Abstract
The thermodynamic properties of tantalum have been evaluated to 5800 K. Selected values include an enthalpy of sublimation of 781 ± 4 kJ/mol at 298.15 K and a boiling point at one atmosphere pressure of 5762 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
This work is one of a series on thermodynamic properties of the elements to appear in the Journal of Phase Equilibria and Diffusion. The articles are expected to be especially useful to those who are updating databases for phase diagram prediction and for other applications as well. Each article in the series will list the properties of a single element, including Ag,[1] Au,[2] Be,[3] Cr, Cu,[4] Hf,[5] Mo, Nb,[6] Ta, Ti, V[7] and W. References are given for papers which have already been published. In addition, the series will also include two summary papers on selected values of the melting points and boiling points of the elements and on the enthalpies and entropies of fusion and transition of the elements.
Inaba[8] determined the superconducting temperature to be 4.4924 K whilst a melting point of 3293 ± 15 K is selected as an average after correction to ITS-90 of values of 3292 K determined by Rudy and Harmon[9] and 3294 K determined by Pemsler[10] where in both cases the material used was of particularly high purity. Although Hiernaut et al.[11] initially also reported a similar value at 3290 K, later Hiernaut et al.[12] reported a much lower value of 3260 K without giving any explanation as to the large difference. Cezairliyan et al.[13] also selected a much lower melting point of 3258 K as a correction to ITS-90 of the value obtained by Malter and Langmuir[14] although no impurity content was given so that this value must be treated with caution. Rudy and Harmon[9] noted that increasing the total impurity content to only 500 ppm (0.05%) resulted in a 30 K drop in the melting point.
Wherever possible values have been corrected to the currently accepted atomic weight of 180.94788 ± 0.00002[15] and to the ITS-90 temperature scale using correction factors of Douglas,[16] Rusby,[17] Rusby et al.[18] and Weir and Goldberg.[19]
Based on dilatometric measurements, Bollinger et al.[20] suggested that all of the three Group 5 elements, vanadium, niobium and tantalum, underwent a martensitic structural distortion just below room temperature leading to a structure which was not cubic. However, although given in smooth form, neither specific heat measurements of Sterrett and Wallace[21] (12-543 K) or electrical resistivity measurements by Williams et al.[22] (80-400 K) showed any evidence of an anomaly that would be involved in a phase transition whilst lattice parameter measurements of Smirnov and Finkel’[23] established that the structure remained body-centered cubic down to at least 127 K. Therefore, until the observations of Bollinger et al.[20] are independently verified and the supposed new low temperature structure is fully characterized then it is assumed that the body-centered cubic structure exists over the whole temperature range (Fig. 1, 2, and 3).
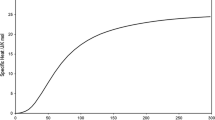
Low temperature specific heat of solid tantalum, taken from Table 15
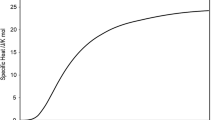
Specific heat of tantalum for 300 < T < 5800 K, taken from Table 16
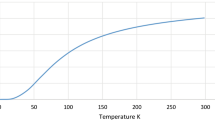
High temperature thermodynamic properties of tantalum for 300 < T < 4300 K, taken from Table 16
Previous comprehensive reviews on tantalum were by Hultgren et al.,[24] Gurvich et al.[25] and JANAF (Chase[26]) and on the solid only by Maglić[27] and Bodryakov.[28]
2 Solid Phase
2.1 Superconducting State—Range 0 to 4.4924 K
In the description of the electronic structure of transition metals in terms of two electronic conduction bands then if the scattering of electrons between the two bands is sufficiently weak then both may separately undergo a transition into the superconducting state with the resultant formation of two energy gaps which is a Type II superconductor. Anomalies which appeared to confirm this behaviour in niobium were summarised by Sellers et al.[29] whilst, in particular, a similar anomaly was observed for tantalum in the specific heat measurements of Satoh et al.[30] However, based on specific heat measurements the anomalies for niobium were shown by Sellers et al.[31] to be due to hydrogen contamination and that with hydrogen removed niobium behaved as a typical Type I superconductor. On this basis, specific heat measurements of Shen[32] were selected for tantalum and below Tc/2, were Tc is the superconducting transition temperature, the electronic contribution to the specific heat (Ces) was fitted to the Bardeen–Cooper–Schrieffer equation which together with two lattice contributions leads to the full representation of the specific heat (Cs) below 2.25 K (Tc/2) as given by Eq 1:
where γ is the normal state electronic coefficient (6.02 mJ/mol K2) and Tc is selected as a working value of 4.467 K as an average of values determined for the samples I and II of Shen.
At Tc, a value of (Cs − Cn)/γ Tc = 1.53 is selected as an average of samples I and II of Shen[32] and when extrapolated to the selected value of Tc leads to Cs − Cn = 41.37 mJ/(mol K) so that Cs = 79.01 mJ/(mol K) at Tc. This value was combined with the value of 13.59 mJ/(mol K) at 2.25 K obtained from Eq 1 and an intermediate value of at the mid-point temperature of 3.369 K obtained by iteration so that at Tc the entropy values of the superconducting state and the normal state are equal. This gives Eq 2 for C s over the range 2.25 K to Tc:
Derived thermodynamic values based on Eq 1 and 2 are given in Table 14 whilst differences from the selected values of other measurements in this region are given in Table 10.
2.2 Normal State—0 to 298.15 K
Low temperature specific heat for tantalum in the normal state is given in terms of the modified Debye equation: Cp = D/T2 + γ T + A T3 + B T5 where D is the nuclear quadrupole coefficient, γ is the electronic coefficient and A and B are lattice contributions where A is usually represented in terms of a limited Debye temperature, θ D , where θ 3 D = (12/5) π4 R/A = 1943.770/A where R is the Gas Constant and A is given in units of J/(mol K4). For D, Shen[32] gives the value 0.01 mJ K/mol which is accepted. Leupold et al.[33] showed that discrepancies in the reported values of γ and θ D could be explained by an abrupt change in the slope of the specific heat values at 7.19 K which was due to the presence of a Kohn anomaly in the phonon density of states. Experimental values of γ and θ D are summarised in Table 1 where the subscripts γ1 and θD1 and γ2 and θD2 distinguish values above and below the transition temperature.
The measurements of Shen[32] and Leupold et al.[33] initially appear to disagree significantly with a preference being given to the values of Shen since the value of θ D at 258 K gave the best agreement with a value of 263.8 K selected by Alers[34] from elastic constant measurements. However, the two sets of measurements were reconciled as follows to cover the range up to 12.5 K:
Above 12.5 K and up to 298.15 K the specific heat measurements of Sterrett and Wallace[21] (12 to 543 K) were selected since these give the best agreement with the high temperature selected values. Because the values were given in a smooth form they were not separately fitted to analytical equations. Laser flash calorimetric measurements of Takahashi and Nakamura[35] (82 to 997 K) agree satisfactorily in the low temperature region trending from 0.1% high at 90 K to 0.7% high at 150 K to 0.4% low at 298.15 K. Thermodynamic values in the normal state below the superconductive transition temperature are also included in Table 14 whilst thermodynamic values at 5 K and above are given in Table 15. The deviations of other specific heat values in the low temperature region are given in Table 11.
2.3 Solid State: 298.15 to 3293 K
The requirement is not only to have a continuity with the low temperature specific heat curve but also to agree with the closely agreeing high temperature enthalpy values of Berezin and Chekhovskoi[36] (2440 to 3238 K) and Arpaci and Frohberg[37] (2653 to 3276 K in the solid range) in order to derive an enthalpy of fusion value which agrees with experimental values.
Specific heat measurements of Rasor and McClelland[38,39] (700 to 3200 K), Lehman[40] (100 to 3200 K) and Taylor and Finch[41,42] (100 to 3195 K) all showed a sudden sharp increase above 2800 K which was not shown in the measurements of Cezairliyan et al.[43] (1900 to 3200 K). Whilst attempts have been made to explain the sharp increase as real behaviour it is considered that it is simply due to sample oxidation or experimental error as evidenced by a similar sudden sharp increase observed in the complimentary thermal expansion measurements of Rasor and McClelland[38,39] when compared to the quality measurements of Petukov et al.[44] (1244 to 2267 K) and Miiller and Cezairliyan[45,46] (1500 to 3200 K). On these grounds, and because the first three sets of measurements differ significantly from those of Cezairliyan et al.,[43] then it is considered that the measured high temperature specific heat values of tantalum are unsatisfactory for representing the thermodynamic properties and therefore instead values are obtained by combining the agreeing drop calorimetry enthalpy measurements of Oetting and Navratil[47] (534 to 1383 K) (8 data points), Berezin and Chekhovskoi[36] (2440 to 3238 K) (11 data points out of 18) and Arpaci and Frohberg[37] (2653 to 3276 K in the solid state) (15 data points out of 19). The first two sets of measurements were directly corrected to ITS-90 whilst the measurements of Arpaci and Frohberg[37] were corrected to the selected melting point. Enthalpy measurements of Conway and Hein[48,49] (1315 to 2622 K) (12 data points out of 19) were also considered but were then rejected since they showed an average bias of 0.5% low compared to the selected equation. The selected values were fitted to Eq 6 which has an overall accuracy as a standard deviation of ± 310 J/mol (0.40%). The selection of this equation to cover the range from 298.15 K to the melting point gave the best continuity with the selected low temperature specific heat values:
Derived specific heat and entropy equations are given in Table 7. Thermodynamic properties derived from Eq 6 are given in Table 16 whilst deviations for specific heat and other enthalpy values are given in Tables 12 and 13 respectively.
2.4 A Comparison of Selected Values at 298.15 K
The comparison is given in Table 2. In the low temperature region above the superconducting temperature, Hultgren et al.[24] mainly combined the specific heat values of Sterrett and Wallace[21] and Kelley[50] whilst JANAF (Chase[26]) combined these two sets of measurements with those of Clusius and Losa.[51] Both Gurvich et al.[25] and in the present review preference is given to the measurements of Sterrett and Wallace.[21]
3 Liquid Phase
Enthalpy measurements of Arpaci and Frohberg[37] (3292 to 3358 K in the liquid state) were corrected to the selected melting point and fitted to Eq 7 with an overall accuracy as a standard deviation of ± 256 J/mol (0.18%):
The derived enthalpy of fusion at 33.883 ± 0.402 kJ/mol can be compared with the wide range of reported values given in Table 3, all of which, apart from the measurement of Arpaci and Frohberg,[37] were determined by rapid pulse heating. However, it is noted that the directly determined value of McClure and Cezairliyan[52] and the derived value of Pottlacher and Seifter[53,54] satisfactorily agree with the selected value.
Because of the very narrow 65 K range over which the drop calorimetry measurements of Arpaci and Frohberg were measured then the derived liquid specific heat is relatively poorly determined as 42.18 ± 4.62 J/(mol K). Other determinations of liquid specific heat used rapid pulse heating and are also given in Table 3 except for the measurements of Lebedev and Mozharov[55] which trend from 69.6 J/(mol K) at 3300 K to 27.7 J/(mol K) at 3900 K. It is considered that the measurements of Pottlacher and Seifter[53,54] at 40.9 J/(mol K) represents an improvement in the rapid pulse heating technique and show a relatively satisfactory agreement with the selected value. Thermodynamic values derived from Eq 7 are also given in Table 16.
4 Gas Phase
4.1 Thermodynamic Properties of the Gas Phase
Selected values are based on the 488 energy levels from the sources given in Table 4 with thermodynamic properties being calculated using the method of Kolsky et al.[56] and the 2014 Fundamental Constants (Mohr et al.[57,58]). Derived thermodynamic values are given in Table 17. Not included in Table 4 are eighteen levels given by Moore[59] which were shown to be spurious by Mocnik et al.[60] and a further five levels which were shown to be spurious by Jaritz et al.[61] It is noted that thermodynamic properties of the gas phase selected by both Gurvich et al.[25] and JANAF (Chase[26]) show differences from the presently accepted values which may be due to the fact that they selected only the energy levels of Moore[57] whereas the present evaluation included a further 228 levels.
4.2 Enthalpy of Sublimation
For values given in the form of the Clausius–Clapeyron equation a “pseudo” Third Law value was calculated by evaluating the enthalpy of sublimation at the temperature extremes and then averaging. Because of a general lack of detail as to what temperature scales were used and problems associated with the exact measurement of temperature then no attempt was made to correct vapor pressure measurements to ITS-90 from what would have been contemporary scales. However, the temperature values of Langmuir and Malter[62] and Fiske[63] were corrected from laboratory scales to IPTS-1948 using the method of Szwarc et al.[64] Values are summarised in Table 5. The selected value of 781 ± 4 kJ/mol is based on the close agreement between the values of Edwards et al.,[65] Gorbatyi and Shuppe[66] and the effusion measurements of Golubtsov and Nesmeyanov[67] with the selected accuracy taking into account the evaporation measurements of Golubtsov and Nesmeyanov[67] and the measurements of Langmuir and Malter,[62] although the latter showed a marked trend with temperature.
5 Vapor Pressure
The vapor pressure equations for the solid as given in Table 6 was evaluated from free energy functions for the solid and the gas at 50 K intervals from 1800 K to 3250 K and the melting point and for the liquid at 50 K intervals from 3300 to 5800 K and the melting point and were fitted to the following equation:
ln (p, bar) = A + B ln(T) + C/T + D T + E T 2
6 Comments on Previous Reviews of the Thermodynamic Properties
Gurvich et al.[25] also appeared to give a preference towards the high temperature solid enthalpy measurements, and the selected values agree fairly satisfactory with the presently accepted values showing a maximum specific heat difference of 2.7% higher at 2800 K. However, JANAF (Chase[26]) gave preference to the high temperature specific heat values of Cezairliyan et al.[43] and as a result selected enthalpy values are on average 1630 J/mol higher than the smoothed experimental values above 3000 K. In the high temperature region, Maglić[27] selected mainly the specific heat measurements of Lehman[40] and Taylor and Finch[41,42] which were rejected here for reasons given in Part 2.3. As a result, selected specific heat values trend from 0.9% low initially to 3.3% low at 1000 K to 16.8% high at 3200 K. Integration of the specific heat equation given by Maglić[27] led to marked enthalpy differences compared to selected values trending 1950 J/mol to 3070 J/mol higher in the narrow range 3000 K to 3200 K. The review by Bodryakov[28] superseded an earlier review by the same author.[68] In the high temperature region, Bodryakov[28] selected specific heat values which differed sinusoidally from the values selected here trending to 2.2% low at 1000 K to 5.8% high at 2400 K to 1.8% low at 3250 K. Although the specific heat curve selected by Bodryakov behaved more naturally than the presently selected curve, integration leads to enthalpy values which exceed smoothed experimental values by an average of 1290 J/mol at 3000 K and above. On these grounds, the selected values of JANAF (Chase[26]), Maglić[27] and Bodryakov[28] are considered as not being representative of the actual high temperature experimental enthalpy values.
7 Summary of Representative Equations
High temperature specific heat equations are given in Table 7, free energy equations are given in Table 8 and transition values involved with the free energy equations in Table 9.
8 Deviations from the Selected Values
Deviations of superconducting specific heat values are given in Table 10 and low temperature normal state specific heat in Table 11. Deviations of high temperature enthalpy and specific heat values in the solid range are given in Tables 12 and 13 respectively.
9 Thermodynamic Tables
Low temperature thermodynamic properties of the solid are given in Tables 14 and 15 and of the high temperature thermodynamic properties of the condensed phases in Table 16. Thermodynamic properties of the gas are given in Table 17 whilst the vapour pressure summary is given in Table 18.
References
J.W. Arblaster, Thermodynamic Properties of Silver, J. Phase Equilib. Diffus., 2015, 36(6), p 573-591
J.W. Arblaster, Thermodynamic Properties of Gold, J. Phase Equilib. Diffus., 2016, 37(2), p 229-245
J.W. Arblaster, Thermodynamic Properties of Beryllium, J. Phase Equilib. Diffus., 2016, 37(5), p 581-591
J.W. Arblaster, Thermodynamic Properties of Copper, J. Phase Equilib. Diffus., 2015, 36(5), p 422-444
J.W. Arblaster, Thermodynamic Properties of Hafnium, J. Phase Equilib. Diffus., 2014, 35(4), p 490-501
J.W. Arblaster, Thermodynamic Properties of Niobium, J. Phase Equilib. Diffus., 2017, 38(5), p 707-722
J.W. Arblaster, Thermodynamic Properties of Vanadium, J. Phase Equilib. Diffus., 2017, 38(1), p 51-64
A. Inaba, Superconductive Transition Point of Tantalum and Niobium as a Reference Temperature, Jpn. J. Appl. Phys., 1980, 19, p 1553-1559
E.Rudy and D.P.Harmon, Ternary Phase Equilibria in Transition Metal-Boron-Carbon-Silicon Systems, Air Force Materials Laboratory, Research and Technology Division, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio, Rept. AFML-TR-65-2, Part I, Volume V (1965)
J.P. Pemsler, Thermodynamics of the Interaction of Niobium and Tantalum with Oxygen and Nitrogen at Temperatures Near the Melting Point, J. Electrochem. Soc., 1961, 108, p 744-750
J.P. Hiernaut, R. Beukers, M. Hoch, T. Matsui, and R.W. Ohse, Determination of the Melting Point and of the Spectral and Total Emissivities of Tungsten, Tantalum and Molybdenum in the Solid and Liquid States using a Six-Wavelength Pyrometer, High Temp. High Press., 1986, 18, p 627-633
J.P. Hiernaut, F. Sakuma, and C. Ronchi, Determination of the Melting Point and the Emissivity of Refractory Metals with a Six-Wavelength Pyrometer, High Temp. High Press., 1989, 21, p 139-148
A. Cezairliyan, J.L. McClure, and A.P. Miiller, Radiance Temperatures (in the Wavelength Range 520-906 nm) of Tantalum at Its Melting Point by a Pulse-Heating Technique, High Temp. High Press., 1993, 25, p 477-484
L. Malter and D.B. Langmuir, Resistance, Emissivities and Melting Point of Tantalum, Phys. Rev., 1939, 55, p 743-747
Commission on Isotopic Abundances and Atomic Weights (CIAAW), Atomic Weights of the Elements 2015, ciaaw.org/atomic-weights.htm, Aug. 2015
T.B. Douglas, Conversion of Existing Calorimetrically Determined Thermodynamic Properties to the Basis of the International Practical Temperature Scale of 1968, J. Res. Natl. Bur. Stand., 1969, 73A, p 451-470
R.L. Rusby, The Conversion of Thermal Reference Values to the ITS-90, J. Chem. Thermodyn., 1991, 23, p 1153-1161
R.L. Rusby, R.P. Hudson, and M. Durieux, Revised Values for (t90–t68) from 630°C to 1064°C, Metrologia, 1994, 31, p 149-153
R.D. Weir and R.N. Goldberg, On the Conversion of Thermodynamic Properties to the Basis of the International Temperature Scale of 1990, J. Chem. Thermodyn., 1996, 28, p 261-276
R.K. Bollinger, B.D. White, J.J. Neumeier, H.R.Z. Sandim, Y. Susuki, C.A.M. dos Santos, R. Avci, A. Migliori, and J.B. Betts, Observation of a Martensitic Structural Distortion in V, Nb and Ta, Phys. Rev. Lett., 2011, 107, p 0755031-0755034
K.F. Sterrett and W.E. Wallace, Heat Capacities, Entropies and Enthalpies of Tantalum between 12 and 550°K, J. Am. Chem. Soc., 1958, 50, p 3176-3177
R.K. Williams, R.S. Graves, T.L. Hebble, D.L. McElroy, and J.P. Moore, Phonon and Electron Components of the Thermal Conductivity of Tantalum at Intermediate Temperatures, Phys. Rev. B, 1982, 26, p 2932-2942
Y.M. Smirnov and V.A. Finkel’, Crystal Structure of Tantalum, Niobium and Vanadium at 110 to 400°K, Zh. Eksp. Teor. Fiz. 49, 1077–1082 (1965) (Sov. Phys. JETP, 1966, 22, 750–753)
R. Hultgren, P.D. Desai, D.T. Hawkins, M. Gleiser, K.K. Kelley, and D.D. Wagman, Selected Values of the Thermodynamic Properties of the Elements, American Society for Metals, Metals Park, 1973
L.V. Gurvich, I.V. Veits, V.A. Medvedev, G.A. Bergman, V.S. Yungman, G.A. Khachkuruzov, V.S. Yorish, O.V. Dorofeeva, E.L. Osina, P.I. Tolmach, I.N. Przhevak’skii, I.I. Nazarenko, N.M. Aristova, E.A. Shenyavskaya, L.N. Gorokhov, A.L. Rogatskii, M.E. Efimov, V.Y. Leonidov, Y.G. Khait, A.G. Efimova, S.E. Tomberg, A.V. Gusarov, N.E. Khandamirova, G.N. Yurkov, L.R. Fokin, L.F. Kuratova, and A.D. Gol’dshtein, in Thermodynamic Properties of Individual Substances, ed. V.P. Glushko, L.V. Gurvich, G.A. Bergman, I.V. Veits, V.A. Medvedev, G.A. Khachkuruzov, and V.S. Yungman “Nauka”, Moscow, Vol. 4, (1982)
M.W. Chase Jr., NIST-JANAF Thermochemical Tables, Fourth Edition, J. Phys. Chem. Ref. Data, Monograph No.9, 1998
K.D. Maglić, Recommended Specific Heat Capacity Functions of Group VA Elements, Int. J. Thermophys., 2003, 24, p 489-500
V.Y. Bodryakov, Correlation between Temperature Dependences of Thermal Expansivity and Heat Capacity up to the Melting Point of Tantalum, Teplofiz. Vys. Temp. 54, 336–342 (2016) (High Temp. 54, 316–321, 2016)
G.J. Sellers, A.C. Anderson, and H.K. Birnbaum, Anomalous Heat Capacities of Niobium and Tantalum Below 1 K, Phys. Rev. B, 1974, 10, p 2771-2776
T. Satoh, A. Sawada, and M. Yamamoto, in Superconducting Specific Heat of Nb-Ta Alloys, Low Temperature Physics – LT 13, Proceedings of the XIIIth International Conference on Low Temperature Physics, University of Colorado, Boulder, Colorado, 21-25 Aug. 1972, Vol. 3: Superconductivity, ed. by K.D. Timmerhaus, W.J. O’Sullivan, and E.F. Hammel (Plenum Press, New York, London, 1974), p 372–376
G.J. Sellers, A.C. Anderson, and H.K. Birnbaum, The Anomalous Heat Capacity of Superconducting Niobium, Phys. Lett. A, 1973, 44, p 173-174
Y.L. Shen, Low Temperature Heat Capacities of Vanadium, Niobium and Tantalum, Ph.D. Thesis, University of California, Lawrence Radiation Laboratory, Berkeley, California. U.S. Atomic Energy Commission, Rept. UCRL-16117, 1965
H.A. Leupold, G.J. Iafrate, F. Rothwarf, J.T. Breslin, D. Edmiston, and T.R. AuCoin, Low-Temperature Specific Heat Anomalies in the Group V Transition Metals, J. Low Temp. Phys., 1977, 28, p 241-261
G.A.Alers, Use of Sound Velocity Measurements in Determining the Debye Temperature of Solids, Physical Acoustics – Principles and Methods, Vol. III, Part B: Lattice Dynamics, W.P.Mason (Ed.), Academic Press, New York, 1965, p 1-42
Y. Takahashi and J. Nakamura, The Heat Capacity of Tantalum from 80 to 1000 K, Thermochim. Acta, 1996, 282–283, p 317-322
B.Y. Berezin and V.Y. Chekhovskoi, The Enthalpy of Tantalum between 2400°K and the Melting Temperature, Izv. Akad. Nauk SSSR Metally (3), 63–65 (1977) (Russ. Metall. Metally (3), 51–53, 1977)
E. Arpaci and M.G. Frohberg, Enthalpy Measurements on Solid and Liquid Tantalum by Levitation Calorimetry, Z. Metallkde., 1982, 73, p 548-551
N.S. Rasor and J.D. McClelland, Thermal Properties of Materials. Part 1. Properties of Graphite, Molybdenum and Tantalum to Their Destruction Temperatures, Wright Air Development Center, Air Research and Development Command, United States Air Force, Wright-Patterson Air Force Base, Ohio, Rept. WADC-TR-56-400, 1957
N.S. Rasor and J.D. McClelland, Thermal Properties of Graphite, Molybdenum and Tantalum to Their Destruction Temperatures, J. Phys. Chem. Solids, 1960, 15, p 17-26
G.W. Lehman, Thermal Properties of Refractory Materials, Wright Air Development Division, Air Research and Development Command, United States Air Force, Wright-Patterson Air Force Base, Ohio, Rept. WADD-TR-60-581, 1960, p 1–19
R.E. Taylor and R.A. Finch, The Specific Heats and Resistivities of Molybdenum, Tantalum and Rhenium from Low to Very High Temperatures, North American Aviation, U.S. Atomic Energy Commission, Rept. NAA-SR-6034, 1961
R.E. Taylor and R.A. Finch, The Specific Heat and Resistivities of Molybdenum, Tantalum and Rhenium, J. Less Common Metals, 1964, 6, p 283-294
A. Cezairliyan, J.L. McClure, and C.W. Beckett, High-Speed (Subsecond) Measurement of Heat Capacity, Electrical Resistivity and Thermal Radiation Properties of Tantalum in the Range 1900 to 3200 K, J. Res. Nat. Bur. Stand., 1971, 75A, p 1-13
V.A. Petukhov, V.Y. Chekhovskoi, and A.G. Mozgovoi, Experimental Study of the Thermal Expansion of Various Construction Materials, Tantalum and Tantalum–Tungsten TV-10 Alloy, Teplofiz. Vys. Temp. 15, 534–538 (1977) (High Temp. 15, 449–452, 1977)
A.P. Miiller and A. Cezairliyan, Transient Interferometric Technique for Measuring Thermal Expansion at High Temperatures: thermal Expansion of Tantalum in the Range 1500-3200 K, Int. J. Thermophys., 1982, 3, p 259-288
A.P. Miiller and A. Cezairliyan, Interferometric Technique for the Subsecond Measurement of Thermal Expansion at High Temperatures: application to Refractory Metals, Int. J. Thermophys., 1991, 12, p 643-656
F.L. Oetting and J.D. Navratil, Enthalpy of Molybdenum and Tantalum from 298-1400 K, J. Chem. Eng. Data, 1972, 17, p 230-231
J.B. Conway and R.A. Hein, Unpublished work quoted by Hoch [49]
M. Hoch, The High Temperature Specific Heat of Body-Centred Cubic Refractory Metals, High Temp. High Press., 1969, 1, p 531-542
K.K. Kelley, The Specific Heat of Tantalum at Low Temperatures and the Effect of Small Amounts of Dissolved Hydrogen, J. Chem. Phys., 1940, 8, p 316-322
K.Clusius and C.G.Losa, Ergebnisse der Tieftemperaturforschung XVI. Die Atom- und Elektronenwärme des Tantals zwischen 10° und 273°K, Z. Naturforschg. 10a, 939–943 (1955)
J.L. McClure and A. Cezairliyan, Measurement of the Heat of Fusion of Tantalum by a Microsecond-Resolution Transient Technique, Int. J. Thermophys., 1994, 15, p 505-511
G. Pottlacher and A. Seifter, Microsecond Laser Polarimetry for Emissivity Measurements on Liquid Metals at High Temperatures–Application to Tantalum, Int. J. Thermophys., 2002, 23, p 1281-1290
G. Pottlacher, High Temperature Thermophysical Properties of 22 Pure Metals, Edition Keiper, Graz, 2010
S.V. Lebedev and G.I. Mozharov, Heat Capacity of Tantalum with Rapid Pulsed Heating by a High-Density Electric Current, Tepolofiz. Vys. Temp. 15, 53–57 (1977) (High Temp. 15, 45–48, 1977)
H.G. Kolsky, R.M. Gilmer, and P.W. Gilles, The Thermodynamic Properties of 54 Elements Considered as Ideal Monatomic Gases. U.S. Atomic Energy Commission Rept. LA 2110 (1957)
P.J. Mohr, D.B. Newell, and B.N. Taylor, CODATA Recommended Values of the Fundamental Physical Constants: 2014, Rev. Mod. Phys., 2016, 88, p 035009-1-035009-73
P.J. Mohr, D.B. Newell, and B.N. Taylor, CODATA Recommended Values of the Fundamental Physical Constants: 2014, J. Phys. Chem. Ref. Data, 2016, 45, p 043102-1-043102-74
C.E.Moore, Atomic Energy Levels, Nat. Bur. Stand. Nat. Stand. Ref. Data Ser., NSRDS-NBS 35, Vol. III, U.S. Govt. Printing Office, Washington, D.C. (1971)
H. Mocnik, B. Arcimowicz, W. Salmhofer, L. Windholz, and G.H. Guthöhrlein, Investigation of the Hyperfine Structure of Ta I-lines (III), Z. Phys. D, 1996, 36, p 129-136
N. Jariz, L. Windolz, D. Messnarz, H. Jäger, R. Engleman, Jr., J.C. Pickering, and H. Jäger, Investigation of the Hyperfine Structure of TaI-Lines (IX), Phys. Scr., 2005, 71, p 611-620
D.B. Langmuir and L. Malter, The Rate of Evaporation of Tantalum, Phys. Rev., 1939, 55, p 748-749
M.D. Fiske, The Temperature Scale, Thermionics, and Thermatomics of Tantalum, Phys. Rev., 1942, 61, p 513-519
R. Szwarc, E.R. Plante, and J.J. Diamond, Vapor Pressure and Heat of Sublimation of Tungsten, J. Res. Nat. Bur. Stand., 1965, 69A, p 417-421
J.W. Edwards, H.L. Johnston, and P.E. Blackburn, Vapor Pressure of Inorganic Substances. IV. Tantalum Between 2624 and 2943°K, J. Am. Chem. Soc., 1951, 73, p 172-174
N.A. Gorbatyi and G.N. Shuppe, On the Effect of Strong Electric Fields (106 v/cm) on the Evaporation and Resistivity of Metals (Mo,Ta,W), Zh. Tekh. Fiz. 28, 623–635 (1958) (Sov. Phys. Tech. Phys. 3, 587–596, 1958)
I.V.Golubtsov and A.N. Nesmeyanov, Investigation of the Evaporation of Tungsten, Molybdenum and Tantalum in a Vacuum, Vestn. Mosk. Univ. Ser. II Khim. (5), 31–33 (1965)
V.Y. Bodryakov, Heat Capacity of Solid Tantalum: Self-Consistent Calculation, Teplofiz. Vys. Temp. 51, 233–242 (2013) (High Temp. 51, 206–214, 2013)
W.H. Keesom and M. Desirant, The Specific Heats of Tantalum in the Normal and in the Superconductive State, Physica, 1941, 8, p 273-288
R.D. Worley, M.W. Zemansky, and H.A. Boorse, Heat Capacities of Vanadium and Tantalum in the Normal and Superconducting Phases, Phys. Rev., 1955, 99, p 447-458
N.M. Wolcott, The Specific Heat of Transition Metals, Conference de Physique des Basses Témperatures, Paris, 2 to 8 Sep. 1955, Centre National de la Recherche Scientifique and UNESCO, Paris, 1956, p 286–289
C. Chou, D. White, and H.L. Johnston, Heat Capacity in the Normal and Superconducting States and Critical Field of Niobium, Phys. Rev., 1958, 109, p 788-796
D. White, C. Chou, and H.L. Johnston, Heat Capacity in the Normal and Conducting States and Critical Field of Tantalum, Phys. Rev., 1958, 109, p 797-802
F.J. Morin and J.P. Maita, Specific Heats of Transition Metal Superconductors, Phys. Rev., 1963, 129, p 1115-1120
J.M. Corsan and A.J. Cook, Electronic Specific Heat and Superconducting Properties of Nb-Ta Alloys, Phys. Lett. A, 1968, 28, p 500-501
S.V. Lebedev, A.I. Savvatimski and Yu.B.Smirnov, Measurement of Latent Heats of Fusion for Refractory Metals, Teplofiz. Vys. Temp. 9, 635–638 (1971) (High Temp. 9, 578–581, 1971)
M.M. Martynyuk and V.I. Tsapkov, Relationship between the Electrical Resistivity of Niobium, Tantalum, Molybdenum and Tungsten and Their Enthalpy, Izv. Akad. Nauk SSSR Metally (6), 63–67 (1974) (Russ. Metall. Metally (6), 52–55, 1974)
S.V. Lebedev and G.I. Mozharov, Determination of the Temperature Dependence of Resistivity of Tantalum in Both the Solid and Liquid States during Rapid Heating with an Electric Current, Teplofiz. Vys. Temp., 1976, 14, p 1266-1269
J.W. Shaner, G.R. Gathers, and C. Minichino, Thermophysical Properties of Liquid Tantalum and Molybdenum, High Temp. High Press., 1977, 9, p 331-343
G.R. Gathers, Correction of Specific Heat in Isobaric Expansion Data, Int. J. Thermophys., 1983, 4, p 149-157
R. Gallob, H. Jäger, and G. Pottlacher, Recent Results on Thermophysical Data of Liquid Niobium and Tantalum, High Temp. High Press., 1985, 17, p 207-213
A. Berthault, L. Arles, and J. Matricon, High-Pressure High-Temperature Thermophysical Measurements on Tantalum and Tungsten, Int. J. Thermophys., 1986, 7, p 167-179
H. Jäger, W. Neff, and G. Pottlacher, Improved Thermophysical Measurements on Solid and Liquid Tantalum, Int. J. Thermophys., 1992, 13, p 83-93
R.S. Hixson, Personal Communication 1990 to Jäger et al. [83]
G.H. Guthöhrlein, H. Mocnik, and L. Windholz, A New Energy Level of the Neutral Tantalum Atom, Z. Phys. D, 1995, 35, p 177-178
D. Messnarz and G.H. Guthöhrlein, Investigation of the Hyperfine Structure of TaI-Lines (IV), Eur. Phys. J. D, 2000, 12, p 269-282
B. Arcimowicz, A. Huss, S. Roth, N. Jaritz, D. Messnarz, G.H. Guthöhrlein, H. Jäger, and L. Windholz, Investigation of the Hyperfine Structure of TaI-Lines (V), Eur. Phys. J. D, 2001, 13, p 187-194
N. Jaritz, H. Jäger, and L. Windholz, Investigation of the Hyperfine Structure of TaI-Lines (VI), Eur. Phys. J. D, 2002, 18, p 267-276
D. Messnarz, N. Jaritz, B. Arcimowicz, V.O. Zilio, R. Engleman, Jr., J.C. Pickering, H. Jäger, G.H. Guthöhrlein, and L. Windholz, Investigation of the Hyperfine Structure of TaI-Lines (VII), Phys. Scr., 2003, 68, p 170-191
N. Jaritz, G.H. Guthöhrlein, L. Windholz, D. Messnarz, R. Engleman, Jr., J.C. Pickering, and H. Jäger, Investigation of the Hyperfine Structure of TaI-Lines (VIII), Phys. Scr., 2004, 69, p 441-450
N. Jaritz, L. Windholz, U. Zaheer, M. Farooq, B. Arcimowicz, R. Engleman, Jr., J.C. Pickering, H. Jäger, and G.H. Guthöhrlein, Investigation of the Hyperfine Structure of TaI-Lines (X), Phys. Scr., 2006, 74, p 211-217
P. Glowacki, Z. Uddin, G.H. Guthöhrlein, L. Windholz, and J. Dembczyński, A Study of the Hyperfine Structure of Ta I, Lines Based on Fourier Transform Spectra and Laser-Induced Fluorescence, Phys. Scr., 2009, 80, p 025301-1-025301-10
T.P.J.H. Babeliowsky, Mass Spectrometric Determination of the Heat of Vaporization of Some Solid Elements, Physica, 1962, 28, p 1160-1169
É.Y. Zandberg, N.I. Ionov, and A.Y. Totegode, Mass-Spectrometric Determination of the Heat of Vaporization of Atoms and Positive Ions in Sublimation of Polycrystalline Rhenium, Tungsten, Tantalum and Molybdenum, Zh. Tekh. Fiz. 35, 1504–1515 (1965) (Sov. Phys. Tech. Phys. 10, 1164–1172, 1966)
N. Sasaki, K. Kubo, and M. Asano, Mass Spectrometric Studies of the Work Function and the Heats of Sublimation of Atom and Positive Ion, Mass Spectrom. (Jpn), 1970, 18, p 1189-1194
E. Gebhardt, H.-D. Seghezzi, and H. Keil, Über die Verdampfungsgeschwindigkeit von Tantalum im Vakuum, Z. Metallkde., 1962, 53, p 524-525
F. Simon and M. Ruhemann, Untersuchungen über die Spezifischen Wärmen bei Tiefen Temperaturen, Z. Phys. Chem., 1927, 129, p 321-348
A. Magnus and H. Holtzmann, Untersuchungen über die Spezifischen Wärme von Tantal, Wolfram und Beryllium zwischen 100 and 900°C, Ann. Physik., 1929, 395, p 585-613
F.M. Jaeger and W.A. Veestra, The Exact Measurement of the Specific Heats of Solid Substances at High Temperature. VI. The Specific Heats of Vanadium, Niobium, Tantalum and Molybdenum, Rec. Trav. Chim., 1934, 53, p 677-687
I.B. Fieldhouse, J.C. Hedge, J.I. Lang, A.N. Takata, and T.E. Watermann, Measurements of Thermal Properties, Armour Research Foundation, Wright Air Development Center, Air Research and Development Command, United States Air Force, Wright-Patterson Air Force Base, Ohio, Rept. WADC-TR-55-495, Part 1 (1956)
M. Hoch, H.L. Johnston, and A. High, Temperature Drop Calorimeter. The Heat Capacities of Tantalum and Tungsten between 1000° and 3000°K, J. Phys. Chem., 1961, 65, p 855-860
M. Pirani, Über die Messung der Spezifischen Wärme fester Körper bei Hohen Temperaturen, Ber. Deut. Physik. Ges., 1912, 10, p 1037-1054
D.B. Langmuir and L. Malter, Specific Heat, Heat of Sublimation and Vapor-Pressure Constant of Tantalum, Phys. Rev., 1939, 55, p 1138
J.H. Boggs, R.A. Knezek, and J.A. Wiebelt, Status Report on a Study of the Use of Furnace Calorimetry for the Rapid Determination of Specific Heats of Solids at High Temperature, U.S. Atomic Energy Commission, Rept. AECU-4282 (1959)
J.H. Boggs and J.A. Wiebelt, An Investigation of a Particular Comparative Method of Specific Heat Determinations in the Temperature Range of 1500 to 2600°F, U.S. Atomic Energy Commission, Rept. TID-5734 (1960)
D.H. Hildenbrand, L.P. Theard, and N.D. Potter, An Experimental Program for Obtaining the Thermodynamic Properties of Propellant Combustion Products. Third Quarterly Technical Summary Report, Aeronutronic Research Laboratories Technical Report, Publ. No. U-1606 (1962)
G.C. Lowenthal, The Specific Heat of Metals Between 1200°K and 2400°K, Aust. J. Phys., 1963, 16, p 47-67
Y.A. Kraftmakher, Specific Heat of Tantalum over the Temperature Range 1200-2900°K, Zh. Prikl. Mekhan. i Tekhn. Fiz. (2), 158–160 (1963)
Y.A. Kraftmakher, The Modulation Method for Measuring Specific Heat, High Temp. High Press., 1973, 5, p 433-454
C. Affortit, Mesure de la Chaleur Spécifique des Métaux Jusqu’à Leur Température de Fusion, Centre d’Etudes Nucleares de Fontenay-aux-Roses, Commissariat à l’Énergie Atomique, Rapp. CEA-R3287 (1967)
L.P. Filippov and R.P. Yurchak, High Temperature Investigation of the Thermal Properties of Solids, Inzh. Fiz. Zh. 21, 561–577 (1971) (J. Eng. Phys 21, 1209–1220, 1971)
L.P. Filippow, Untersuchung der Thermischen Eigenschaften im Stoff an der Moskauer Universität, Int. J. Heat Mass Transfer, 1973, 16, p 865-885
A.A. Kulish and L.P. Filippov, Determination of the Thermophysical Properties of Group V Metals at High Temperatures by Means of a Study of Deformation Vibrations of Plates, Teplofiz. Vys. Temp. 16, 602–610 (1978) (High Temp. 16, 512–519, 1978)
K.E. Gilchrist and S.D. Preston, Thermophysical Property Measurements on Some Neutron Absorbing Materials, High. Temp. High Press., 1979, 11, p 643-651
N.D. Milošević, G.S. Vuković, D.Z. Pavičić, and K.D. Maglić, Thermal Properties of Tantalum Between 300 and 2300 K, Int. J. Thermophys., 1999, 20, p 1129-1136
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arblaster, J.W. Thermodynamic Properties of Tantalum. J. Phase Equilib. Diffus. 39, 255–272 (2018). https://doi.org/10.1007/s11669-018-0627-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0627-2