Abstract
The phase diagram of the Li-Bi binary system was amended by x-ray analysis and differential scanning calorimetry. The formation of the binary compound Li2Bi was confirmed and its structure determined by x-ray single crystal diffraction. The Li2Bi compound crystallizes with the Mg2Ga structure type (space group P-62c, hP18, a = 8.0712(4) Å, c = 6.8352(3) Å). The analysis of the interatomic distances together with electronic structure calculations using the tight-binding linear muffin-tin orbital atomic spheres approximations (TB-LMTO-ASA) indicate the dominance of a metallic type of bonding, although there is a weak partially ionic bonding, caused by charge transfer from Li to Bi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The binary, ternary and multicomponent lithium alloys and intermetallic compounds have been extensively studied in different fields and applications, including electrode materials for lithium-ion batteries, hydrogen storage materials, lightweight alloys and others.[1-12] In spite of intensive studies on lithium-containing binary systems, not all equilibrium phases are known yet. The Li-Zn[13,14] and the Li-In[15] systems are two examples with new binary phases established recently. The phase diagram of the system Li-Bi was first investigated by Grube et al.,[16] mainly based on thermal analysis and electrical resistivity measurements. The LiBi and Li3Bi compounds were identified by Zintl and Brauer,[17] and their crystal structures were determined by x-ray diffraction.
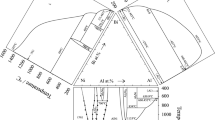
The peritectic melting and polymorphic transformation of LiBi was also confirmed by Weppner and Huggins.[18] The Li3Bi phase melts congruently and was later also prepared as solid films by the direct reaction of Bi(s) and Li(g) at low pressure and 200 °C.[19] The thermodynamic properties of Li-Bi alloys are described in references.[18,20-23] The assessed Li-Bi phase diagram is based on data from Sangster and Pelton[24] and shown in Fig. 1(a).
The phase diagram of Li-Bi system redrawn from[27] (a) and amendment (b)
The thin films of Li-Bi alloys were prepared by Hiratani et al.[25] It was found that a new compound Li2Bi was formed in addition to two known intermetallic compounds, LiBi and Li3Bi. The x-ray diffraction patterns after heat treatment showed that this new compound was frozen in a meta-stable state at room temperature.
This paper includes new results on the Li-Bi phase diagram. The synthesis and crystal structure of the binary phase Li2Bi are described.
Experimental Details
Lithium and bismuth, all with a nominal purity higher than 99.9% w/w, were used as starting materials for syntheses of Li75Bi25, Li66.7Bi33.3 and Li50Bi50 binary alloys. All preparation steps were performed in a glove box under dried argon atmosphere. The melting of the metals was carried out within sealed tantalum crucibles in an induction furnace at 1100 °C. After 10 min, the sample was rapidly cooled down to room temperature by removing the crucible from the furnace. After thermal treatment at 200 °C during 400 h, the sample could easily be separated from the opened tantalum container. No side-reaction of the alloy with the crucible was detected. The brittle dark-gray metallic alloys are very sensitive to the humidity in air. The amount of Li-loss during sample preparation in hermetically closed crucibles can be estimated, based on previous studies with successive detailed chemical analyses of similar systems.[6-8,26] Accordingly, a lithium loss up to 1% versus the nominal composition can be expected. In addition, for the alloys the content of lithium was determined by means of Flapho-4 flame photometer (Carl Zeiss Jena) with interference filter (671 nm).
X-ray powder diffraction of the samples was carried out using a STOE STADI/P powder diffractometer (Mo Kα1-radiation, step scanning). Rietveld refinements, based on x-ray powder diffraction data (XRPD), were performed with the FULLPROF program.[27]
X-ray singe crystal diffraction was used for detailed crystal structure refinements. The intensity data were collected by an automatic four-circle diffractometer Xcalibur from Oxford Diffraction, equipped with CCD detector and graphite- monochromatized Mo Kα radiation. Scans were taken in ω mode, and CrysalisRed[28] was used for analytical absorption corrections. The crystal structure was solved by direct methods and refined using the SHELX-97 program package.[29,30]
The electronic structures of the compounds were calculated using the tight-binding linear muffin-tin orbital (TB-LMTO) method in the atomic spheres approximation (TB-LMTO-ASA,[31-33] using the experimental crystallographic data reported here. The exchange and correlation were interpreted in the local density approximation.[34] All the figures and graphics concerning electron structure calculations were generated using wxDragon.[35]
The thermal DSC-TG-DTG analysis was carried out with the NETZSCH STA-449 simultaneous thermal analyzer. Samples of approximately 0.030 g were heated in corundum crucibles. The heating was performed under static conditions in argon atmosphere in the range of 30-600 °C with the temperature rate increase of 5 K/min. They provided the 0.5 °C precision in reading of temperature.
Both 6Li and 7Li nuclear magnetic resonance (NMR) spectroscopy has been extensively applied on inorganic and intermetallic lithium-containing compounds. The 6Li isotope has as smaller nucler spin (I = 1), a smaller magnetogyric ratio, and a much smaller nuclear quadrupole moment. Therefore, sharp linewidths are achieved compared to the 7Li isotope. Thus, 6Li NMR was used to get a better resolution in the spectrum. The 6Li magic angle spinning (MAS) NMR spectra were recorded with 2.5 mm zirconia rotors at room temperature with a single-pulse sequence, a RF pulse length of 2 µs, and a rotation frequency of 5 kHz. Chemical shifts are given relative to that of an aqueous 1 M 6LiCl solution.
Results and Discussion
Phase Diagram of Li-Bi System
A re-examination of the Li-Bi system, based on differential scanning calorimetry, x-ray diffraction analysis and flame photometry of binary alloys (Tables 1, 2), revealed a more complex phase diagram in the composition range between LiBi and Li3Bi than originally reported (Fig. 1b). The XRPD phase analysis shows that Li75Bi25 cast alloy is single-phase with cubic BiF3 structure type. The Li50Bi50 cast alloy as a main phase consists of a tetragonal LiBi (AuCu structure type) and two Li2Bi and Li3Bi additional phases the content of which was significantly reduced after annealing. Even after annealing the Li66.7Bi33.3 alloy was three-phase, and the content of the main Li2Bi hexagonal phase was less than 50%. This is caused by such conditions as low temperature (200 °C) at which the process of phase formation in solid state cannot proceed rapidly. Good correlation between starting nominal composition and lithium content obtained by flame photometry for Li75Bi25 and Li50Bi50 annealed alloys is observed (Table 1). The difference between nominal composition and composition obtained by flame photometry is slightly higher for three phase Li66.7Bi33.3 alloy. The presence of Li2Bi hexagonal phase in cast alloys and increase in the content of the annealed alloys suggest that it is the stable phase in contrast to results of the authors,[28] who observed this compound in thin films of Li-Bi alloys as a meta-stable phase. This compound forms at 211 °C by peritectoid reaction:
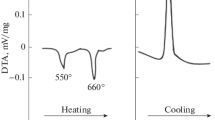
Powder patterns from a Li2Bi alloy, measured sequentially with increasing temperature show that the hexagonal phase exists from room temperature up to 211 °C (Fig. 2). Above this temperature all powder patterns reflected only the coexistence of the tetragonal LiBi and the cubic Li3Bi phases. The results of the Rietveld refinements of the as cast and annealed at 200 °C Li2Bi alloy are shown Fig. 3. In addition to the reflections from the new hexagonal phase reflections from the known cubic and tetragonal phases are detected. The annealing of this alloy during 400 h at 200 °C increase the Li2Bi phase content from 18% (for cast alloy) up to 47%. Subsequent continuation of annealing slightly increases the content of this phase. Differential scanning calorimetry determined the accurate temperature of the peritectoid reaction at 211 °C (Fig. 4).
Observed (red circles), calculated (black line) and difference (bottom blue line) x-ray powder diffraction patterns for Li66.7Bi33.3 as cast alloy (a) and annealed at 200 °C (b). Vertical bars indicate the Bragg positions of the corresponding phases. Symbols for phase identification: ▲—Li2Bi, ■—LiBi and ●—Li3Bi (Color figure online)
For the already known phases Li3Bi and LiBi congruent and peritectic reactions with the liquid melt (L) were confirmed at temperatures of 1145 and 423 °C, respectively:
For the equiatomic LiBi phase Grube et al.[16] observed two invariant reactions at 415 and 400 °C, assigned to the peritectic melting of LiBi and the polymorphic transformation α-LiBi ⇌ β-LiBi, respectively. According to our DSC data these temperature are 423 and 401 °C respectively. The tetragonal structure (AuCu structure type, P4/mmm) of the low temperature modification α-LiBi was confirmed in this study and more precise unit cell parameters could be refined: a = 3.36104(3), c = 4.24404(6) Å. The refined lattice parameter for cubic Li3Bi (BiF3 structure type, Fm-3m) at room temperature is a = 6.7063(2) Å. All known crystallographic data for solid phases in the Li-Bi system are summarized in Table 2, together with the types of reaction and the temperature of formation.
Crystal and Electronic Structure of the Li2Bi Binary Compound
From the annealed alloy of nominal composition Li66.7Bi33.3 a small prismatic single crystal was isolated by mechanical fragmentation. The structure solution by direct methods and subsequent structure refinement was performed using the SHELX97 software package. The structure was solved after an analytical absorption correction in the non-centrosymmetric space group P-62c, since the solution and refinement in centrosymmetric variants were not satisfactory. Selected information on single crystal x-ray data collection and refinements is given in Table 3, and the fractional atomic coordinates and thermal displacement parameters of the Li2Bi compound are compiled in Table 4.
The structure of title compound crystallizes in the Mg2Ga-type.[36] Atoms of bismuth in the Li2Bi structure occupy the 4f and the 2b sites, which are occupied by gallium atoms in Mg2Ga. The lithium atoms occupy 6h and 6g sites as the magnesium atoms in Mg2Ga. The unit cell of the Li2Bi structure and the coordination polyhedra of all representative atoms are shown in Fig. 5(a). The coordination polyhedron of Li1 has 13 vertices and is a tricapped pentagonal prism [Li1Bi5Li8]. The coordination polyhedron of Li2 is an anticuboctahedron [Li2Bi4Li8]. For Bi1 the coordination polyhedron is a trigonal prism, in which the quadrilateral facets are centered by lithium atoms and the one base facet centered by a Bi atom [Bi1Li9Bi]. Bi2 is surrounded by 11 atoms and the coordination polyhedra is a trigonal prism, in which the quadrilateral facets are centered by lithium and the two bases triangular facets by Bi atoms [Bi2Li9Bi2].
The crystal structure of Li2Bi (Mg2Ga-type) compound is also closely related to the Mg2In (or Fe2P) structure type which has a twice shorter unit cell dimension c. In this structure, both non-equivalent indium atoms have the same atomic coordination (c.n. = 11), while in the Li2Bi compound the atoms of p-element (Bi) have different coordination numbers c.n. = 10 and c.n. = 11. This difference probably is the reason for doubling of unit cell dimension c.
At the refinement of the Li2Bi in the Mg2In (or Fe2P) structure model proposed by Hiratani et al.[28] the R-factors are growing almost twice more (R = 0.054 in compare to R = 0.023 for Mg2Ga type), and all refinement parameters are worse. Also our experimental data clearly shown that the model with P-62c space group is more correct, because the single crystal data consist enough visible reflexes which are absent in the model with P-62m space group, such as 011, 013, 023, −143 etc.
The structure of Li2Bi (Fig. 5b) can be described by two different atomic networks: The lithium atoms form three-dimensional 63-nets with Bi atoms distributed in the hexagonal channels. Bismuth atoms form Kagome 36-nets.
Li2Bi belongs to the ‘classical’ Zintl phases, because it contains an alkaline metal (Li) and a p-element (Bi), it is electronically balanced (lithium atoms form positively charged 2n[Li] δ+ polycations, which compensate the negative charge of n[Bi] 2δ− polyanions) and has a very narrow or no homogeneity width (see phase diagram, Fig. 1). Electronic structure calculations using the TB-LMTO-ASA confirm the Zintl concept and a charge transfer from Li to Bi. The electron localization function (ELF) mapping and isosurfaces for Li2Bi are shown in Fig. 6(a) and (b). Generally, in the case of a fully delocalized electron the ELF value is equal 0 and in the case of a fully localized electron equal 1. For the investigated phases the ELF is numerically bound between 0 (around Li) and 0.953 (around Bi). Thus, despite the partial ionization of all atoms, there are still sufficient delocalized electrons available that provide a dominant metallic bonding. A significant decrease of the density of states (DOS) at the Fermi level (Fig. 7a) indicates a semi-metallic behavior. Such a behavior is typical for Zintl phases, which are poor conductors or semiconductors.
The quantitative evaluation of the bonding strength between the different types of atoms in Li2Bi was obtained from the crystal orbital Hamilton population (COHP) and integrated COHP (iCOHP) calculations (Fig. 7b; Table 4). From the COHP curves the strongest interactions are concluded between Bi-Bi atoms. The Bi1-Bi1 distance (2.9720 Å) is shorter than Bi2-Bi2 (3.4176 Å), indicating an increase of the bond strength and an increases of the -iCOHP parameter from 1.727 to 0.754 eV, respectively. The interactions between Li and Bi atoms are somewhat weaker and for the shortest distances within 0.363-0.585 eV (Table 5).
An analysis of the interatomic distances together with electronic structure calculations indicates the dominance of a metallic type of bonding, although there is also a weak partially ionic bonding, caused by charge transfer from Li to Bi.
The 6Li MAS NMR spectrum of Li2Bi (Fig. 8) is dominated by a main peak at +15.4 ppm. The large positive shift is a so-called Knight shift resulting from interaction of the Li nucleus with delocalized electrons, further confirming the metallic character of the sample. Some minor contribution was observed at about 3 ppm, probably from an impurity phase or an interstitial Li site in the structure with very low occupation probability.
References
B. Scrosati and J. Garche, Lithium Batteries: Status, Prospects and Future, J. Power Sources, 2010, 195, p 2419-2430
C.-M. Park, J.-H. Kim, H. Kim, and H.-J. Sohn, Li-Alloy Based Anode Materials for Li Secondary Batteries, Chem. Soc. Rev., 2010, 39, p 3115-3141
F. Thoss, L. Giebeler, S. Oswald, H. Ehrenberg, and J. Eckert, Study on the Reversible Li-Insertion of Amorphous and Partially Crystalline Al86Ni8La6 and Al86Ni8Y6 Alloys as Anode Materials for Li-Ion Batteries, Electrochim. Acta, 2012, 60, p 85-94
I. Chumak, G. Dmytriv, V. Pavlyuk, S. Oswald, J. Eckert, H. Trill, and H. Eckert, Li(Al1-zZnz) Alloys as Anode Materials for Rechargeable Li-Ion Batteries, J. Mater. Res., 2010, 25, p 1492-1499
V. Pavlyuk, G. Dmytriv, I. Chumak, O. Gutfleisch, I. Lindemann, and H. Ehrenberg, High Hydrogen Content Super-Lightweight Intermetallics from the Li-Mg-Si System, Int. J. Hydrogen Energy, 2013, 38, p 724-737
G. Dmytriv, H. Pauly, H. Ehrenberg, V. Pavlyuk, and E. Vollmar, Homogeneity Range of the NaTl-type Zintl Phase in the Ternary System Li-In-Ag, J. Solid State Chem., 2005, 178, p 2825-2831
V. Pavlyuk, G. Dmytriv, I. Chumak, H. Ehrenberg, and H. Pauly, The Crystal Structure of the LiAg2In Compound, J. Solid State Chem., 2005, 178, p 3303-3307
V. Pavlyuk, G. Dmytriv, I. Tarasiuk, H. Pauly, and H. Ehrenberg, The Ternary Indide Li278(In, Ag)154: A New n = 6 Variant of Cubic n × n×n W-Type Superstructures, Intermetallics, 2007, 15, p 1409-1415
V. Pavlyuk, G. Dmytriv, I. Tarasiuk, I. Chumak, H. Pauly, and H. Ehrenberg, Li8Cu12+xAl6−x (x = 1.16): A New Structure Type Related to Laves Phases, Acta Crysallogr., 2008, C64, p 15-17
V. Pavlyuk, G. Dmytriv, I. Tarasiuk, I. Chumak, H. Pauly, and H. Ehrenberg, Li12Cu16+xAl26−x (x = 3.2): A New Intermetallic Structure Type, Acta Crysallogr., 2008, C64, p 73-75
V. Pavlyuk, G. Dmytriv, I. Tarasiuk, I. Chumak, H. Pauly, and H. Ehrenberg, Polymorphism of LiAg. Solid State, Science, 2010, 12, p 274-280
G. Dmytriv, V. Pavlyuk, H. Pauly, J. Eckert, and H. Ehrenberg, New Real Ternary and Pseudoternary Phases in the Li-Au-In-System, J. Solid State Chem., 2011, 184, p 1328-1332
V. Pavlyuk, I. Chumak, and H. Ehrenberg, Polymorphism of Li2Zn3, Acta Crysallogr., 2012, B68, p 34-39
V. Pavlyuk, I. Chumak, L. Akselrud, S. Lidin, and H. Ehrenberg, LiZn4−x (x = 0.825) as a (3 + 1)-Dimensional Modulated Derivative of Hexagonal Close Packing, Acta Crysallogr., 2014, B70, p 212-217
V. Pavlyuk, I. Chumak, and H. Ehrenberg, Binary Lithium Indides Li22−x In8+x (x = 0.1), Li11−x In4+x (x = 1.05), and Li10−x In2+x (x = 1.59) with Clusters, Eur. J. Inorg. Chem., 2014, 12, p 2053-2064
G. Grube, H. Vosskühler, and H. Schlecht, Electrical Conductivity and Phase Diagrams of Binary Alloys. 12. The Bi-Li System, Z. Elektmchem., 1934, 40, p 270-274
E. Zintl and G. Brauer, Metals and Alloys. 14. Constitution of Bi-Li Alloys, Z. Elektmchem., 1935, 41, p 297-303
W. Weppner and R.A. Huggins, Thermodynamic Properties of the Intermetallic Systems Li-Sb and Li-B, J. Electrochem. Soc., 1978, 125, p 7-14
R. Gobrecht, On Li Bismuthide, Ann. Phys. Ser., 1967, 20(5/6), p 262-264
W. Seith and O. Kubaschewski, The Heats of Formation of Several Alloys, Z. Elektmchem., 1937, 43, p 743-749
M.S. Foster, S.E. Wood, and C.E. Crouthamel, Thermodynamics of Binary Alloys. I. The Bi-Li System, Inorg. Chem., 1964, 3, p 1428-1431
B. Predel and G. Oehme, Calorimetric Study of Liquid Li-Tl, In-Li and Bi-Li Alloys, Z. Metallkd., 1979, 70, p 618-623
A. Neubert, H.R. Ihle, and K.A. Gingerich, Thermodynamic Study of the Molecules BiLi and PbLi by Knudsen Effusion Mass Spectrometry, J. Chem. Phys., 1980, 73, p 1406-1409
J. Sangster and A.D. Pelton, The Bi-Li (Bismuth-Lithium) System, J. Phase Equilibria, 1991, 12, p 447-450
M. Hiratani, Y. Ito, K. Miyauchi, and T. Kudo, Thin Film Formation of Li-Bi Alloy and Identification of a New Intermetallic Compound, Mater. Res. Bull., 1988, 23, p 1739-1746
H. Pauly, A. Weiss, and H. Witte, The Crystal Structure of the Ternary Intermetallic Phases Li2 EX (E = Cu, Ag, Au; X = Al, Ga, In, Tl, Si, Ge, Sn, Pb, Sb, Bi), Z. Metallk., 1968, 59, p 47-58
J. Rodriguez-Carvajal, Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction, Physica B, 1993, 192, p 55-69
CrysAlis PRO Agilent, CrysAlis PRO, Agilent Technologies, Yarnton, 2011
G.M. Sheldrick, SHELXS, Program for the Solution of Crystal Structures, University of Goettingen, Goettingen, 1997
G.M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Goettingen, Goettingen, 1997
O.K. Andersen, Linear Methods in Band Theory, Phys. Rev. B, 1975, 12, p 3060-3083
O.K. Andersen and O. Jepsen, Explicit, First-Principles Tight-Binding Theory, Phys. Rev. Lett., 1984, 53, p 2571-2574
O.K. Andersen, Z. Pawlowska, and O. Jepsen, Illustration of the Linear-Muffin-Tin-Orbital Tight-Binding Representation: Compact Orbitals and Charge Density in Si, Phys. Rev. B, 1986, 34, p 51-53
U. von Barth and L. Hedin, A Local Exchange-Correlation Potential for the Spin Polarized Case, I. J. Phys. C, 1972, 5, p 1629-1714
B. Eck, wxDragon 1.6.6, Aachen, 1994-2010, http://www.ssc.rwth-aachen.de, Accessed Apr 7, 2013
K. Frank and K. Schubert, Kristallstruktur von Mg2Ga und Mg2Tl, J. Less Common Met., 1970, 20, p 215-221
P. Villars and K. Cenzual, Pearson’s Crystal, Version 1.0, Release 2007/8, ASM International, Materials Park
Acknowledgments
Financial support from the National Science Centre, Poland (No. 2014/15/B/ST8/00101) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlyuk, V., Sozanskyi, M., Dmytriv, G. et al. Amendment of the Li-Bi Phase Diagram Crystal and Electronic Structure of Li2Bi. J. Phase Equilib. Diffus. 36, 544–553 (2015). https://doi.org/10.1007/s11669-015-0409-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-015-0409-z