Abstract
The phase equilibria in the Ni-Al-Bi ternary system at 800 and 1000 °C were experimentally determined by x-ray diffraction and electron probe microanalysis. The results show that: Bi is almost insoluble in any Ni-Al compounds and (Ni) phase. There are five and four three-phase regions existing in the isothermal section at 800 and 1000 °C, respectively. The Ni-Al binary compounds are all in equilibrium with the liquid phase in these two isothermal sections. No ternary compound was found at 800 and 1000 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the deterioration of the environment and the increasing depletion of fossil fuels, hydrogen energy is receiving close attention as a clean and pollution-free energy source. Al-based alloys have been widely used due to their low cost, high hydrogen production, and environmental protection. Aluminum and its alloys are recognized to be one of the most suitable metals applicable for future hydrogen production.[1,2,3] According to previous studies, adding one or two low melting point metals,[4, 5] oxides[6, 7] and salts[8, 9] is one of the methods to activate metallic aluminum. Al-based alloys ball milled with low-melting-point metals,[10] active metals[11] and other additives[12] have good hydrogen production performance. The Al-Bi binary system is monotectic and there is a miscibility gap in the system. During the gas atomization process, the liquid phase separated into two phases (the Bi-rich liquid phase and the Al-rich liquid phase) during the cooling process, which will greatly promote the hydrolysis performance of aluminum.[13] In addition, the addition of Ni can significantly improve the production of hydrogen in Al-H2O reaction.[14]
The three binary phase diagrams of Ni-Al,[15] Ni-Bi[16] and Al-Bi[17] constituting the Ni-Al-Bi ternary system are shown in Fig. 1 and the information of stable solid phases and their crystal structures in three binary systems are summarized in Table 1. Okamoto et al.[15] plotted the complete Ni-Al binary phase diagram. Peng et al.[18] evaluated thermodynamic assessments for the Ni-Al binary system. The Ni-Bi subsystem is relatively simple, with no intermetallic compounds at 800 and 1000 °C. Vassilev et al.[19, 20] optimized the Ni-Bi binary system. For the Al-Bi subsystem, Mirkovic et al.[21] revised the experimental data available. Liu et al.[22] further conducted a thermodynamic evaluation on the Al-Bi binary system and found no intermetallic compounds in this binary system.
Although there have been many investigations of the above subsystems, there is no information about Ni-Al-Bi ternary system in the relevant literature. The Ni-Al-Bi ternary system is an important subsystem of Al-based hydrogen production materials and studying its phase equilibria is very useful for the design of hydrogen production components. Hydrogen production experiments or other experiments often need to be performed at high temperatures. Therefore, it is necessary to obtain the phase equilibrium information at high temperature, which will provide a theoretical basis for the thermodynamic calculation of the Ni-Al-Bi ternary system. The main objective in the present work is to establish the isothermal section of Ni-Al-Bi ternary system at 800 and 1000 °C.
2 Experimental Procedure
The phase relationship of the Ni-Al-Bi ternary system was deduced by studying the phase composition of the alloys. The compositions of ternary alloys were determined according to the binary phase diagram. Pure metals, nickel (99.9 wt.%), aluminum (99.9 wt.%) and bismuth (99.9 wt.%), were used as raw materials and the bulk buttons were prepared by arc melting under high purity argon atmosphere, using a non-consumable tungsten electrode. The mass of each sample was about 15 g. To achieve homogeneity, the ingots were turned over and remelted at least five times.
The molten alloys were cut into small pieces, and then put into an alumina crucible to prevent the sample from contacting and reacting with the glass tube, and then sealed in a quartz tube with a small amount of titanium chips to prevent the samples from oxidizing during heating in a gradient furnace. Specimens were annealed at 800 and 1000 °C for different times. The annealing time depended on the annealing temperature and the composition of specimens. At the end of the heat treatment, the samples reaching phase equilibria were quenched into iced water for subsequent sample characterization analysis.
After standard metallographic preparation, the backscatter electron (BSE) and the composition analysis of each equilibrium phase were carried out by electron probe microanalysis (EPMA, JXA-8100, JEOL, Japan). High purity metals were used as standard, and the measurements were carried out at a voltage of 20kV and a current of 1.0×10-8 A. Energy Dispersive Spectrometry (EDS) was used to determine the compositions of the liquid phase. The liquid phase was measured by EDS analysis at least 12 times. The average value was taken as the result. The crystal structure analysis was conducted using x-ray diffraction (XRD) on a Philips Panalytical X-pert diffractometer (Cu Kα radiation at 40 kV and 40 mA). The data were collected in the range of 2θ from 10° to 90° at a step size of 0.01°.
3 Result and Discussion
3.1 Phase Equilibria at 800 °C
To determine the isothermal section of the Ni-Al-Bi ternary system at 800 °C, a total of 12 alloys were prepared. The equilibrium composition of each phase is shown in Table 2. All the mentioned chemical compositions in this work were given in the form of an atomic fraction (at.%). The L1(Bi-rich) and L2(Al-rich) mentioned in this work represent the liquid phase of Bi and the liquid phase of Al, respectively. The different phases in the alloy can easily be distinguished by studying the morphology, contrast, and chemical composition of the alloy. In most cases, observations using EPMA coupled with EDS analyses are sufficient to identify most of the equilibrium phases. However, the relevant x-ray diffraction patterns were analyzed for the final identification of the phases.[23] Typical BSE images and x-ray diffraction patterns of the Ni-Al-Bi equilibrated alloys quenched from 800 °C are shown in Fig. 2 and 3, respectively.
A BSE image of the alloy Ni20Al70Bi10 annealed at 800 °C is presented in Fig. 2(a), showing three-phase co-existence of L1(Bi-rich) (white) + L2(Al-rich) (black) + Al3Ni (gray). The x-ray diffraction pattern of Ni20Al70Bi10 can further confirm the three-phase equilibrium state of the alloy, which is shown in Fig. 3(a). At 800 °C, the L1(Bi-rich) phase and L2(Al-rich) phase are both liquid phases, which are obtained during the quenching process. Because Al-Bi binary system is monotectic with a miscibility gap, the liquid phase is separated into two phases during quenching, namely the L1(Bi-rich) and the L2(Al-rich). Due to the presence of two liquid phases, the deviation of EPMA detection is relatively large. Therefore, for the accuracy of the experiment, three alloys of different compositions were melted in this three-phase region. The detection results are shown in Table 2, taking the average value as the result.
In the Ni31Al66Bi3 alloy annealed at 800 °C, the three-phase equilibrium of L1(Bi-rich) (white) + Al3Ni (black) + Al3Ni2 (gray) is identified, as shown in Fig. 2(b). The corresponding x-ray diffraction pattern is shown in Fig. 3(b). Three alloys with different components were also melted in this phase region for the accuracy of the experiment, and the test results were not much different. In Fig. 2(c), there is a two-phase microstructure of L1(Bi-rich) (white) + AlNi (black) in alloy Ni54Al34Bi12 that was annealed at 800 °C. The three-phase microstructure consisting of L1(Bi-rich) (white) + AlNi (black) + AlNi3 (gray) is observed in the Ni61Al26Bi13 alloy annealed at 800 °C, and the x-ray diffraction results show very good consistency in Fig. 3(c).
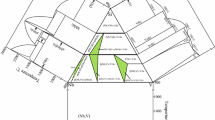
Based on the above experimental data, the 800 °C isothermal section diagram was established, which is shown in Fig. 4. Four three-phase regions of (Ni) + AlNi3 + L1(Bi-rich), AlNi3 + AlNi + L1(Bi-rich), Al3Ni2 + Al3Ni + L1(Bi-rich) and Al3Ni + L1(Bi-rich) + L2(Al-rich) were experimentally determined at 800 °C, and they were marked with different symbols. An undetermined three-phase region of AlNi + Al3Ni2 + L1(Bi-rich) is indicated by dashed lines. The maximum solubility of Al in the L1(Bi-rich) phase was measured to be about 1 at.%, and the solubility of Bi in the L2(Al-rich) phase was measured to be about 0.3 at.%. Bi is almost insoluble in other Ni-Al compounds and (Ni) phase.
3.2 Phase Equilibria at 1000 °C
In the present study, a total of 9 samples with different composition were prepared in order to determine the phase equilibria at 1000 °C in the Ni-Al-Bi ternary system. The equilibrium composition of each phase was shown in Table 3. BSE images and x-ray diffraction patterns for most of the Ni-Al-Bi ternary specimens are shown in Fig. 5 and 6, respectively.
Figure 5(a) illustrates that a three-phase equilibrium of L1(Bi-rich) (white) + L2(Al-rich) (black) + Al3Ni2 (gray) exists in alloy Ni33Al64Bi3 annealed at 1000 °C. The x-ray diffraction patterns analysis further matches up with them, as shown in Fig. 6(a). To further prove the accuracy of the data, another alloy composition was selected in this three-phase region. The results obtained by these two alloys are similar, and the average value is taken as the result on the isothermal section. Two two-phase microstructures of Al3Ni2 (gray) +L1(Bi-rich) (white) and (Ni) (gray) + L1(Bi-rich) (white) were found in alloys Ni40Al54Bi6 and Ni86Al6Bi8, respectively Fig. 5(b,d). The x-ray diffraction pattern shown in Fig. 6(b) confirms that there is a two-phase equilibrium in alloy Ni40Al54Bi6. As shown in Fig. 5(c, a) three-phase equilibrium of L1(Bi-rich) (white) + AlNi (black) + (Ni) (gray) was discovered in the alloy Ni80Al18Bi2. The x-ray diffraction results show very good consistency in Fig. 6(c).
Based on the EPMA analyses and the x-ray diffraction pattern of the above-mentioned alloys, the phase diagram of the Ni-Al-Bi ternary system at 1000 °C is determined as shown in Fig. 7. Three three-phase regions of (Ni) + AlNi3 + L1(Bi-rich), AlNi3 + AlNi + L1(Bi-rich) and Al3Ni2 + L1(Bi-rich) + L2(Al-rich) were obtained. Like 800 °C, the three-phase region of AlNi + Al3Ni2 + L1(Bi-rich) was not determined by the designed alloys. From Fig. 7, the L1(Bi-rich) phase is in equilibrium with all phases of Ni-Al binary system, and Bi is almost insoluble in any Ni-Al compounds and (Ni) phase.
4 Conclusion
-
1.
The isothermal sections of the Ni-Al-Bi ternary system at 800 and 1000 °C were experimentally determined. There are five and four three-phase regions existing at 800 and 1000 °C, respectively. No ternary compound is found.
-
2.
The Al-Bi binary system is monotectic with a miscibility gap, and the liquid phase is separated into L1(Bi-rich) phase and L2(Al-rich) phase during quenching.
-
3.
The solid solubility of Bi in Ni-Al binary compounds is very small.
-
4.
The Ni-Al compounds are all in equilibrium with the L1(Bi-rich) phase in these two isothermal sections.
Reference
D. Qiao, Y. Lu, Z. Tang, X. Fan, T. Wang, T. Li, and P.K. Liaw, The Superior Hydrogen-Generation Performance of Multi-component Al Alloys by the Hydrolysis Reaction, Int. J. Hydrogen Energy, 2019, 44(7), p 3527–3537
X. Guan, Z. Zhou, P. Luo, F. Wu, S. Dong, Hydrogen Generation from the Reaction of Al-based Composites Activated by Low-melting-point Metals/Oxides/Salts with Water, Energy, 188, (2019)
H.Z. Wang, D.Y.C. Leung, M.K.H. Leung, and M. Ni, A Review on Hydrogen Production Using Aluminum and Aluminum Alloys, Renew. Sustain. Energy Rev., 2009, 13(4), p 845–853
W. Wang, W. Chen, X.M. Zhao, D.M. Chen, and K. Yang, Effect of Composition on the Reactivity of Al-rich Alloys with Water, Int. J. Hydrogen Energy, 2012, 37(24), p 18672–18678
F. Zhang, R. Yonemoto, M. Arita, and Z. Horita, Hydrogen Generation from Pure Water Using Al-Sn Powders Consolidated Through High-pressure Torsion, J. Mater. Res., 2016, 31(6), p 775–782
Z.-Y. Deng, Y.-F. Liu, Y. Tanaka, J. Ye, and Y. Sakka, Modification of Al Particle Surfaces by gamma-Al2O3 and Its Effect on the Corrosion Behavior of Al, J. Am. Ceram. Soc., 2005, 88(4), p 977–979
P. Dai, X. Zhao, D. Xu, C. Wang, X. Tao, X. Liu, and J. Gao, Preparation, Characterization, and Properties of Pt/Al2O3/Cordierite Monolith Catalyst for Hydrogen Generation from Hydrolysis of Sodium Borohydride in a Flow Reactor, Int. J. Hydrogen Energy, 2019, 44(53), p 28463–28470
Y. Jia, J. Shen, H. Meng, Y. Dong, Y. Chai, and N. Wang, Hydrogen Generation Using a Ball-Milled Al/Ni/NaCl Mixture, J. Alloy. Compd., 2014, 588, p 259–264
S.S. Razavi-Tousi, and J.A. Szpunar, Effect of Addition of Water-Soluble Salts on the Hydrogen Generation of Aluminum in Reaction with Hot Water, J. Alloy. Compd., 2016, 679, p 364–374
M.Q. Fan, F. Xu, and L.X. Sun, Hydrogen Generation by Hydrolysis Reaction of Ball-Milled Al−Bi Alloys, Energy Fuels, 2007, 21(4), p 2294–2298
M. Fan, F. Xu, and L. Sun, Studies on Hydrogen Generation Characteristics of Hydrolysis of the Ball Milling Al-based Materials in Pure Water, Int. J. Hydrogen Energy, 2007, 32(14), p 2809–2815
Y. Liu, X. Wang, Z. Dong, H. Liu, S. Li, H. Ge, and M. Yan, Hydrogen Generation from the Hydrolysis of Mg Powder Ball-milled with AlCl3, Energy, 2013, 53, p 147–152
Y. Liu, X. Liu, X. Chen, S. Yang, and C. Wang, Hydrogen Generation from Hydrolysis of Activated Al-Bi, Al-Sn Powders Prepared by Gas Atomization Method, Int. J. Hydrogen Energy, 2017, 42(16), p 10943–10951
J. Liang, L.J. Gao, N.N. Miao, Y.J. Chai, N. Wang, and X.Q. Song, Hydrogen Generation by Reaction of Al-M (M = Fe Co, Ni) with Water, Energy, 2016, 113, p 282–287
H. Okamoto, Al-Ni (Aluminum-Nickel), Journal of Phase Equilibria, 1993, 14(2), p 257
P. Nash, The Bi-Ni (Bismuth-Nickel) System, Bulletin of Alloy Phase Diagrams, 1985, 6(4), p 345–347
A.J. McAlister, The Al-Bi (Aluminum-Bismuth) System, Bulletin of Alloy Phase Diagrams, 1984, 5(3), p 247–250
J. Peng, P. Franke, D. Manara, T. Watkins, R.J.M. Konings, and H.J. Seifert, Experimental Investigation and Thermodynamic Re-assessment of the Al-Mo-Ni System, J. Alloy. Compd., 2016, 674, p 305–314
G.P. Vassilev, X.J. Liu, and K. Ishida, Experimental Studies and Thermodynamic Optimization of the Ni-Bi System, J. Phase Equilib. Diffus., 2005, 26(2), p 161–168
G.P. Vassilev, and J. Romanowska, Bismuth Activity Measurements and Thermodynamic Re-optimization of the Ni-Bi System, Int. J. Mater. Res., 2007, 98(6), p 468–475
D. Mirkovic, J. Grobner, I. Kaban, W. Hoyer, and R. Schmid-Fetzer, Integrated Approach to Thermodynamics, Phase Relations, Liquid Densities and Solidification Microstructures in the Al-Bi-Cu System, Int. J. Mater. Res., 2009, 100(2), p 176–188
H.X. Liu, C.P. Wang, Y. Yu, X.J. Liu, Y. Takaku, I. Ohnuma, R. Kainuma, and K. Ishida, Experimental Investigation and Thermodynamic Calculation of the Phase Equilibria in the Al-Bi-Sn Ternary System, J. Phase Equilib. Diffus., 2012, 33(1), p 9–19
C. Xu, F. Yin, M. Zhao, Y. Liu, and X. Su, Phase Equilibria of the Zn-Bi-Ni System at 600 °C and 750 °C, J. Alloy. Compd., 2010, 506(1), p 125–130
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Grant No. 51771158) and the Development and Reform Commission of Shenzhen Municipality.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M.S., Wei, H.T., Qiu, C.R. et al. Phase Equilibria in the Ni-Al-Bi Ternary System. J. Phase Equilib. Diffus. 42, 321–328 (2021). https://doi.org/10.1007/s11669-021-00885-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-021-00885-x