Abstract
Phase equilibria in the CaO-ZnO-Fe2O3 system have been investigated at 1483-1673 K (1210-1400 °C) for oxide liquid in equilibrium with air and solid oxide phases: (a) zincite (Zn,Fe,Ca)O1+x; (b) spinel (Zn,Fe,Ca)Fe2O4; (c) lime (Ca,Zn)O; (d) calcium ferrites dissolving zinc oxide: brownmillerite Ca2(Fe,Zn)2O5−x, and calcium diferrite (Ca,Zn)Fe4O7. High-temperature equilibration on inert metal (platinum) substrates, followed by quenching and direct measurement of the Ca, Zn and Fe concentrations in the phases with the electron probe x-ray microanalysis (EPMA) has been used to accurately characterize the system in equilibrium with air. All results are projected onto the CaO-ZnO-“FeO1.5” plane for presentation purposes. The present paper presents systematic characterization of liquidus over a wide range of compositions in this system in equilibrium with air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The present experimental studies of the gas–slag–solid oxide phase equilibria in the CaO-ZnO-Fe2O3 “low-order” system is an important part of an integrated thermodynamic modeling and experimental research program for the Pb-Zn-Fe-Cu-Ca-Si-Al-Mg-O multicomponent system, important for the lead smelting and recycling.
Only one study of the subsolidus phase relations in this system has been found in the literature.[1] There are no previous studies of the liquidus in this system found in literature; this fact is likely to be related to the difficulties of preventing crystallisation of the liquid phase on quenching these highly fluid, silica-free slags.
In the present study experiments were focussed on a composition range having low liquidus temperature that is found to be located in the central part of the CaO-ZnO-FeO1.5 triangle: zincite (ZnO dissolving Fe2O3, FeO and CaO), spinel (Zn,Fe,Ca)Fe2O4+y, lime (CaO dissolving ZnO), brownmillerite (Ca2Fe2O5 dissolving ZnO), and calcium diferrite (CaFe4O7 dissolving ZnO) primary phase fields at 1483-1673 K (1210-1400 °C).
2 Experimental Technique and Procedure
The experimental procedure and apparatus have been described in detail in previous publications by PYROSEARCH.[2,3,4] Initial mixtures were made by mixing high-purity powders of Fe2O3 (99.945 wt.% purity), ZnO (99.8 wt.% purity), CaCO3 (99.9-99.99 wt.% purity), supplied by Alfa Aesar, MA, USA. Envelopes made of platinum foil (> 99.9% purity, 0.05 mm thickness, provided by Johnson Matthew, Australia) were used for equilibration of slags.
The technique of equilibration experiments and parameters of electron probe x-ray microanalysis (JEOL 8200L EPMA; Japan Electron Optics Ltd., Tokyo, Japan) were similar to.[2] The sample was suspended in the hot zone of the furnace by Kanthal support wire (0.7 or 1-mm diameter). Samples were pre-melted for 10-45 min (see Table 1) at 20-130 K above the equilibration temperature, to form a homogeneous slag. This was followed by equilibration at the final target temperature for the required time (1-20 h, see Table 1), to allow solid phases to crystallize from the melts.
At the end of the equilibration process, the sample was rapidly quenched in iced calcium chloride brine (< 253 K (− 20 °C)). The specimen was then washed thoroughly in water and ethanol before being dried on a hot plate, mounted in epoxy resin, and then cross-sections were prepared using conventional metallographic polishing techniques.
Hematite (Fe2O3), wollastonite (CaSiO3) (supplied by Charles M. Taylor Co., Stanford, CA, USA), and zincite (ZnO, sintered from powder) standards were used for calibration of EPMA. The ratios of the different oxidation states of iron cations were not measured with EPMA, and the iron oxide concentrations were recalculated as FeO1.5 for presentation purposes only. Stoichiometric calcium ferrites Ca2Fe2O5, CaFe2O4 and CaFe4O7 were prepared to test the JEOL ZAF correction in the Ca-Fe-O system. Systematic overestimation of CaO concentration was found after application of the standard JEOL ZAF correction for all compounds: apparent CaO concentrations were 50.6 ± 0.2 mol.% in Ca2Fe2O5, 33.9 ± 0.3 mol.% in CaFe2O4, and 20.5 ± 0.3 mol.% for CaFe4O7. Later study by Cheng et al.[5] confirms these values for Ca2Fe2O5. Similar to procedures described previously by the authors,[6, 7] an additional correction polynomial has been developed to adjust the CaO concentrations to stoichiometric (50, 33.33, and 20 mol.%, respectively) and applied to all measurements:
where x is molar fraction of cation.
No reference stoichiometric compounds are available in the Ca-Zn-O and Zn-Fe-O systems, so the correction is extended into ternary in a simplified way:
In studies of several ZnO- and Fe2O3-containing systems,[2,8,9,10] it was found that a significant fraction of apparent solubility of ZnO and Fe2O3 in other solid phases (e.g. tridymite SiO2) was not a real solubility but an effect of secondary fluorescence. According to the current hypothesis of the authors, derived from the review by Llovet et al.[11] bremsstrahlung (continuum) x-rays from electrons decelerating in particles can excite Zn or Fe atoms in the surrounding ZnO- or Fe2O3-containing matrix (at least several hundreds of microns around), and then those could emit characteristic radiation. To resolve this issue, several experiments for unreacted small particles pelletized in a matrix of Zn- or Fe-containing powder have been conducted, with the following results:
Apparent 1.45 mol.% “FeO1.5” is measured by EPMA in pure ZnO particles (average size 50 micron) surrounded by Fe2O3;
Apparent 1.64 mol.% “ZnO” is measured by EPMA in Ca2(Fe,Zn)2O5−x particles (average size 30 micron) surrounded by ZnO.
Similar to,[8,9,12] the following correction for the secondary x-ray fluorescence therefore has been developed and applied in the present study to the compositions in the phases of CaO-ZnO-“FeO1.5” system measured by EPMA:
Other calcium ferrites were only found in equilibrium with low-ZnO slag, so correction was not applied for them.
To ensure the achievement of equilibrium in the samples, the four-point test approach[4, 13] was used, including: (1) varying equilibration time; (2) confirming homogeneity of each phase; (3) approaching equilibrium from different directions (if available); (4) analyzing possible reactions that may not complete during equilibration or interfere with achievement of equilibrium. The approach used to obtain accurate, repeatable, and objective measurements by EPMA-line analysis was similar to the one described by Nikolic et al.[14] in which the average composition of the liquid phase is calculated from 20 points measured in a well-quenched area within 15 microns from the surface, with the allowed limit of standard deviation < 1 wt.%. The accuracy of the EPMA measurements was estimated to be within 1 wt. %, and the typical detection limit of minor components was estimated to be about 0.01 wt.%.[11]
3 Results and Discussion
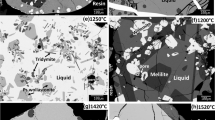
Examples of micrographs of quenched CaO-ZnO-“FeO1.5” samples are given in Fig. 1(a), (b), (c), (d), (e) and (f).
Backscattered scanning electron micrographs of quenched slag in the CaO-ZnO-“FeO1.5” system in equilibrium with one or two crystalline phases and air, p(O2) = 0.21 atm. (a) Liquid + low-Fe zincite. (b) Liquid + high-Fe zincite. (c) Liquid + spinel + brownmillerite Ca2Fe2O5. (d) Liquid + spinel + “2CaO·3FeO1.5” phase. (e) Liquid + lime + low-Fe zincite. (f) Liquid + lime + brownmillerite Ca2Fe2O5
The results of experiments after correction according to Eq 1, 2, and 3 are reported in Table 1. The phase liquidus surface constructed based on the present results and studies[5,8,16,17] for the binary joins is given in Fig. 2(a) and (b). Isotherms and boundaries at high temperatures are estimated with FactSage model.[15] Isotherms are plotted as thin solid lines, boundaries as thick solid lines, and tielines as dashed lines. All phases observed in the system in the present study are listed in Table 2.
Liquidus surface of the CaO-ZnO-“FeO1.5” system in air, p(O2) = 0.21 atm. All temperatures are in °C and compositions in mol.%. Isotherms and boundaries at high temperatures are estimated with FactSage model,[15], experimental points (brown labels) on the binary joins are from Ref 5, 8, 16, and 17. (a) Complete diagram; (b) zoomed low-ZnO part
The liquidus surface is characterized by three ternary eutectics:
Lime + zincite + brownmillerite at ~ 1264 °C
Zincite (high-Fe, see details below) + brownmillerite + spinel at ~ 1249 °C
Spinel + brownmillerite + calcium diferrite (Ca,Zn)Fe4O7 at ~ 1208 °C
Calcium diferrite (Ca,Zn)Fe4O7 is expected to form through peritectic reactions:
Spinel + hematite = calcium diferrite + liquid at ~ 1284 °C (estimation subject to large uncertainty)
Brownmillerite + CaFe2O4 = calcium diferrite + liquid at ~ 1214 °C.
Liquidus of calcium diferrite goes up on addition of ZnO due to dissolution of high ZnO in this solid solution phase exceeding its concentrations in slag. Unlike the data,[1] the phase combination spinel + CaFe2O4 was not observed in the present study, probably due to the difference in temperature.
Saddle points are predicted between zincite + brownmillerite and spinel + brownmillerite.
3.1 Composition of Zincite
Previous studies of solubility of iron oxides in zincite in air by Hansson et al.[18] reported the predominating form to be “FeO1.5” rather than “FeO” from the wet chemistry analysis, and a significant increase in iron oxide solubility in zincite was observed above 1250 °C. Jak et al.[19,20,21,22] measured the concentration of iron oxide in zincite in equilibrium with Pb-Zn-Fe-Si-O slag and spinel; again, there is a sharp change of solubility of “FeO1.5” in zincite at ~ 1260 °C from < 2 mol.% Fe/(Fe + Zn) to 10-30%. Recently, the same trend for iron oxide solubility in zincite was observed in the ZnO-“FeO1.5”-SiO2,[8] PbO-ZnO-“FeO1.5”,[23] PbO-ZnO-“FeO1.5”-SiO2 and CaO-ZnO-“FeO1.5”-SiO2 (at low CaO/SiO2 ratios) systems[24] in air. This is represented as “experimental trend” dashed line in Fig. 3. Note that the data reported by Hansson et al.[18] from experiments in fully solid samples, are considered to overestimate the solubility of “FeO1.5” in zincite due to small size of the neighbouring crystals and secondary x-ray fluorescence effect; the data with slag phase present[8, 19,20,21,22,23,24] are considered more reliable.
The public FactSage thermodynamic database[25,26,27,28] describes a smooth increase of “FeO1.5” dissolved in zincite with increasing concentration of iron in slag, and the CaO solubility in zincite would decrease with increasing concentration of iron in slag in the CaO-ZnO-“FeO1.5” system. However, the present experimental results demonstrate significantly different trends. Both “FeO1.5” and CaO solubility has been found to be low (< 1.3 mol.% “FeO1.5” and < 3.3 mol.% CaO) for low-Fe slag (< 39 mol.% FeO1.5). At higher Fe in slag, solubility of both FeO1.5 and CaO rapidly increases to ~ 27% and ~ 8%, respectively. Remarkably, ~ 3 times more CaO is dissolved in zincite despite less CaO in slag. The morphology of zincite changes from “spherical” to “elongated”—the latter called “High-Fe zincite”.
The transition of low-Fe zincite to high-Fe zincite is not affected by PbO or SiO2, but is promoted by CaO. This corresponds to change of morphology of zincite crystals from round-shaped (Fig. 1a) to plate-like (Fig. 1b), which strongly affects the mechanical properties of zinc sinters. This feature was studied previously[18]—both types of zincite have the same crystal group (hexagonal), but different c/a ratios. It may be considered as a second order phase transition (cooperative effect of dissolved FeOx making the structure more flexible to include more dissolved species, such as FeOx itself and CaO). This likely second order transition has not yet been included in any thermodynamic model—significant efforts are required to introduce a second “High-Fe zincite” phase with current FactSage database.
3.2 Compositions of Other Solid Phases
-
1.
The mutual effect of Zn and Ca in spinel has been observed for the first time. CaO concentration reaches maximum 7 mol.% when there is 20 mol.% ZnO in spinel (in equilibrium with 30% CaO slag and brownmillerite). At higher ZnO in spinel, it competes with CaO for divalent sites. At lower ZnO in spinel, the equilibrium of spinel with such high-CaO slags is not possible anymore, since calcium diferrite and hematite phases form; therefore, CaO in spinel decreases.
-
2.
The substitution of ZnO for FeO1.5 in brownmillerite Ca2(Fe,Zn)2O5−x has been observed, up to 2.0 mol.% ZnO is dissolved. CaO concentration remains constant at 50 mol.%.
-
3.
The substitution of ZnO for CaO in dicalcium ferrite (Ca,Zn)Fe4O7 has been observed, up to 5 mol.% ZnO is dissolved. FeO1.5 concentration remains constant at 80 mol.%.
-
4.
An unusual compound 2CaO·3(FeO1.5, ZnO) or Ca4Fe6O13 has been observed in one sample; the zinc-free end-member 2CaO·3FeO1.5 is unknown. Further study is needed to investigate the nature of this phase.
-
5.
Up to 10.5% ZnO is observed to dissolve in lime within the studied conditions; at higher temperatures, higher solubility has been reported by Xia et al.[17] 23 mol.% ZnO. The solubility of iron oxide in lime does not exceed 0.5 mol.%.
In summary, present experimental study has produced the first comprehensive dataset for the Ca-Zn-Fe-O system in air that is essential for the lead and zinc smelting and recycling industry, and provides valuable new data that can be used to optimize thermodynamic databases of these systems.
4 Conclusions
New phase equilibria information in the CaO-ZnO-“FeO1.5” system in air has been obtained. The studied range of temperatures covered 1483-1673 K (1210-1400 °C), and included the equilibria of slag with one or two crystalline phases: zincite, spinel, lime, calcium ferrites [brownmillerite Ca2(Fe,Zn)2O5−x, calcium diferrite (Ca,Zn)Fe4O7, etc.]. This is the first systematic study of the Ca-Zn-Fe-O system in air, as a part of the multicomponent system Pb-Zn-Fe-Cu-Si-Ca-Al-Mg-O, essential for the lead and zinc smelting and recycling industries.
References
H. Bolio-Arceo and F.P. Glasser, Zinc Oxide in Cement Clinkering: Part 1. Systems CaO-ZnO-Al2O3 and CaO-ZnO-Fe2O3, Adv. Cem. Res., 1998, 10(1), p 25-32
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Pb-Fe-Si-O System in Equilibrium with Metallic Pb, Metall. Mater. Trans. B, 2018, 49(1), p 159-180
E. Jak, P.C. Hayes, and H.-G. Lee, Improved Methodologies for the Determination of High Temperature Phase Equilibria, Korean J. Min. Mater. Inst. (Seoul), 1995, 1(1), p 1-8
E. Jak, Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field, in 9th International Conference on Molten Slags, Fluxes and Salts (MOLTEN12) (The Chinese Society for Metals, Beijing, China, 2012)
S. Cheng, Shevchenko M., and E. Jak, Phase equilibria of the CaO-“Fe2O3”-SiO2 system in air. Private communication, PyroSearch, The University of Queensland, 2019
M. Shevchenko, Chen J., and E. Jak. Establishing additional correction for quantitative EPMA measurements in the system PbO-SiO2, in AMAS 2017, 14th Biennial Australian Microbeam Analysis Symposium (QUT, Brisbane, Australia, 2017)
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Pb-Fe-Ca-O System in Air, J. Phase Equilib. Diffus., 2019, https://doi.org/10.1007/s11669-018-0703-7
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Zn-Fe-Si-O System in Air, Int. J. Mater. Res. IJMR, 2019, https://doi.org/10.3139/146.111779
M. Shevchenko and E. Jak, Experimental Liquidus Study of the Binary PbO-ZnO and Ternary PbO-ZnO-SiO2 Systems, Ceram. Int., 2018, 45(6), p 6795-6803. https://doi.org/10.1016/j.ceramint.2018.12.172
M. Shevchenko and E. Jak, Experimental Liquidus Study of the Ternary CaO-ZnO-SiO2 System. Metall. Mater. Trans. B, 2019, 50(6), p 2780-2793
X. Llovet et al., Secondary Fluorescence in Electron Probe Microanalysis of Material Couples, J. Phys. D Appl. Phys., 2012, 45(22), p 225301/1-225301/12
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Pb-Fe-Si-O System in Air, J. Phase Equilib. Diffus., 2019, https://doi.org/10.1007/s11669-019-00727-x
M. Shevchenko et al., Liquidus of “FeO”-SiO2-PbO slags in equilibrium with air and with metallic lead, in Molten 2016, 10th International Conference on Molten Slags, Fluxes and Salts (Seattle, Washington, 2016), pp. 1221-1228
S. Nikolic, P.C. Hayes, and E. Jak, Experimental Techniques for Investigating Calcium Ferrite Slags at Metallic Copper Saturation and Application to the Systems “Cu2O”-”Fe2O3” and “Cu2O”-CaO at Metallic Copper Saturation, Metall. Mater. Trans. B, 2009, 40B(6), p 892-899
M. Shevchenko and E. Jak, Thermodynamic Optimization of the ZnO-FeO-Fe2O3-CaO System. Private communication, PyroSearch, The University of Queensland, 2019
X.G. Liu, Experimental phase equilibria studies in oxide systems for copper smelting slags, in School of Chemical Engineering (University of Queensland, Brisbane, Australia, 2012), p. 108
L. Xia, Z. Liu, and P. Taskinen, Phase Equilibrium Study of the CaO-ZnO System, J. Am. Ceram. Soc., 2016, 99(11), p 3809-3815
R. Hansson, P.C. Hayes, and E. Jak, Phase Equilibria in the ZnO-Rich Area of the Fe-Zn-O System in Air, Scand. J. Metall., 2004, 33(5), p 294-304
E. Jak and P.C. Hayes, Experimental Study of Phase Equilibria in the PbO-ZnO-”Fe2O3”-CaO-SiO2 System in Air for High Lead Smelting Slags (CaO/SiO2 = 0.35 and PbO/(CaO + SiO2) = 5.0 by Weight), Metall. Mater. Trans. B, 2002, 33B(6), p 817-825
E. Jak and P.C. Hayes, Experimental Liquidus in the PbO-ZnO-”Fe2O3”-(CaO + SiO2) System in Air, with CaO/SiO2 = 0.35 and PbO/(CaO + SiO2) = 3.2, Metall. Mater. Trans. B, 2002, 33B(6), p 851-863
E. Jak and P.C. Hayes, The Effect of the CaO/SiO2 Ratio on the Phase Equilibria in the ZnO-”Fe2O3”-(PbO + CaO + SiO2) System in Air: CaO/SiO2 = 0.1, PbO/(CaO + SiO2) = 6.2, and CaO/SiO2 = 0.6, PbO/(CaO + SiO2) = 4.3, Metall. Mater. Trans. B, 2003, 34B(4), p 369-382
E. Jak et al., Experimental Study of Phase Equilibria in the PbO-ZnO-”Fe2O3”-(CaO + SiO2) System in Air for the Lead and Zinc Blast Furnace Sinters (CaO/SiO2 Weight Ratio of 0.933 and PbO/(CaO + SiO2) Ratios of 2.0 and 3.2), Metall. Mater. Trans. B, 2003, 34B(4), p 383-397
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Ternary PbO-ZnO-“FeO1.5” and PbO-CaO-ZnO systems in Air. Private communication, PyroSearch, The University of Queensland, 2018.
M. Shevchenko and E. Jak, Experimental Study of the CaO-ZnO-“FeO 1.5”-SiO 2, PbO-ZnO-“FeO 1.5”-SiO 2, PbO-CaO-ZnO-SiO 2Liquidus in Air and PbO-CaO-“FeO”-SiO 2in Equilibrium with Pb and Fe Metals. Private communication, PyroSearch, The University of Queensland, 2018.
www.factsage.com. 2007 (Montreal).
E. Jak et al., Thermodynamic modelling of the system PbO-ZnO-FeO-Fe2O3-CaO-SiO2 for zinc/lead smelting, in Proceedings of the 5th International Conference on Molten Slags, Fluxes and Salts (Iron and Steel Society, AIME, Sydney, Australia, 1997)
E. Jak et al., Coupled Experimental and Thermodynamic Modelling Studies for Metallurgical Smelting and Coal Combustion Slag Systems, Metall. Mater. Trans. B, 2000, 31B(4), p 621-630
S.A. Decterov et al., Thermodynamic modeling of the Al2O3-CaO-CoO-CrO-Cr2O3-FeO-Fe2O3-MgO-MnO-NiO-SiO2-S system and applications in ferrous process metallurgy, in SAIMM Symposium Series S36 (VII International Conference on Molten Slags, Fluxes & Salts) (The South African Institute of Mining and Metallurgy, Johannesburg, Republic of South Africa, 2004)
Acknowledgments
The authors would like to thank Nyrstar (Australia), Outotec Pty Ltd (Australia), Aurubis AG (Germany), Umicore NV (Belgium), and Kazzinc Ltd, Glencore (Kazakhstan), and Australian Research Council Linkage project LP150100783 for their financial support for this research. The authors are grateful to Prof. Peter C. Hayes (UQ) for valuable comments and suggestions, and to Ms. Suping Huang for assistance with conducting experiments, and to the staff of the University of Queensland Centre for Microanalysis and Microscopy (CMM) for their support in maintenance and operation of scanning and electron microprobe facilities in the Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shevchenko, M., Jak, E. Experimental Liquidus Studies of the CaO-ZnO-Fe2O3 System in Air. J. Phase Equilib. Diffus. 40, 779–786 (2019). https://doi.org/10.1007/s11669-019-00766-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-019-00766-4