Abstract
The Co–Fe–Zr system and its Co–Zr subsystem were optimized using the CALculation of PHAse Diagram (CALPHAD) approach. The substitutional solution model was used for describing the phases liquid, fcc_A1, bcc_A2 and hcp_A3. Two Laves phases were modeled as (Co,Fe,Zr)2(Co,Fe,Zr)1, and the phases CoFe and CoZr with the bcc_B2 crystal structure were described as the ordered one of bcc_A2 in the formula (Co,Fe,Va,Zr)0.5(Co,Fe,Va,Zr)0.5Va3. With limited solubility ranges, all other phases were treated as the line compounds (Co,Fe)mZrn. An excellent agreement between the reported and calculated results was reached. The reliable thermodynamic parameters of the Co–Fe–Zr system were acquired, which can be well applied to various thermodynamic calculations and materials design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Co–Fe–Zr system is a basic and key system in hydrogen storage materials, amorphous alloys, high-entropy alloys, and magnetic materials.
In the international thermonuclear experimental reactor (ITER) project, the intermetallic compound ZrCo is intended to replace uranium for the storage and delivery of tritium.[1] Fe substitution for Co can enhance the durability against the hydrogen-induced disproportionation, which makes it more favorable to store tritium.[2] And cobalt, iron and zirconium are the principal elements in amorphous alloys[3,4,5,6] and high-entropy alloys.[7,8,9] Furthermore, Hoque et al.[10] have reported a two-phase magnetic Co11Zr2–Co23Zr6 system and the addition of Fe can improve its magnetic and mechanical properties.
The further development of such novel materials is largely dependent on the accurate phase equilibria information and detailed thermodynamic description of the Co–Fe–Zr system. Recently, the Co–Fe–Zr isothermal sections between 1273 and 1573 K have been experimentally studied by Wang et al.[11], and the Co–Fe, Co–Zr and Fe–Zr subsystems have been thermodynamically assessed by several researchers.[12,13,14] Whereas the Co–Zr thermodynamic parameters.[13] still need to be adjusted because of the inappropriate thermodynamic model of CoZr and low-temperature decomposition of CoZr3. As a result, the current study is aimed to develop reasonable thermodynamic descriptions and obtain a self-consistent set of thermodynamic parameters for the Co–Zr and Co–Fe–Zr systems using the CALPHAD approach.
2 Literature Review
2.1 Co–Fe System
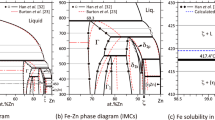
Guillermet,[15] Ohnuma et al.[16] and Turchanin et al.[17] carried out the thermodynamic studies of the Co–Fe system, but their calculated magnetic properties did not completely reproduce the experimental data.[18,19,20] Wang et al.[12] conducted the first-principles calculations and heat capacity measurements and performed a detailed thermodynamic assessment of the Co–Fe system. Their thermodynamic parameters[12] are directly adopted in the current work. Fig. 1 presents the Co–Fe phase diagram assessed by Wang et al.[12]
Co–Fe phase diagram assessed by Wang et al.[12]
2.2 Co–Zr System
Chart and Putland,[21] Saunders and Miodownik[22] and Bratberg and Jansson[23] performed the early thermodynamic optimizations of the Co–Zr system, but the thermochemical data were not considered in their work. Liu et al.[24] carried out the detailed experimental determination and proper thermodynamic assessment of the Co–Zr system; however, the reported mixing enthalpies of liquid phase[25,26,27] were still not taken into consideration. Durga and Kumar[28] first coupled bcc_A2 and CoZr using an order-disorder model and improved the Co–Zr thermodynamic description. Kosorukova et al.[29] and Semenova et al.[30] performed the experimental investigations on the controversial part of the Co–Zr phase diagram including the phase transition and phase stability of CoZr3. Given the available phase equilibria and thermochemical information[24,25,26,27,28,29,30,31,32,33,34,35,36,37], Agraval et al.[13] conducted a novel Co–Zr thermodynamic assessment. Nevertheless, the thermodynamic parameters of CoZr and CoZr3[13] still required to be revised to match the common model of the order-disorder transition and avoid the low-temperature decomposition of CoZr3. As a result, the Co–Zr thermodynamic modeling is updated in this work.
The Co–Zr phase diagram was first studied by Pechin et al.,[31] who identified the five compounds Co11Zr2, Co4Zr (also called as Co23Zr6), λ2, CoZr and CoZr2. Bataleva et al.[32] used metallography, EPMA, XRD and DTA to carry out the experimental study on the Co–Zr phase diagram, especially the liquidus temperatures. The reported phase diagrams[31, 32] were contradictory in the Zr-rich part owing to the presence of CoZr3[32]. Liu et al.[24] adopted the same method as Bataleva et al.[32] to measure the phase equilibria of the Co–Zr system in the complete composition ranges above 1073 K. CoZr3 was found to form peritectically from liquid + bcc_A2 at 1253 K,[32] but peritectoidically from CoZr2 + bcc_A2 at 1254 K.[24] Kosorukova et al.[29] also reported the temperatures of four invariant reactions using metallography, XRD and DTA. Semenova et al.[30] determined the CoZr2–Zr region of the Co–Zr phase diagram using the same methods as Kosorukova et al.,[29] and their experimental results supported the conclusion[24] that CoZr3 formed by the peritectoid reaction CoZr2 + bcc_A2 → CoZr3 at 1254 K. For the development of the present description, all phase equilibria data[24, 29,30,31,32] are considered.
The enthalpies of formation of the Co–Zr compounds were studied by several researchers using direct synthesis reaction calorimetry[33, 34], differential calorimetry,[35] solution calorimetry[36] and first-principles calculations[37]. In the update process, the reported thermochemical data related to CoZr, CoZr2 and CoZr3 are taken into consideration.
2.3 Fe–Zr System
The early thermodynamic studies of the Fe–Zr system were performed by Servant et al.[38] and Jiang et al.[39] The Fe–Zr phase diagram was measured using metallography, EPMA, XRD and DTA by Stein et al.,[40] who suggested that Fe23Zr6 should be a metastable phase and its existence in the Fe–Zr system was mainly due to the stabilization of oxygen. Guo et al.[41] accepted their experimental results[40] and then performed a new thermodynamic assessment of the Fe–Zr system. But Yang et al.[42] and Lu et al.[43] thought Fe23Zr6 as a stable phase and re-modeled the Fe–Zr system. Saenko et al.[14] considered the experimental and theoretical thermochemical properties,[44,45,46] determined the heat capacity of FeZr2 from 220 to 450 K, agreed with Stein et al.[40] that Fe23Zr6 was not an equilibrium phase, and carried out the thermodynamic re-modeling of the Fe–Zr system. Their calculated results reproduced the experimental data better, hence the thermodynamic parameters of Saenko et al.[14] are directly adopted in this work. Fig. 2 is the Fe–Zr phase diagram assessed by Saenko et al.[14]
Fe–Zr phase diagram assessed by Saenko et al.[14]
2.4 Co–Fe–Zr System
The isothermal section of the Co–Fe–Zr system at 1273 K was reported by Panteleimonov et al.,[47] Two continuous solid solutions (Co,Fe)Zr2 and λ2 and three three-phase regions (Co,Fe)Zr2 + λ2 + CoZr, λ2 + Co4Zr + Fe3Zr and Co4Zr + Fe3Zr + fcc(Co,Fe) were identified, but the stability of FeZr2 was inconsistent with that in the Fe–Zr system[40]. Mishenina et al.[48] investigated some equilibrated alloys to determine the Co–Fe–Zr isothermal section at 770 K, but the FeZr2 stability was also in discrepancy with that in the Fe–Zr system[40]. In addition, Mishenina et al.[48] did not report the bcc_A2/CoFe phase boundaries from the Co–Fe system and the occurrence of Co11Zr2 and CoZr3. Wang et al.[11] measured four isothermal sections in the entire composition ranges at 1273, 1373, 1473 and 1573 K utilizing metallography, EPMA, XRD and DSC, in which liquid and λ2 formed the continuous solid solutions between 1273 and 1573 K from the Co–Zr to Fe–Zr corner. Four reported isothermal sections[11] are in good accordance with the corresponding subsystems and thus accepted in the optimization process.
3 Thermodynamic Models
3.1 Unary Phases
The lattice stability parameters of Co, Fe and Zr were taken from the SGTE Pure 5.1 Database.[49]
3.2 Solution Phases
The substitutional solution model was adopted to describe the solution phases ϕ (ϕ = liquid, fcc_A1, bcc_A2 and hcp_A3):
where xi represents the molar fraction of i (i = Co, Fe, Zr); means the excess Gibbs energy, generally expanded using a Redlich–Kister type polynomial;[50] \({}^{mag}G_{m}^{\phi }\) presents the magnetic part of the Gibbs energy, expanded using a Hillert-Jarl type formalism.[51]
where the jth interaction parameters \({}^{j}L_{{{\text{Co}},{\text{Fe}}}}^{\phi }\), \({}^{j}L_{{{\text{Co}},Zr}}^{\phi }\), \({}^{j}L_{{{\text{Fe}},{\text{Zr}}}}^{\phi }\), \({}^{j}L_{{{\text{Co}},{\text{Fe}},{\text{Zr}}}}^{\phi }\) are taken from Refs.[12,13,14] or optimized in the present work.
3.3 Intermetallic Phases
Two Laves phases were described as (Co,Fe,Zr)2(Co,Fe,Zr)1 using a usual two-sublattice model. Moreover, four intermetallic phases Co11Zr2, (Co,Fe)23Zr6, (Co,Fe)1Zr2 and (Co,Fe)1Zr3 were modeled as the line compounds in the formula (Co,Fe)mZrn due to their limited homogeneity ranges.[11] Taking the Laves phase λ2 as an example, its Gibbs energy is given as follows:
where \(y_{*}^{^{\prime}}\) and \(y_{*}^{{^{\prime\prime}}}\) are the site fractions of * (* = Co, Fe and Zr); \(G_{r:s}^{{{\uplambda }_{{2}} }}\) represent the Gibbs energy in its SER state, hcp_A3 for Co, bcc_A2 for Fe or hcp_A3 for Zr; \({}^{j}L_{u,v:w}^{{{\uplambda }_{{2}} }}\) and \({}^{j}L_{w:u,v}^{{{\uplambda }_{{2}} }}\) are the assessed jth interaction parameters in this work.
The triple-defect mechanism is important for bcc_B2 when considering its site ordering.[52] In order to combine with bcc_B2, the vacancies should occupy sites on each sublattice in bcc_A2. Two intermetallic phases CoFe and CoZr had the ordered bcc_B2 crystal structure and a single Gibbs energy function derived by Ansara et al.[53] was proposed to describe the disordered bcc_A2 and ordered bcc_B2 simultaneously:
where \(G_{m}^{{{\text{bcc\_A2}}}} (x_{i} )\) means the Gibbs energy of bcc_A2; \(G_{m}^{bcc\_B2} (y_{i}^{^{\prime}} ,y_{i} )\) and \(G_{m}^{{{\text{bcc\_B2}}}} (x_{i} )\) are the contribution of bcc_B2 and the one from bcc_A2 to bcc_B2, respectively. When \(x_{i}^{{}} = y_{i}^{^{\prime}} = y_{i}\) (i = Co, Fe, Va and Zr), the phase is bcc_A2, otherwise it would be bcc_B2.
4 Result and Discussion
In the present work, the Thermo-Calc software package[54] is used for optimizing the Co–Zr and Co–Fe–Zr systems. The crystal structures and thermodynamic models of each phase are presented in Table 1.
In the optimization process, Fe23Zr6 is treated as a metastable phase in the Fe–Zr system according to the detailed experimental results[40]. And the value a of \(G_{{\text{Fe:Zr}}}^{{\left( {\text{Co,Fe}} \right)_{{{23}}} {\text{Zr}}_{{6}} }}\)(=a + bT) is set to − 680411.0 based on the reported enthalpy of formation from the ab-initio calculations[43], and the value b is set to a suitable one to satisfy the metastable state in the Fe–Zr system.
Figure 3 and Table 2 present the calculated phase diagram and invariant reactions of the Co–Zr system superimposed with the previous work[13] and experimental data.[24, 29,30,31,32] In comparison with the former work,[13] the low-temperature decomposition of CoZr3 has been solved. The calculated enthalpies of formation of the Co–Zr compounds at 298 K in comparison with the previous work[13] and experimental data[33,34,35,36,37] are presented in Table 3. Nearly all the reported enthalpies of formation are well reproduced. The calculated enthalpy of formation of CoZr2 is more positive than the reported one[13] to avoid the low-temperature decomposition of CoZr3.
The Co–Fe–Zr thermodynamic parameters are summarized in Table 4. Figure. 4, 5, 6, 7 show the four calculated Co–Fe–Zr isothermal sections at 1273, 1373, 1473, and 1573 K together with the experimental data.[11] Most of the experimental information is well reproducible, but there still exist a few acceptable discrepancies. The calculated phase boundaries of the liquid phase at 1573 K show large inconsistency with the experimental data[11] probably because of the experimental uncertainty at a very high temperature. With the stability limit of CoZr2 at 1473 K, the two-phase region CoZr2 + λ2 at 1373 K is impossible to fully meet the experimental data.[11] The three-phase regions Co23Zr6 + Co11Zr2 + fcc(Co,Fe) at 1273 and 1373 K disagree with the experimental information.[11] Although great efforts were made, the value b of \({}^{{0}}L_{{\text{Co,Fe:Zr}}}^{{{\text{Co}}_{{{11}}} {\text{Zr}}_{{2}} }}\) (=a + bT) would need to be -60 J/(mol K) to fit this three-phase region. This value leads to the occurrence of Co11Zr2 at 1573 K and cannot be accepted in this work. Therefore, further studies are recommended to determine the phase relations about Co11Zr2 below 1373 K. Additionally, the tie-lines λ2 + Co23Zr6 at 1273, 1373, 1473, and 1573 K have some differences from the experimental data,[11] which is due to the experimental homogeneity range and corresponding Gibbs energy of λ2.
Calculated isothermal section at 1273 K of the Co–Fe–Zr system compared with the experimental data[11].
Calculated isothermal section at 1373 K of the Co–Fe–Zr system compared with the experimental data[11].
Calculated isothermal section at 1473 K of the Co–Fe–Zr system compared with the experimental data[11].
Calculated isothermal section at 1573 K of the Co–Fe–Zr system compared with the experimental data[11].
The calculated liquidus surface projection and corresponding invariant reactions of the Co–Fe–Zr system are also shown in Fig. 8 and Table 5.
5 Conclusion
In the current study, the thermodynamic descriptions of the Co–Zr and Co–Fe–Zr systems have been carried out using the CALPHAD approach in accordance with the experimental phase equilibria and thermochemical information. There is a satisfactory agreement between the experimental and calculated results. The reliable Co–Fe–Zr thermodynamic parameters are first acquired, which can be used as a cost-effective tool for materials design and processing.
References
F. Wang, R. Li, C. Ding, W. Tang, Y. Wang, S. Xu, R. Yu, and Y. Wu, F. Wang, R. Li, C. Ding, W. Tang, Y. Wang, S. Xu, R. Yu, and Y. Wu, Recent Progress On The Hydrogen Storage Properties of ZrCo-Based Alloys Applied in International Thermonuclear Experimental Reactor (ITER), Prog. Nat. Sci. Mater., 2017, 27(1), p 58–65
R.A. Jat, R. Singh, S.C. Parida, A. Das, R. Agarwal, S.K. Mukerjee, and K.L. Ramakumar, R.A. Jat, R. Singh, S.C. Parida, A. Das, R. Agarwal, S.K. Mukerjee, and K.L. Ramakumar, Structural and Hydrogen Isotope Storage Properties of Zr–Co–Fe Alloy, Int. J. Hydrogen Energy, 2015, 40(15), p 5135–5143
C. Xie, W. Li, J. Luo, Y. Yang, and S. Li, C. Xie, W. Li, J. Luo, Y. Yang, and S. Li, Development of MAgnetic and Ductile Fe-Co-Zr-Mo-Cr Glassy Alloy Without Metalloid Elements, J. Non-Cryst. Solids, 2018, 482, p 213–216
P. Yu, J.Z. Zhang, and L. Xia, P. Yu, J.Z. Zhang, and L. Xia, Fe87Zr7B4Co2 Amorphous Alloy with Excellent Magneto-caloric Effect Near Room Temperature, Intermetallics, 2018, 95, p 85–88
A.P. Srivastava, D.A. Babu, A. Verma, A.A. Deshmukh, A. Kaushal, and U.A. Palikundwar, Understanding the Effect of Hf on Thermal Stability and Glass Forming Ability of Fe572Co308Zr7-xHfxB4Cu1 (x = 3, 5, and 7) Metallic Glasses, J. Non-Cryst. Solids, 2019. https://doi.org/10.1016/j.jnoncrysol.2018.09.016
K. Kotynia, P. Pawlik, K. Filipecka, and J. Filipecki, K. Kotynia, P. Pawlik, K. Filipecka, and J. Filipecki, Calorimetric and Structural Analysis of the Zr–Fe–Co–B–Mo–W Amorphous Alloys Doped With Gadolinium, J. Alloy. Compd., 2020, 842, p 155940
P. Gong, S. Wang, F. Li, and X. Wang, P. Gong, S. Wang, F. Li, and X. Wang, Kinetics of Glass Transition and Crystallization of a Zr40Hf10Ti4Y1Al10Cu25Ni7Co2Fe1 Bulk Metallic Glass with High Mixing Entropy, Metall. Mater. Trans. A, 2018, 49, p 2918–2928
J. Liu, Z. Xing, H. Wang, X. Cui, G. Jin, and B. Xu, J. Liu, Z. Xing, H. Wang, X. Cui, G. Jin, and B. Xu, Microstructure and Fatigue Damage Mechanism of Fe–Co–Ni–Al–Ti–Zr High-entropy Alloy Film by Nanoscale Dynamic Mechanical Analysis, Vacuum, 2019, 159, p 516–523
C. Chen, H. Zhang, Y. Fan, W. Zhang, R. Wei, S. Guan, T. Wang, B. Kong, T. Zhang, and F. Li, C. Chen, H. Zhang, Y. Fan, W. Zhang, R. Wei, S. Guan, T. Wang, B. Kong, T. Zhang, and F. Li, Crystallization and Corrosion Resistance of Zr–Ti–Y–Al–Cu–Ni–Co–Fe Complex Multi-component Bulk Metallic Glasses, Intermetallics, 2020, 118, p 106688
S.M. Hoque, S.K. Makineni, A. Pal, S.A. Rahman, S. Hossain, R. Islam, P. Ayyub, and K. Chattopadhyay, S.M. Hoque, S.K. Makineni, A. Pal, S.A. Rahman, S. Hossain, R. Islam, P. Ayyub, and K. Chattopadhyay, Two Phase Ferromagnetic Composites in Co-Zr and Co-Zr-Fe Systems Containing Anti-Phase Domain Imparting Very High Strength, Mater. Res. Bull., 2018, 97, p 61–70
C.P. Wang, Y. Yu, H.H. Zhang, H.F. Hu, and X.J. Liu, C.P. Wang, Y. Yu, H.H. Zhang, H.F. Hu, and X.J. Liu, Experimental Determination of the Phase Equilibria in the Co–Fe–Zr Ternary System, J. Alloy. Compd., 2011, 509(13), p 4470–4477
J. Wang, X. Lu, N. Zhu, and W. Zheng, J. Wang, X. Lu, N. Zhu, and W. Zheng, Thermodynamic and Diffusion kinetic Studies of the Fe-Co System, Calphad, 2017, 58, p 82–100
P. Agraval, L. Dreval, M. Turchanin, and T. Velikanova, P. Agraval, L. Dreval, M. Turchanin, and T. Velikanova, Thermodynamic Assessment of the Co-Zr System, J. Phase Equilib. Diffus., 2020, 41, p 491–499
I. Saenko, A. Kupravaa, A. Udovsky, and O. Fabrichnaya, I. Saenko, A. Kupravaa, A. Udovsky, and O. Fabrichnaya, Heat Capacity Measurement of Zr2Fe and Thermodynamic Re-assessment of the Fe–Zr System, Calphad, 2019, 66, p 101625
A.F. Guillermet, A.F. Guillermet, Critical Evaluation of the Thermodynamic Properties of the Iron-Cobalt System, High Temp. High Press., 1987, 19(5), p 477–499
I. Ohnuma, H. Enoki, O. Ikeda, R. Kainuma, H. Ohtani, B. Sundman, and K. Ishida, I. Ohnuma, H. Enoki, O. Ikeda, R. Kainuma, H. Ohtani, B. Sundman, and K. Ishida, Phase Equilibria in the Fe–Co Binary System, Acta Mater., 2002, 50(2), p 379–393
M.A. Turchanin, L.A. Dreval, A.R. Abdulov, and P.G. Agraval, M.A. Turchanin, L.A. Dreval, A.R. Abdulov, and P.G. Agraval, Mixing Enthalpies of Liquid Alloys and Thermodynamic Assessment of the Cu–Fe–Co System, Powd. Metall. Met. Ceram., 2011, 50(1–2), p 98–116
M.F. Collins, and J.B. Forsyth, M.F. Collins, and J.B. Forsyth, The Magnetic Moment Distribution in Some Transition Metal Alloys, Philos. Mag., 1963, 8(87), p 401–410
D.I. Bardos, D.I. Bardos, Mean Magnetic Moments in bcc Fe-Co Alloys, J. Appl. Phys., 1969, 40(3), p 1371–1372
M. Kogachi, N. Tadachi, H. Kohata, and H. Ishibashi, M. Kogachi, N. Tadachi, H. Kohata, and H. Ishibashi, Magnetism and Point Defect in B2-type CoFe Alloys, Intermetallics, 2005, 13(5), p 535–542
T. Chart, and F. Putland, T. Chart, and F. Putland, A Thermodynamically Calculated Phase Diagram for the Co–Cr–Zr System, Calphad, 1979, 3(1), p 9–18
N. Saunders, and A.P. Miodownik, N. Saunders, and A.P. Miodownik, Thermodynamic Aspects of Amorphous Phase Formation, J. Mater. Res., 1986, 1(1), p 38–46
J. Bratberg, and B. Jansson, J. Bratberg, and B. Jansson, Thermodynamic Evaluation of the c-co-w-hf-zr System for Cemented Carbides Applications, J. Phase Equilib. Diffus., 2006, 27(3), p 213–219
X.J. Liu, H.H. Zhang, C.P. Wang, and K. Ishida, X.J. Liu, H.H. Zhang, C.P. Wang, and K. Ishida, Experimental Determination and Thermodynamic Assessment of the Phase Diagram in the Co–Zr System, J. Alloy. Compd., 2009, 482(1–2), p 99–105
Yu.O. Esin, OYu. Sidorov, M.G. Valishev, and P.V. Geld, Yu.O. Esin, OYu. Sidorov, M.G. Valishev, and P.V. Geld, The Enthalpies of Formation of Molten Zirconium Alloys with Cobalt, TVT, 1989, 27(2), p 394–396
R. Lück, H. Wang, and B. Predel, R. Lück, H. Wang, and B. Predel, Calorimetric Determination of the Mixing Enthalpy of Liquid Cobal-Zirconium Alloys, Z. Anorg. Allg. Chem., 1993, 619(3), p 447–452
M.A. Turchanin, and P.G. Agraval, M.A. Turchanin, and P.G. Agraval, Enthalpies of Mixing of Titanium, Zirconium and Hafnium Liquid Alloys with Cobalt, Rasplavy, 2002, 2, p 8–16
A. Durga, and K.C. Hari Kumar, A. Durga, and K.C. Hari Kumar, Thermodynamic Optimization of the Co–Zr System, Calphad, 2010, 34(2), p 200–205
Kosorukova, T., Agraval, P., Ivanchenko, V., Turchanin, M, (2010) Experimental reinvestigations and thermodynamic assessment of the Co–Zr system, XI International Conference on Crystal Chemistry of Intermetallic Compounds, May 30–June 2, National University of Lviv, p 52
O.L. Semenova, V.M. Petyukh, and O.S. Fomichev, O.L. Semenova, V.M. Petyukh, and O.S. Fomichev, The Constitution of Co–Zr Phase Diagram, Powder Metall. Met. Ceram., 2016, 54(9–10), p 583–589
W.H. Pechin, D.E. Williams, and W.L. Larsen, W.H. Pechin, D.E. Williams, and W.L. Larsen, The Zirconium-Cobalt Alloy System, Trans. ASM, 1964, 57, p 464–473
S.K. Bataleva, V.V. Kuprina, V.V. Burnasheva, V.Y. Markiv, G.N. Ronami, and S.M. Kurnetsova, S.K. Bataleva, V.V. Kuprina, V.V. Burnasheva, V.Y. Markiv, G.N. Ronami, and S.M. Kurnetsova, Phase Diagram of Cobalt– Zirconium System, Moscow Univ. Chem. Bull., 1970, 25(5), p 33–36
J.C. Gachon, and J. Hertz, J.C. Gachon, and J. Hertz, Enthalpies of Formation of Binary Phases in the Systems FeTi, FeZr, CoTi, CoZr, NiTi, and NiZr, by Direct Reaction Calorimetry, Calphad, 1983, 7(1), p 1–12
Q. Guo, and O.J. Kleppa, Q. Guo, and O.J. Kleppa, Standard Enthalpies Of Formation Of Some Alloys Formed Between Group IV Elements and Group VIII Elements, Determined by High-Temperature Direct Synthesis Calorimetry II Alloys of (Ti, Zr, Hf) with (Co, Ni), J Alloy Compd, 1998, 269(1–2), p 181–186
P.A. Gomozov, Y.V. Zasypalov, and B.M. Mogutnov, P.A. Gomozov, Y.V. Zasypalov, and B.M. Mogutnov, Enthalpies of Formation Of Intermetallic Compounds with CsCl Structure (CoTi, CoZr, CoAl, NiTi), Russ. J. Phys. Chem., 1986, 60(8), p 1122–1124
R. Klein, P.A.G. O’Hare, and I. Jacob, R. Klein, P.A.G. O’Hare, and I. Jacob, Standard Molar Enthalpies of Formation of Alloys in the Pseudobinary System Zr(AlxCo1-x)2 at the Temperature 298.15 K, J Alloy Compd, 1997. https://doi.org/10.1016/S0925-8388(97)00226-0
P.R. Ohodnicki Jr., N.C. Cates, D.E. Laughlin, M.E. McHenry, and M. Widom, P.R. Ohodnicki Jr., N.C. Cates, D.E. Laughlin, M.E. McHenry, and M. Widom, Ab Initio Theoretical Study Of Magnetization And Phase Stability of the (Fe Co, Ni)23B6 and (Fe Co, Ni)23Zr6 Structures of Cr23C6 and Mn23Th6 Prototypes, Phys. Rev. B, 2008, 78(14), p 144414
C. Servant, C. Gueneau, and I. Ansara, C. Servant, C. Gueneau, and I. Ansara, Experimental and Thermodynamic Assessment of the Fe–Zr System, J. Alloy. Compd., 1995, 220(1–2), p 19–26
M. Jiang, K. Oikawa, T. Ikeshoji, L. Wulff, and K. Ishida, M. Jiang, K. Oikawa, T. Ikeshoji, L. Wulff, and K. Ishida, Thermodynamic Calculations of Fe-Zr and Fe-Zr-C Systems, J. Phase Equilib., 2001, 22(4), p 406–417
F. Stein, G. Sauthoff, and M. Palm, F. Stein, G. Sauthoff, and M. Palm, Experimental Determination Of Intermetallic Phases, Phase Equilibria, And Invariant Reaction Temperatures in the Fe-Zr System, J. Phase Equilib., 2002, 23(6), p 480–494
C. Guo, Z. Du, C. Li, B. Zhang, and M. Tao, C. Guo, Z. Du, C. Li, B. Zhang, and M. Tao, Thermodynamic Description of the Al–Fe–Zr System, Calphad, 2008, 32(4), p 637–649
Y. Yang, L. Tan, H. Bei, and J.T. Busby, Y. Yang, L. Tan, H. Bei, and J.T. Busby, Thermodynamic Modeling And Experimental Study of the Fe–Cr–Zr System, J. Nucl. Mater., 2013, 441(1–3), p 190–202
H. Lu, N. Zou, X. Zhao, J. Shen, X. Lu, and Y. He, H. Lu, N. Zou, X. Zhao, J. Shen, X. Lu, and Y. He, Thermodynamic Investigation of the Zr-Fe-Nb System and its Applications, Intermetallics, 2017, 88, p 91–100
K. Ali, A. Arya, P.S. Ghosh, and G.K. Dey, K. Ali, A. Arya, P.S. Ghosh, and G.K. Dey, A First Principles Study of Cohesive, Elastic and Electronic Properties Of Binary Fe–Zr Intermetallics, Comp. Mater. Sci., 2016, 112, p 52–66
K. Ali, P.S. Ghosh, and A. Arya, K. Ali, P.S. Ghosh, and A. Arya, A DFT Study of Structural, Elastic and Lattice Dynamical Properties of Fe2Zr and FeZr2 Intermetallics, J. Alloy. Compd., 2017, 723(5), p 611–619
B.O. Mukhamedov, I. Saenko, A.V. Ponomareva, M.J. Kriegel, A. Chugreev, A. Udovsky, O. Fabrichnaya, and I.A. Abrikosov, B.O. Mukhamedov, I. Saenko, A.V. Ponomareva, M.J. Kriegel, A. Chugreev, A. Udovsky, O. Fabrichnaya, and I.A. Abrikosov, Thermodynamic and Physical Properties Of Zr3fe And Zrfe2 Intermetallic Compounds, Intermetallics, 2019, 109, p 189–196
L.A. Panteleimonov, O.G. Burtseva, and V.V. Zubenko, L.A. Panteleimonov, O.G. Burtseva, and V.V. Zubenko, The Iron-Cobalt-Zirconium System, Moscow Univ. Chem. Bull., 1981, 36(6), p 89–90
I.V. Mishenina, E.F. Kazakova, E.M. Sokolovskaya, and N.Y. Tolmachiova, I.V. Mishenina, E.F. Kazakova, E.M. Sokolovskaya, and N.Y. Tolmachiova, Isothermal Cross-Section Of Phase Diagram of the Fe-Co-Zr System at 770 K, Moscow Univ. Chem. Bull., 1996, 51(1), p 52–54
SGTE Pure Elements (Unary) Database, Version 5.1, 〈https://www.thermocalc.com/academia/researchers/assessment-of-thermodynamic-data/〉, visited July 2 2020.
O. Redlich, and A.T. Kister, O. Redlich, and A.T. Kister, Algebraic Representation Of Thermodynamic Properties and The Classification of Solutions, Ind. Eng. Chem., 1948, 40(2), p 345–348
M. Hillert, and M. Jarl, M. Hillert, and M. Jarl, A Model For Alloying Effects In Ferromagnetic Metals, Calphad, 1978, 2(3), p 227–238
N. Dupin, and I. Ansara, N. Dupin, and I. Ansara, On The Sublattice Formalism Applied to the B2 Phase, Z. Metallkd., 1999, 90(1), p 76–85
I. Ansara, N. Dupin, H.L. Lukas, and B. Sundman, I. Ansara, N. Dupin, H.L. Lukas, and B. Sundman, Thermodynamic Assessment Of The Al–Ni System, J. Alloy. Compd., 1997, 247(1), p 20–30
B. Sundman, B. Jansson, and J.O. Andersson, B. Sundman, B. Jansson, and J.O. Andersson, The Thermo-Calc Databank System, Calphad, 1985, 9(2), p 153–190
Acknowledgment
This work was supported by Scientific Research Starting Foundation for Advanced Talents of Jiangxi University of Science and Technology (Grant No. 205200100063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, C., Wang, H. Thermodynamic Descriptions of the Co–Zr and Co–Fe–Zr Systems. J. Phase Equilib. Diffus. 42, 77–90 (2021). https://doi.org/10.1007/s11669-020-00859-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00859-5