or control (Table 1). Two 1 × 1 m quadrats were established within each subplot to monitor the emergence and disappearance of tree seedlings. Details of the study plots are described in Itô and Hino (2004, 2005). In 1999, we established an additional plot (10 × 10 m) to make the sample size larger. It contained eight 1 × 1 m quadrats corresponding to each of the eight treatments. The total number of quadrats was initially 80, and this increased to 88 in 1999 due to the eight additional quadrats. In the present paper, however, we have only used the data from the deer-exclusion quadrats to isolate the effects of dwarf bamboo, and we have not distinguished between the exclusion or presence of mice because our previous studies showed insignificant effects of mice on seedling survival (Itô and Hino 2004, 2005). As a result, we used data from 40 quadrats (1997–1999) or 44 quadrats (2000–2004).
We began monitoring the seedlings in the initial 80 quadrats in 1997, and in the eight supplemental quadrats in 2000. We marked all tree seedlings that emerged in the 1 × 1 m quadrats from 1997 to 2003 and checked their status (alive or dead) every 0.5–2 months from 1997 to 2004, except during the winter when the plots were under snow cover.
The abundance of S. nipponica was estimated from the aboveground parts of this plant every fall from 1997 to 2003 in each subplot where the dwarf bamboo had not been removed. Six sample plots covering a circle of radius 10 cm were randomly selected within each subplot. In each sampling plot, the S. nipponica plants were clipped at ground level, and the dry weights of these samples were measured. The biomass value for each plot is represented by the mean of the six samples.
Statistical analysis
We developed a statistical model to explain the relationship between seedling survival and the biomass of the dwarf bamboo using a generalized linear mixed model (GLMM) approach:
where i is the plot number, j is the cohort number, k is the individual number, p ijk is the probability of survival, w ijk is the aboveground biomass of the dwarf bamboo (kg d.w./m2) where the seedling is located, and a ijk is the age of the seedling (years). β w and β a are fixed effect coefficients for w and a, respectively, and β is the intercept. ω i and ω j correspond to the random effects of the ith plot and jth cohort, respectively. ɛ ijk is the individual error factor for a given individual, cohort, and plot. Equation 1 can be transformed into
where f(w, a) is the linear predictor.
In this analysis, we defined survival and death based on whether a seedling that emerged in or survived until a given year was found alive or dead, respectively, in the next spring. We used the value of the aboveground biomass of the dwarf bamboo in the plot corresponding to the treatment and the plot in the year when survival was assessed. This analysis was conducted for each tree species using the lmer function provided by the Matrix package for the R software package (version 2.2.1; R Development Core Team 2005) with Laplace’s approximation method. The number of seedlings of Fagus crenata was only high enough to use this analysis in 1999, so we used only this cohort and did not allocate a random cohort factor to the analysis of this species.
We then analyzed the relationships between the aboveground biomass of dwarf bamboo and the number of emerging seedlings for cohorts that emerged in 2002 for A. homolepis and F. lanuginosa f. serrata. There were not enough Fagus crenata seedlings to emerge after 1999 to permit this analysis. A GLMM procedure with a logarithmic link function and a Poisson error distribution was used for this analysis. For both species, the number of seedlings that emerged in 2002 was used as the dependent variable, and the aboveground biomass of the dwarf bamboo was used as the independent variable. The differences between plots were allocated to the random factor, which only affected the intercept.
Results and discussion
In the 40 quadrats in the deer-exclusion plots established in 1997, the aboveground biomass of S. nipponica increased from an average of 0.09 kg/m2 (range 0.07–0.12) in 1997 to 0.65 kg/m2 (range 0.40–0.87) in 2003. This large change and the interplot variation in dwarf bamboo biomass caused differential survival of the tree seedlings and enabled us to analyze these relationships for each tree species and age class. Table 2 shows the sample sizes for each cohort and each species.
For all three-tree species, increasing the biomass of the dwarf bamboo decreased the seedling survival (Fig. 1), and the fixed-effect coefficients differed significantly from zero (Table 3). The more dense the dwarf bamboo, the more severely it shaded the forest floor (Itô and Hino 2004). Differences in shade tolerance among the tree species might thus explain their different responses to bamboo biomass. The fixed-effect coefficients for age also differed significantly from zero for A. homolepis and F. lanuginosa. The positive values for this coefficient mean that survival improved with increasing seedling age, and the ratio of these two coefficients was about 1/10. This meant that one year of seedling growth could compensate for 0.1 kg/m2 of aboveground biomass of Sasa.
a–c Estimated relationships between the aboveground biomass of dwarf bamboo (Sasa nipponica) and seedling survival in each age class. aAbies homolepis, bFraxinus lanuginosa f. serrata, and cFagus crenata. Curves for age classes are not shown for F. crenata because age was not a statistically significant parameter (see Table 3)
)
Of the three species, the survival of the F. crenata seedlings was affected most strongly by dwarf bamboo. The survival curve for this species decreased more steeply than those for the other two species in Fig. 1 (i.e., survival decreased most rapidly with increasing dwarf bamboo biomass). Specifically, an estimated 89.9% of seedlings of F. crenata would survive for one year if all dwarf bamboo was removed (1/[1 + exp(−2.19+19.5 × 0)] = 0.899). However, only 0.05% were estimated to survive at a biomass of 0.5 kg/m2, which was the minimum biomass attained by the dwarf bamboo in the deer-exclusion plots. At a typical dwarf bamboo biomass of 0.1 kg d.w./m2, and in the presence of deer foraging at our study site, 56.0% were estimated to survive. This survival rate is comparable to that of 0–1 year seedlings of F. crenata in the bright location after the simultaneous death of dwarf bamboo (59.4%; Nakashizuka 1988). However, if the dwarf bamboo was to grow to 0.2 kg d.w./m2 (double the current natural state), the survival rate would decrease to 15.3%.
Dwarf bamboo had the least effect on seedling survival of the coniferous species A. homolepis, which had the most gently sloping curve in Fig. 1. The survival rate for seedlings younger than one year with complete removal of dwarf bamboo was 67.9%. While this was lower than that of F. crenata (Fig. 1), they survived better for a higher density of dwarf bamboo. An estimated 28.9% of A. homolepis seedlings younger than one year would survive to the following year, even at 0.5 kg d.w./m2 of dwarf bamboo biomass, versus a value of ~0% for F. crenata. The estimated 52.3% survivorship at a dwarf bamboo biomass of 0.2 kg d.w./m2 is also much higher than that for F. crenata (15.3%). This survival rate indicates that the capacity for regeneration in this species is as strong as the value reported for F. crenata by Nakashizuka (1988) under the condition of simultaneous death of dwarf bamboo, but this would not require complete elimination of dwarf bamboo.
Fraxinus lanuginosa f. serrata had survivorship curves with slopes that were intermediate between those of the other two species (Fig. 1). For this species, an estimated 82.6% of the seedlings younger than one year would survive in plots where dwarf bamboo was removed, and 18.6% would survive at 0.5 kg d.w./m2 of dwarf bamboo biomass. The estimated survival rate for seedlings younger than one year at a dwarf bamboo biomass of 0.2 kg d.w./m2 is 58.5%, which is equivalent to that of A. homolepis.
Increasing aboveground biomass of the dwarf bamboo also significantly decreased the number of emerging seedlings in A. homolepis and F. lanuginosa (P<0.001; Table 4). When the aboveground biomass of dwarf bamboo reached 0.5 kg d.w./m2, the number of emerging seedlings of A. homolepis was about half the natural level observed at the current dwarf bamboo biomass of 0.1 kg d.w./m2 (Fig. 2; exp(−1.43 × 0.5)/exp(−1.43 × 0.1) = 0.564). F. lanuginosa revealed a similar relationship. Dense dwarf bamboo generates a large amount of litter, and increased litter is known to inhibit seed germination in many plant species (Xiong and Nilsson 1999). S. nipponica retains its aboveground parts for about 18–20 months (Agata and Kamata 1979), so an aboveground biomass of 0.5 kg d.w./m2 for this plant contributes about 0.3–0.33 kg/m2/year of litter to the forest floor. This value was larger than the amount of litter fall from trees in this site (0.2268 kg/m2/year; Furusawa et al. 2003). This additional supply of leaf litter may influence seedling emergence of the various tree species differently, though the responses of A. homolepis and F. lanuginosa were not so different.
In conclusion, dwarf bamboo appears to function as an ecological filter because different tree species responded differently to its biomass in terms of seedling emergence and survival. In addition, our quantitative analysis revealed some implications for forest restoration in areas with large deer populations. In many areas of Japan, including the Ôdaigahara region, large-scale deer exclosures have been established to prevent deer grazing from impeding forest restoration. However, this may lead to rapid growth of understory plants, often reaching densities that can adversely affect tree regeneration. In areas where S. nipponica dominates the forest floor, exclosure allows it to rapidly reach an aboveground biomass greater than 0.5 kg d.w./m2. Our results indicate that this level of biomass would prevent F. crenata from regenerating and would greatly reduce seedling emergence and survival of A. homolepis and F. lanuginosa. If we assume that about 50% survival of seedlings is required for successful regeneration, dwarf bamboo biomass must be kept below 0.2 kg d.w./m2 for A. homolepis and F. lanuginosa and below 0.1 kg d.w./m2 for F. crenata. Accordingly, forest managers must somehow reduce the biomass or the area of S. nipponica where deer exclosures have been established. In forests where dwarf bamboo dominates the forest floor, excluding it from some parts of the forest floor will facilitate seedling establishment and forest regeneration. In addition, preserving suitable seedbeds such as fallen logs for Abies species (Narukawa and Yamamoto 2002) and tip-up mounds for F. crenata (Peters et al. 1992) will also enhance seedling establishment.
References
Agata W, Kamata E (1979) Ecological characteristics and dry matter production of some native grasses in Japan I. Annual growth pattern of Sasa nipponica community. J Jpn Grassl Sci 25:103–109 (in Japanese with English summary)
Cross JR (1981) The establishment of Rhododendron ponticum in the Killarney Oakwoods, S. W. Ireland. J Ecol 69:807–824
Furusawa H, Miyanishi H, Kaneko S, Hino T (2003) Movement of soil and litter on the floor of a temperate mixed forest with an impoverished understory grazed by deer (Cervus nippon centralis Temminck). J Jpn For Soc 85:318–325 (in Japanese with English summary)
George LO, Bazzaz FA (1999a) The fern understory as an ecological filter: growth and survival of canopy-tree seedlings. Ecology 80:846–856
George LO, Bazzaz FA (1999b) The fern understory as an ecological filter: emergence and establishment of canopy-tree seedlings. Ecology 80:833–845
Hiura T, Sano J, Konno Y (1995) Age structure and response to fine-scale disturbances of Abies sachalinensis, Picea jezoensis, Picea glehnii, and Betula ermanii growing under the influence of a dwarf bamboo understory in northern Japan. Can J For Res 26:289–297
Itô H, Hino T (2003) Stand structure of a mixed forest in Mt. Ôdaigahara. Appl For Sci 12:163–165 (in Japanese)
Itô H, Hino T (2004) Effects of deer, mice and dwarf bamboo on the emergence, survival and growth of Abies homolepis (Piceacea) seedlings. Ecol Res 19:217–223
Itô H, Hino T (2005) How do deer affect tree seedlings on a dwarf bamboo-dominated forest floor? Ecol Res 20:121–128
Koike T (1986) Photosynthetic responses to light intensity of deciduous broad-leaved tree seedlings raised under various artificial shade. Environ Control Biol 24:51–58
Lei TT, Semones SW, Walker JF, Clinton BD, Nilsen ET (2002) Effects of Rhododendron maximum thickets on tree seed dispersal, seedling morphology, and survivorship. Int J Plant Sci 163:991–1000
Maeji I, Yokoyama S, Shibata E (1999) Population density and range use of sika Deer, Cervus nippon, on Mt. Ohdaigahara, Central Japan. J For Res 4:235–239
Maguire DA, Forman RTT (1983) Herb cover effects on tree seedling patterns in a mature hemlock-hardwood forest. Ecology 64:1367–1380
Nakashizuka T (1987) Regeneration dynamics of beech forests in Japan. Vegetatio 69:169–175
Nakashizuka T (1988) Regeneration of beech (Fagus crenata) after the simultaneous death of undergrowing dwarf bamboo (Sasa kurlensis). Ecol Res 3:21–35
Nakashizuka T (1991) Population dynamics of coniferous and broad-leaved trees in a Japanese temperate mixed forest. J Veg Sci 2:413–418
Nakashizuka T, Numata M (1982) Regeneration process of climax beech forests I. Structure of a beech forest with the undergrowth of Sasa. Jpn J Ecol 32:57–67
Narukawa Y, Yamamoto S (2002) Effects of dwarf bamboo (Sasa sp.) and forest floor microsites on conifer seedling recruitment in a subalpine forest, Japan. For Ecol Manag 163:61–70
Peters R, Nakashizuka T, Ohkubo T (1992) Regeneration and development in beech-dwarf bamboo forest in Japan. For Ecol Manag 55:35–50
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rooney TP (2001) Deer impacts on forest ecosystems: a North American perspective. Forestry 74:201–208
Smith LL, Vankat JL (1991) Communities and tree seedling distribution in Quercus rubra- and Prunus serotina-dominated forests in southwestern Pennsylvania. Am Midl Nat 126:294–307
Takatsuki S (1983) The importance of Sasa nipponica as a forage for Sika deer (Cervus nippon) in Omote-Nikko. Jpn J Ecol 33:17–25
Takatsuki S (1986) Food habits of Sika Deer on Mt. Goyo, Northern Honshu. Ecol Res 1:119–128
Takatsuki S (1990) Dwarf bamboos as food for Sike Deer in Japan. Bamboo J 8:56–63
Taylor AH, Qin Z (1988) Regeneration patterns in old-growth Abies-Betula forests in the Wolong Natural Reserve, Sichuan, China. J Ecol 76:1204–1218
Taylor AH, Huan J, Zhou S (2004) Canopy tree development and undergrowth bamboo dynamics in old-growth Abies-Betula forests in southwestern China: a 12-year study. For Ecol Manag 200:347–360
Wada N (1993) Dwarf bamboos affect the regeneration of zoochorous trees by providing habitats to acorn-feedking rodents. Oecologia 94:403–407
Waller DM, Alverson WS (1997) The white-tailed deer: a keystone herbivore. Wildl Soc Bull 25:217–226
Xiong S, Nilsson C (1999) The effects of plant litter on vegetation: a meta-analysis. J Ecol 87:984–994
Yokoyama S, Shibata E (1998a) Characteristics of Sasa nipponica grassland as a summer forage rosouce for sika deer on Mt Ohdaigahara, central Japan. Ecol Res 13:193–198
Yokoyama S, Shibata E (1998b) The effects of sika-deer browsing on the biomass and morphology of a dwarf bamboo, Sasa nipponica, in Mt. Ohdaigahara. For Ecol Manag 103:49–56
Acknowledgments
We are grateful to H. Furusawa, Y. Takahata, A. Ueda, T. Shimada, S. Chikaguchi, and S. Narayama (Forestry and Forest Products Research Institute) for their support, the staff of the Ôdaigahara Visitors’ Center of the Ministry of Environment, and S. and S. Tagaito (Ôdai Shrine) for their kind cooperation during our fieldwork. We also thank Prof. E. Shibata (Nagoya University), Prof. T. Nakashizuka (Research Institute for Humanity and Nature), Prof. K. Kikuzawa (Ishikawa Prefectural University), Dr. T. Kubo (Hokkaido University) for their helpful comments on our research. This study was supported by grants from the Ministry of Environment (Environmental Research by National Research Institutes of Government Ministries and Agencies, 1999–2002) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 14206019, 2002–2005).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Itô, H., Hino, T. Dwarf bamboo as an ecological filter for forest regeneration. Ecol Res 22, 706–711 (2007). https://doi.org/10.1007/s11284-006-0066-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-006-0066-0