Abstract
The phase equilibria of the Sn-Zn-Sb ternary system at 400 and 500 °C were experimentally determined by electron probe microanalyzer, x-ray diffraction and differential scanning calorimetry. In this study, the ternary compound of (Zn,Sn)Sb is confirmed at 400 and 500 °C. Additionally, a liquid phase region is found at 400 °C with composition ranges of 9.09-12.86 at.% Zn and 31.47-34.22 at.% Sn, which becomes a prolongation of the main liquid as the temperature is raised to 500 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerns over the toxicity of lead have resulted in motivation to eliminate the use of solders containing Pb, and to multifarious efforts to replace lead-containing solder.[1] Since Sn-Zn based alloys have low melting temperature, good properties and are inexpensive, they have been considered as a good candidate to replace Sn-Pb eutectic alloys.[2-4] Also Sb and Zn are common components in alloys developed for lead-free soldering.[5,6] Thus Sn-Zn-Sb ternary alloys may be potential lead-free solders, and knowledge of its phase equilibrium will be necessary for the development of Sn-Zn-Sb ternary alloys.
In the previous experimental work, Zobac et al.[7] obtained information on the thermodynamic stability of the coexisting phase by using DTA method at 200, 250 and 350 °C.[7] However the phase equilibrium and the microstructure of the Sn-Zn-Sb ternary system at higher temperature are lacking. Positioning of the phase fields with mutual solubility of all three components and specification of the stability range of the ternary phase depend on the experimental research of the Sn-Zn-Sb ternary system.[7]
In the present work, the phase equilibrium of the Sn-Zn-Sb system at 400 and 500 °C were experimentally determined by using electron probe microanalyzer (EPMA), x-ray diffraction (XRD) and differential scanning calorimetry (DSC). The obtained results are expected to give a better understanding on microstructures in the Sn-Zn-Sb alloys.
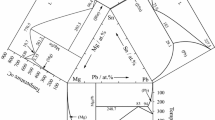
Three binary systems Sb-Zn,[8] Sb-Sn [9] and Sn-Zn,[10] constituting the Sn-Zn-Sb ternary system, are shown in Fig. 1. The Sb-Zn binary system has been studied by many researchers.[11-15] The Sb-Zn binary phase diagram assessed by Li et al.[8] is adopted in this work due to the good agreement with the experimental data. The Sb-Zn binary system has six intermediate phases, namely SbZn, γ-Sb3Zn4, β-Sb3Zn4, α-Sb3Zn4, β-Sb2Zn3 and α-Sb2Zn3, respectively. The phase diagram of the Sn-Zn binary system is a simple system without any intermediate phase. The Sb-Sn [9,16-19] binary system has two intermediate phases, Sb2Sn3 and SbSn phases. There are three peritectic reactions, L + Sb2Sn3 ↔ Sn, L + SbSn ↔ Sb2Sn3, and L + Sb ↔ SbSn in the Sb-Sn binary system, occuring at 243, 323 and 424 °C, respectively. The information of all stable solid phases in the three binary and ternary systems mentioned above is summarized in Table 1. It is notable that Sb2SnZn phase can exist as a polymorph as reported by Tengå et al.[21]: chalocopyrite phase (space group: I-42d) below 240 °C, and sphalerite phase (space group: F-43m) above 240 °C.
Binary phase diagrams constituting the Sn-Zn-Sb ternary system[8]
Experimental Procedures
Sn-Zn-Sb alloys were prepared using pure antimony (99.8 wt.%), zinc (99.8 wt.%) and tin (99.9 wt.%) in sealed transparent quartz capsules. In order to reduce the volatilization of zinc and ensure the samples homogeneity, the capsules were placed in argon atmosphere at 300 °C for 24 h. After that, the alloys were annealed at 800 °C for 72 h.
Afterwards, the samples were cut into small pieces. All samples were sealed in quartz capsules that are evacuated and backfilled with argon gas. The samples were annealed at 400 and 500 °C, respectively. On the basis of the temperature and composition of the samples, time of the heat treatment varied from several hours to several days. At the end of the heat treatment, the samples were quenched into ice water.
Compositions of the phases were determined using EPMA (JXA-8100R, JEOL, Japan). Pure elements were used as standards and the measurements were carried out at a voltage of 20 kV and a current of 20 nA. Microprobe measurements for each equilibrium phase were taken on seven points to get high accuracy. The composition of the liquid phase was determined via area analysis using EDS at 20.0 kV. Seven area measurements on the liquid phase were made to decrease the statistical error. At the same time, the maximum and minimum values were removed, and the final values were the average of the remaining values. The constituent phases of the alloys were further determined by analyzing XRD patterns generated by a Phillips PANalytical X-pert diffractometer, operating at 40.0 kV and 30 mA with Cu Kα radiation. The data were collected in the range of 2θ from 20° to 90° at a step size of 0.0167°. The phase transformation temperature was determined using DSC (DSC-404C, NETZSCH, Germany) test. And the measurements were conducted using small pieces of specimens ranging from 18 to 44 mg at a heating and cooling rates of 20 °C/min in a flowing argon atmosphere with sintered Al2O3 as reference.
Results and Discussion
Microstructure and Phase Equilibria
Back-scattered electron (BSE) images of typical ternary Sn-Zn-Sb alloys are shown Fig. 2(a)-(j). Phase identification is based on the equilibrium composition measured by EPMA and crystal structure determined by XRD analysis. For the Sn20Zn70Sb10 (at.%) alloy quenched from 400 °C, three phases microstructure is observed, as showed in Fig. 2(a). EPMA result indicates that the dark phase is (Zn), the grey one is β(Sb3Zn4), and the white one is Liquid (denoted as “L” in this study). This liquid phase is located in the Sn-rich region. Another liquid phase region is found in the Sb-rich region at 400 °C and will be discussed later in the following. Figure 2(b) shows three phases microstructure of the SbZn + (Zn,Sn)Sb + L in the Sn35Zn32Sb33 (at.%) alloy. These results have been confirmed by XRD analysis. XRD patterns of the Sn35Zn32Sb33 (at.%) alloy show characteristic peaks of Sn phase. So the existence of a liquid phase is confirmed. In the quenching process, it is inevitable the liquid phase decomposing to several solid phase, probably into Sn and either SbSn or Zn phase. After analyzing XRD result, we find that the characteristic peaks corresponding to SbSn or Zn phase are covered by those of SbZn phase. Although the liquid decomposes into either SbSn or Zn phase is reasonable from the binary phase diagrams, we haven’t found clear evidence to confirm the existence of SbSn or Zn phase. As shown in Fig. 3(a), it can be seen the characteristic peaks corresponding to Sn, SbZn and (Zn,Sn)Sb phases are well distinguished by different symbols. The BSE micrograph of the Sn20Zn40Sb40 (at.%) alloy annealed at 400 °C for 4 days is showed in Fig. 2(c), where two different phases (SbZn + L) are distinguished. Three-phase microstructure of the L + (Zn,Sn)Sb + SbZn in the Sn26Zn23Sb51(at.%) alloy annealed at 400 °C for 4 days is shown in Fig. 2(d). BSE image of the Sn10Zn20Sb70 (at.%) alloy annealed at 400 °C for 18 days is presented in Fig. 2(e), showing coexistence of three phases consisted of SbZn + (Sb) + L. To confirm the existence of the liquid phase, a DSC test for Sn10Zn20Sb70 (at.%) alloy was then carried out. As shown in Fig. 4, it can be seen that there is only one melting peak in the heating curve, in which the onset temperature is 396.4 °C. Clearly, the liquid phase appearing at 400 °C for this alloy is proved reasonable since the temperature applied in the phase equilibrium experiments is slightly higher than that in the DSC test. Figure 2(f) shows three-phase equilibrium microstructure consisted of the L + (Zn,Sn)Sb + SbSn for the Sn50Zn10Sb40 (at.%) alloy annealed at 400 °C. This observation is consistent with the analysis of XRD in Fig. 3(b). In Fig. 3(b), the diffraction peaks of the Sn phase, (Zn,Sn)Sb phase and SbSn phase are well identified and marked by different symbols.
Typical ternary BSE images obtained from: (a) Sn20Zn70Sb10 (at.%) alloy annealed at 400 °C for 4 days; (b) Sn35Zn32Sb33 (at.%) alloy annealed at 400 °C for 18 days; (c) Sn20Zn40Sb40 (at.%) alloy annealed at 400 °C for 4 days; (d) Sn26Zn23Sb51 (at.%) alloy annealed at 400 °C for 4 days; (e) Sn10Zn20Sb70 (at.%) alloy annealed at 400 °C for 18 days; (f) Sn50Zn10Sb40 (at.%) alloy annealed at 400 °C for 4 days; (g) Sn3Zn20Sb77 (at.%) alloy annealed at 500 °C for 30 days; (h) Sn20Zn50Sb30 (at.%) alloy annealed at 500 °C for 2 days; (i) Sn20Zn45Sb35 (at.%) alloy annealed at 500 °C for 2 days; (j) Sn65Zn10Sb25 (at.%) alloy annealed at 500 °C for 2 days
In addition, the microstructure of Sn3Zn20Sb77 (at.%) alloy annealed at 500 °C for 30 days is shown in Fig. 2(g), where there exists a two-phase equilibrium ((Sb) + SbZn). In the Sn20Zn50Sb30 (at.%) alloy quenched from 500 °C, the two-phase microstructure (Sb2Zn3 + L) is observed (Fig. 2h). Figure 2(i) shows the two-phase microstructure (Sb3Zn4 + L) of the Sn20Zn45Sb35 (at.%) alloy annealed at 500 °C for 2 days. In the Sn65Zn10Sb25 (at.%) alloy quenched from 500 °C, a two-phase microstructure of the L + (Zn,Sn)Sb is observed in Fig. 2(j). The XRD pattern of this sample is presented in Fig. 3(c), confirming the coexistence of two phases consisted of L + (Zn,Sn)Sb.
Isothermal Sections
All phase equilibria and related equilibrium compositions of the Sn-Zn-Sb ternary system at 400 and 500 °C determined in the present work by EPMA are summarized in Table 2. Based on the experimental data mentioned above, two isothermal sections at 40 and 500 °C have been constructed as presented in Fig. 5(a) and (b), respectively. Undetermined three-phase regions have dashed outlines.
In the isothermal section at 400 °C shown in Fig. 5(a), eight three-phase regions, two liquid regions and one ternary phase are experimentally determined. The solubility of Sn and Sb in (Zn) is extremely low, and the solubility of Sn in the α(Sb3Zn4) and SbZn phase is about 1.69 and 1.29 at.% through the determined isothermal section. In addition, the solubility of Zn in the (Sb) is measured to be approximately 1.33 at.%, and the solubility of Zn in the SbSn phase is measured to be about 2.22 at.%. It is interesting that a region of liquid phase appears at 400 °C in the composition ranges of 9.09-12.86 at.% Zn and 31.47-34.22 at.% Sn, which becomes a prolongation of the main liquid field when the temperature is raised up to 500 °C.
In the isothermal section at 500 °C (Fig. 5b), three three-phase regions of the L + (Zn,Sn)Sb + SbZn, L + (Sb) + SbZn and L + (Zn,Sn)Sb + SbZn are experimentally determined in this work. Compared with the isothermal section at 400 °C, the phase region of the ternary phase (Zn,Sn)Sb phase does not seem to be significantly different. In the previous study, Rinco et al.[20] considered the (Zn,Sn)Sb could only be stable below 759 K (486 °C). In addition, Tenga et al.[21] thought that (Zn,Sn)Sb intermetallic phase decomposes peritectically at about 360 °C, and cannot exist stably at 400 and 500 °C. This experimental result is different from the previous literature, because the (Zn,Sn)Sb phase can exist stably as a equilibrium phase at 400 and 500 °C, as measured by EPMA and substantiated by the XRD results, respectively.
Conclusions
Two isothermal sections of the Sn-Zn-Sb ternary system at 400 and 500 °C were experimentally determined. The newly determined phase equilibria will provide additional support for the thermodynamic assessment of this Sn-Zn-Sb system. The conclusions drawn from the present study are as follows:
-
(1)
A liquid phase region is found at 400 °C with composition ranges of 9.09-12.86 at.% Zn and 31.47-34.22 at.% Sn, and the area of liquid phase becomes a prolongation of the main liquid field when the temperature is raised to 500 °C.
-
(2)
The ternary compound of (Zn,Sn)Sb is confirmed to exist stably at 400 and 500 °C.
References
M.E. Loomans, S. Vaynman, G. Ghosh, and M.E. Fine, Investigation of Multi-component Lead-Free Solders, J. Electron. Mater., 1994, 23, p 741-746
M. Abtew and G. Selvaduray, Lead-Free Solders in Microelectronics, Mater. Sci. Eng. R, 2000, 27, p 95-141
C.W. Huang and K.L. Lin, Interfacial Reactions of Lead-Free Sn-Zn Based Solders on Cu and Cu Plated Electroless Ni-P/Au Layer Under Aging at 150 °C, J. Mater. Res., 2004, 19, p 3560-3568
S.W. Chen, C.H. Wang, S.K. Lin, and C.N. Chiu, Phase Diagrams of Pb-free Solders and Their Related Materials Systems, J. Mater. Sci., 2007, 18, p 19-37
T.C. Chang, M.C. Wang, and M.H. Hon, Thermal Properties and Interfacial Reaction Between the Sn-9Zn-xAg Lead-Free Solders and Cu Substrate, Metall. Mater. Trans. A, 2005, 36, p 3019-3029
D.R. Frear and P.T. Vianco, Intermetallic Growth and Mechanical Behavior of Low and High Melting Temperature Solder Alloys, Metall. Mater. Trans. A, 1994, 25, p 1509-1523
O. Zobac, J. Sopousek, J. Bursik, A. Zemanova, and P. Roupcova, Experimental Study of the Sb-Sn-Zn Alloy System, Metall. Mater. Trans. A, 2014, 45, p 1181-1188
J.B. Li, M.C. Record, and J.C. Tedenac, A Thermodynamic Assessment of the Sb-Zn System, J. Alloy. Compd., 2007, 438, p 171-177
B. Predel and W. Schwermann, Constitution and Thermodynamics of Antimony-Tin System, J. Inst. Met., 1971, 99, p 169-173
Z. Moser, J. Dutkiewicz, W. Gasior, and J. Salawa, The Sn-Zn (Tin-Zinc) System, J. Phase Equilib., 1985, 6, p 330-334
G. Vuillard and J.P. Piton, Transformations of Intermetallic Phases of the Antimony-Zinc Systems, Comput. Rend. Acad. Sci., 1966, 263, p 1018-1021
X.J. Liu, C.P. Wang, I. Ohnuma, R. Kainuma, and K. Ishida, Thermodynamic Assessment of the Phase Diagrams of the Cu-Sb and Sb-Zn Systems, J. Phase Equilib., 2000, 21, p 432-442
V. Izard, M.C. Record, J.C. Tedenac, and S.G. Fries, Discussion on the Stability of the Antimony Zinc Binary Phases, CALPHAD, 2001, 25, p 567-581
F. Adjadj, E. Belbacha, M. Bouharkat, and A. Kerboub, Crystallographic Study of the Intermediate Compounds SbZn, Sb3Zn4 and Sb2Zn3, J. Alloy. Compd., 2006, 419, p 267-270
F. Adjadj, E. Belbacha, and M. Bouharkat, Differential Calorimetric Analysis of the Binary System Sb-Zn, J. Alloy. Compd., 2007, 430, p 85-91
F.E. Gallagher, The Alloys of Antimony and Tin, J. Phys. Chem., 1906, 10, p 93-98
K. Iwasé, N. Aoki, and A. Osawa, Sci. Rep. Tóhoku Univ. Bd, 1931, 20, p 353-368
A. Stegherr, Doktor-Ingenieurs Genehmigte, Dissertation. Technischen Hochschute, Aachen, 1969
H. Ohtani, K. Okuda, and K. Ishida, Thermodynamic Study of Phase Equilibria in the Pb-Sn-Sb System, J. Phase Equilib., 1995, 16, p 416-429
C. Rinco, Order-Disorder Transition in Ternary Chalcopyrite Compounds and Pseudobinary Alloys, Phys. Rev. B, 1992, 45, p 12716
A. Tengå, F.J. García-García, and A.S. Mikhaylushkin, Sphalerite-Chalcopyrite Polymorphism in Semimetallic ZnSnSb2, Chem. Mater., 2005, 17, p 6080-6085
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 51031003 and 51171159) and the Ministry of Education of China (Grant No. 20120121130004). The support from National Key Basic Research Program of China (973 Program, 2012CB825700), the Aviation Industry Corporation of China and Xiamen City Department of Science & Technology (Grant No. 3502Z20121153) are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Xu, Y., Yang, S. et al. Experimental Determination of Phase Equilibria in the Sn-Zn-Sb System. J. Phase Equilib. Diffus. 36, 350–356 (2015). https://doi.org/10.1007/s11669-015-0387-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-015-0387-1