Abstract
Phase equilibria of the binary PbO-CaO and ternary PbO-CaO-SiO2 systems have been investigated at 870-1655 °C for oxide liquid in equilibrium with air and solid oxide phases: tridymite or cristobalite SiO2, pseudowollastonite CaSiO3, dicalcium silicate (Ca,Pb)2SiO4, tricalcium silicate (Ca1−xPbx)3SiO5 (x < 0.03), new phase tricalcium-lead silicate (Ca0.8Pb0.2)3SiO5 (Ca12Pb3Si5O25), lime CaO, and calcium plumbate Ca2PbO4, covering the ranges of concentrations not studied before. High-temperature equilibration on primary phase or inert metal (platinum, gold) substrates, followed by quenching and direct measurement of the Pb, Ca and Si concentrations in the phases with the electron probe x-ray microanalysis (EPMA) has been used to accurately characterize the system. Liquidus phase equilibrium data of the present authors for the PbO-CaO-SiO2 system are essential to obtain a self-consistent set of parameters of thermodynamic models for all phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The present study of the PbO-CaO-SiO2 system is a part of a wider research program aimed at characterization of phase equilibria and thermodynamic properties of the seven-component PbO-ZnO-FeO-Fe2O3-Cu2O-CaO-SiO2 gas-slag-solid-metal system for the zinc/lead/copper smelting industry using integrated experimental investigations and thermodynamic modelling. This overall research program has already been described in previous publications of the present authors.[1] Although the pure PbO-CaO-SiO2 system itself may have limited value for industry, its study provides essential fundamental information for creating an accurate thermodynamic database, as its components combined normally constitute more than half of lead–zinc sinters and slags by molar ratio.
2 Literature Review
2.1 The Pb-Ca-O System
The studies available in literature[2,3,4,5,6] are reviewed in the previous assessment by Jak et al.[7,8] The only stable compound, calcium plumbate Ca2PbO4 (2CaO·PbO2), contains lead in highest oxidation state (Pb4+) and therefore its stability depends on p(O2). Ca2PbO4 was confirmed to be unstable at lower p(O2), < 0.001 atm.[7] Recent studies[9,10] confirm the previous data. No attempt to investigate the liquidus by microanalysis of quenched samples in this system has been found in literature, due to difficulty of quenching of the silica-free glasses, corrosive nature of such slags against platinum, and reactivity with water (usual quenching medium). Development of the phase equilibria study methodology by the authors, described below in detail, makes such investigation possible for the first time.
2.2 The PbO-CaO-SiO2 System
A literature review and an extensive experimental study of solid–liquid phase equilibria of the “PbO”-CaO-SiO2 system in air has been performed by Jak.[7,8] Over one hundred experimental liquidus points over the composition range 0-66 mol.% SiO2 and 0-42 mol.% CaO in tridymite and quartz SiO2, wollastonite and pseudowollastonite CaSiO3, dicalcium silicate (Ca,Pb)2SiO4, ganomalite Pb3(Ca,Pb)2Si3O11, calcium barysilite Pb8CaSi6O21, margarosanite Ca2PbSi3O9, and massicot PbO primary phase fields were obtained. Several solid phases reported in the early studies of the PbO-CaO-SiO2 liquidus[11,12] (3CaO·PbO·SiO2, 2CaO·3PbO·SiO2, and solutions along the PbSiO3-CaSiO3 and Pb2SiO4-CaSiO3 joins) were not confirmed by Jak.[7] Samples containing lime CaO and calcium plumbate Ca2PbO4 were not quenched since the technique of sufficiently fast quenching was not developed yet, so the corresponding samples gave information on the phase assemblage in general but not on compositions of slags. Dicalcium silicate and ganomalite were reported to have wide ranges of solid solutions in the system.
The previous experimental study[7] did not involve the high-temperature, low-PbO parts of the tridymite/cristobalite, pseudowollastonite, and dicalcium silicate primary phase fields. Present study, therefore, is focused on these in the temperature range 1473-1928 K (1200-1655 °C). Another area of the present study includes low-SiO2 part of the liquid in equilibrium with calcium plumbate, lime, dicalcium silicate and tricalcium silicate.
The dataset[7] has been acquired while the information about systematic deviations of JEOL 8200 EPMA ZAF correction for the PbO-SiO2[13,14] and PbO-CaO systems was not clearly available yet. For certain slag compositions, these deviations reach 1.5 mol.% overestimation of SiO2 concentrations. Therefore, an additional purpose of selected experiments for the PbO-CaO-SiO2 liquidus in the current study is to verify the applicability of the correction to the data measured 25 years ago[7] with the same instrument and at virtually the same conditions, so that the currently accepted correction technique can be applied for the whole dataset, including previous results.[7]
3 Experimental Part
The experimental procedure and apparatus have been described in detail in previous publications by PYROSEARCH.[1,15,16,17] Initial mixtures were made by mixing high-purity powders of CaCO3 (99.9 wt.% purity), PbO (99.9 wt.% purity), SiO2 (99.9 wt.% purity), supplied by Alfa Aesar, MA, USA.
Three types of substrates were used for equilibration, depending on the conditions:
-
1.
Pt foil envelopes or wires (> 99.9% purity, 0.05 mm thickness, provided by Johnson Matthew, Australia) were used for the slags in the primary phase fields of pseudowollastonite and dicalcium silicate at 1553-1708 K (1280-1435 °C), cristobalite at 1903-1928 K (1630-1655 °C), and lime at 1373-1573 K (1100-1300 °C).
-
2.
Au foil envelopes (99.99% purity, 0.127 mm thickness, provided by Sigma Aldrich, Australia) were used for the low-SiO2 PbO-CaO-SiO2 slag in equilibrium with calcium plumbate and other solids at 1143-1273 K (870-1000 °C).
-
3.
SiO2 open crucibles (prepared from ampoule sealed by melting with hydrogen–oxygen torch and then with a small hole drilled at the top, to reduce PbO loss due to evaporation and accidental contamination from dust in the furnace) were used for the PbO-CaO-SiO2 slags in equilibrium with tridymite and cristobalite at 1473-1743 K (1200-1470 °C).
An additional measure to reduce the evaporation of PbO from the samples and to achieve equilibrium was preparation of the PbO-SiO2 (60 mol.% SiO2) master-slag by heating the oxide mixture in Pt crucibles at 1173 K (900 °C). The obtained glassy slag was quenched and ground to fine powder in an agate mortar.
The technique of equilibration experiments, quenching, sample preparation, and parameters of electron probe x-ray microanalysis (JEOL 8200L EPMA; Japan Electron Optics Ltd., Tokyo, Japan) is similar to Ref 1. The sample was suspended in the hot zone of the furnace on a support wire made of Kanthal [0.7 or 1-mm diameter, for T < 1753 K (1480 °C)] or platinum–rhodium alloy [0.5-mm diameter, for T > 1753 K (1480 °C)]. Samples were pre-melted for short time (15-60 min, see Table 1) at 10-50 K above the equilibration temperature, to form a homogeneous slag. This was followed by equilibration at the final target temperature and atmosphere condition for the required time (from 0.75 to 5 h).
Preliminary FactSage calculation[8] showed that if a bulk composition is selected close to the “PbO”-CaO binary system (74 mol.% PbO, 24% CaO and 2% SiO2), the liquid phase at 1143-1573 K (870-1300 °C) will contain very low SiO2 concentrations (~ 0.1 mol.%). Most SiO2 will form a separate solid phase such as (Ca,Pb)2SiO4, and the equilibrium of two essentially SiO2-free phases (liquid and lime or Ca2PbO4) will not be affected by the SiO2-containing phase. Therefore, instead of studying the pure “PbO”-CaO binary system, low-SiO2 bulk compositions are prepared in the present study, since it allows to obtain additional information on the first SiO2-containing phase forming at minor addition of SiO2 to the “PbO”-CaO binary. The highest temperature sample in the PbO-CaO(-low SiO2) system at 1573 K (1300 °C) was done without premelt and for 20 min only; even then, the evaporation loss of PbO was significant, that delineates the upper temperature limit of silica-free PbO-rich slags to be investigated with the current technique.
Wollastonite (CaSiO3) (supplied by Charles M. Taylor Co., Stanford, CA) and K-456 lead silicate glass (71 wt. pct. PbO, supplied by NIST) standards were used for Si, Pb and Ca calibration of the EPMA. PbSiO3 and Pb2SiO4 (obtained as a part of experimental study of the PbO-SiO2 system[14]), and Ca2PbO4 were used as additional reference points in the PbO-SiO2 and Ca-Pb-O systems. Additional correction was developed and applied for the values after the JEOL 8200 EPMA ZAF correction:
4 Results and Discussion
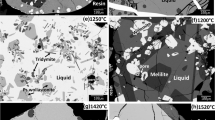
Examples of micrographs of quenched PbO-CaO-SiO2 samples are given in Fig. 1(a), (b), (c), and (d).
The experimental results are reported in Table 1 and compared to the optimized thermodynamic model predictions[18] for the binary “PbO”-CaO and ternary “PbO”-CaO-SiO2 liquidus in Fig. 2, 3, and 4. As there is one phase (Ca2PbO4) based on PbO2 instead of PbO, the diagrams are strictly projections from the oxygen corner onto the “PbO”-CaO line and “PbO”-CaO-SiO2 plane, respectively.
“PbO”-CaO phase diagram in air (high-PbO part) with present experimental points at 1143-1573 K (870-1300 °C), compared to a recent model[18]
“PbO”-CaO-SiO2 phase diagram in air (low-SiO2, high-PbO part) with present experimental points at 1143-1573 K (870-1300 °C), compared to a recent model.[18] Note that Pb is present as PbO in slag and all solid phases except Ca2PbO4 (2CaO·PbO2)
Liquidus projection of the “PbO”-CaO-SiO2 system in air, with data[7] (recalculated using newly established correction for EPMA measurement) and current experimental results. Isotherms and boundary lines outside the experimentally studied range of compositions are calculated using a recent optimised thermodynamic model.[18] Note that Pb is present as PbO in slag and all solid phases except Ca2PbO4 (2CaO·PbO2)
The results of the present work for the “PbO”-CaO system agree with the most recent data[10] for invariant reactions: eutectic Liquid + O2 (0.21 atm) = PbO + Ca2PbO4 at 1129 ± 2 K (856 ± 2 °C) and peritectic Liquid + CaO + O2 (0.21 atm) = Ca2PbO4 at 1258 ± 5 K (985 ± 5 °C).
Although the FactSage model[18] predicts a significant range of two immiscible liquids over the cristobalite primary phase field, the experiment at the highest temperature, 1928 K (1655 °C) and 6 mol.% PbO still gave single liquid in equilibrium with cristobalite. Therefore, the immiscibility range is much more narrow than expected, i.e. less than 6 mol.% PbO addition suppresses the immiscibility in the CaO-SiO2 system.
Four solid phases in the PbO-CaO-SiO2 system show significant ranges of solid solutions:
Among those, C3S is identified as a solution stable in equilibrium with liquid above ~ 1225 °C in present study for the first time. Analysis of the dataset[7] shows no difference in solubility behavior of PbO in wollastonite and pseudowollastonite.
A new phase (Ca0.8Pb0.2)3SiO5 or Ca12Pb3Si5O25 has been discovered. Its primary phase field occupies a narrow range between the Ca2PbO4 and C2S fields at 1143-1273 K (870-1000 °C) at low SiO2 concentrations. It does not form a continuous solution with C3S, as the latter dissolves no more than 3 mol.% PbO (compared to 15% in Ca12Pb3Si5O25), and is stable only above ~ 1225 °C. In the intermediate range at 1323-1473 K (1050-1200 °C) the low-SiO2 liquid is in equilibrium with lime and C2S. In all low-SiO2 experiments at 870-1300 °C, the concentration of SiO2 in liquid, lime and Ca2PbO4 was below the EPMA uncertainty limit (0.1 mol.%), confirming the initial FactSage prediction and allowing to plot these points on the “PbO”-CaO binary diagram.
Attempts to reach the primary phase of rankinite were not successful, since at 1708 K (1435 °C) liquid was still observed in equilibrium with pseudowollastonite and C2S. This indicates that addition of PbO lowers stability of rankinite compared to pseudowollastonite and C2S, and the primary phase field of rankinite is limited by a narrow and short range close to the CaO-SiO2 binary.
5 Conclusions
New phase equilibria information in the “PbO”-CaO and the “PbO”-CaO-SiO2 systems in air has been obtained. The studied range of temperatures covered 1173-1928 K (870-1655 °C), and included the equilibria of slag with one or two crystalline phases: tridymite, cristobalite SiO2, pseudowollastonite (Ca,Pb)SiO3, dicalcium silicate (Ca,Pb)2SiO4, tricalcium silicate (Ca,Pb)3SiO5, lime CaO, calcium plumbate Ca2PbO4, and a new phase (Ca0.8Pb0.2)3SiO5. This is an important update of the previous study of this system[7] that allows to create a more accurate thermodynamic model of this system as well as a foundation for the thermodynamic database of the multicomponent system Pb-Zn-Fe-Cu-Si-Ca-Al-Mg-O, essential for the lead smelting and recycling industries.
References
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Pb-Fe-Si-O System in Equilibrium with Metallic Pb, Metall. Mater. Trans. B, 2017, 49(1), p 159-180
U. Kuxmann and P. Fischer, Beitrag zur Kenntnis der Zustandsdiagramme PbO-Al2O3, PbO-CaO, PbO-SiO2, Erzmetall (Z. Erzbergbau Metallhuettenwes), 1974, 27(11), p 533-537
H. Kitaguchi, J. Takada, K. Oda, and Y. Miura, Equilibrium Phase Diagram for the System PbO-CaO-CuO, J. Mater. Res., 1990, 5(5), p 929-931
D. Araten, Some Molten Ionic Oxides as Chemical Reagents, J. Appl. Chem. (London), 1968, 18(4), p 118-121
M. Koishi, A Study of the Hydration of Calcium Orthoplumbate.VII. The Mechanism of the Formation of the Hydrate, Bull. Chem. Soc. Jpn, 1966, 39(11), p 2406-2410
V.M. Tromel, Die kristallstruktur der verbindungen vom Sr2PbO4-typ, Z. Anorg. Allg. Chem., 1969, 371, p 237-247
E. Jak, N. Liu, and P.C. Hayes, Experimental study of Phase Equilibria in the Systems PbOx-CaO and PbOx-CaO-SiO2, Metall. Mater. Trans. B, 1998, 29B(3), p 541-553
E. Jak, S.A. Decterov, P.C. Hayes, and A.D. Pelton, Thermodynamic Optimisation of the Systems CaO-Pb-O and PbO-CaO-SiO2, Can. Metall. Q., 1998, 37(1), p 41-47
K.T. Jacob and K.P. Jayadevan, Measurement of Gibbs Energy of Formation of Ca2PbO4 Using a Solid-State Cel with Three Electrodes, J. Mater. Chem., 1997, 7(12), p 2407-2413
R.O. Suzuki, J.-I. Nagata, and D. Risold, Experimental Phase Equilibria in the PbOx-CaO System, J. Am. Ceram. Soc., 1998, 81(9), p 2493-2496
S.C. Samanta and F.A. Hummel, Phase Equilibria in the System CaO-PbO-SiO2, J. Am. Ceram. Soc., 1976, 59(3–4), p 157-160
G.A. Mikirticheva, R.G. Grebenshchikov, and S.K. Kuchaeva, Calcium Silicate (CaSiO3)-Lead Silicate (PbSiO3) Phase System, Zh. Neorg. Khim., 1989, 34(8), p 2135-2139 (in Russian)
M. Shevchenko, J. Chen, E. Jak, Establishing additional correction for quantitative EPMA measurements in the system PbO-SiO2, in AMAS 2017, 14th Biennial Australian Microbeam Analysis Symposium, 6-10 February, 2017 (Brisbane, Australia), QUT, Brisbane, pp 94–95
M. Shevchenko and E. Jak, Experimental Phase Equilibria Studies of the PbO-SiO2 System, J. Am. Ceram. Soc., 2018, 101(1), p 458-471
T. Hidayat, H.M. Henao, P.C. Hayes, and E. Jak, Phase Equilibria Studies of the Cu-Fe-O-Si System in Equilibrium with Air and with Metallic Copper, Metall. Mater. Trans. B, 2012, 43, p 1034-1045
E. Jak, P.C. Hayes, and H.-G. Lee, Improved Methodologies for the Determination of High Temperature Phase Equilibria, Korean J. Miner. Mater. Inst. (Seoul), 1995, 1(1), p 1-8
E. Jak, Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field, in 9th International Conference on Molten Slags, Fluxes and Salts (MOLTEN12), 2012 (Beijing, China), The Chinese Society for Metals, W077
M. Shevchenko and E. Jak, Thermodynamic optimization of the binary PbO-CaO and ternary PbO-CaO-SiO 2 systems, Personal communication, The University of Queensland, Brisbane, 2018
Acknowledgments
The authors would like to thank Nyrstar (Australia), Outotec Pty Ltd (Australia), Aurubis AG (Germany), Umicore NV (Belgium), and Kazzinc Ltd, Glencore (Kazakhstan), and Australian Research Council Linkage project LP150100783 for their financial support for this research. The authors are grateful to Prof. Peter C. Hayes (UQ) for valuable comments and suggestions, to Ms. Suping Huang (UQ) for assistance with conducting experiments, and to the staff of the University of Queensland Centre for Microanalysis and Microscopy (CMM) for their support in maintenance and operation of scanning and electron microprobe facilities in the Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shevchenko, M., Jak, E. Experimental Liquidus Study of the Binary PbO-CaO and Ternary PbO-CaO-SiO2 Systems. J. Phase Equilib. Diffus. 40, 148–155 (2019). https://doi.org/10.1007/s11669-019-00705-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-019-00705-3