Abstract
(CoCrFeNi)94Ti1.5Al4.5 high entropy alloy (HEA) coatings were prepared on Q235 steel substrate by plasma cladding and plasma spraying method to investigate the effect of minor addition of Ti, Al elements and different microstructure evolution on the corrosion resistance. After 18 h of annealing at 800 °C, the microstructure of the cladded HEA coatings presented typical dendrite structure, and the precipitated phases appeared in the interdendrite region. The corrosion current densities for the cladded CoCrFeNi coating, (CoCrFeNi)94Ti1.5Al4.5 coatings without and with annealing and plasma sprayed (CoCrFeNi)94Ti1.5Al4.5 HEA coatings were 0.040, 0.166, 0.016, and 1.276 μA/cm2, respectively, while the corrosion potentials were approximately the same. The corrosion resistance of the cladded coatings was better than that of the sprayed coating. With the addition of Ti and Al elements, the passive region was broadened. The corrosion resistance was improved after annealing, whereas galvanic corrosion was easily formed between precipitates and dendrite region, which was prone to pitting corrosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, high entropy alloys (HEAs) have become a research hotspot in the field of materials. Their unique "high entropy effect" can effectively inhibit the nucleation and growth of second phases (Ref 1), making them easier to form single-phase disordered solid solution structures such as BCC, FCC, and HCP structures (Ref 2,3,4,5). Therefore, HEAs have more excellent properties than traditional alloys. Early research on HEAs mainly focused on the trade-off between strength and ductility, and then gradually shifted to both strength and other properties (Ref 6), such as wear resistance (Ref 7,8,9), oxidation resistance (Ref 10), and corrosion resistance (Ref 11,12,13). These excellent properties can be combined with surface modification technology to obtain wide industrial applications (Ref 14). Among these surface modification technologies, plasma cladding technology has attracted increasing attention in the preparation of HEA coatings due to its advantages of low thermal deformation, low dilution of substrate materials, and good interfacial bonding strength (Ref 15, 16).
For corrosion-resistant HEA coatings, due to the high ionic resistivity in the surface layer, it can form a barrier to prevent the penetration of erosion agent, minimize the electrochemical reaction under the coating, and then provide corrosion protection (Ref 17). To prepare high performance corrosion resistance HEA coatings, it is necessary to understand the effect of composition and microstructure on corrosion resistance. CoCrFeNi-based HEA system has excellent corrosion resistance. Its single solid solution structure can avoid pitting or galvanic corrosion induced by element segregation, and it is also conducive to the growth of passive film (Ref 6, 18). Although the distribution of elements in the CoCrFeNi HEA is uniform, the formed passive films have a weak bonding force and insufficient stability (Ref 19), which impairs the corrosion resistance. Therefore, it is necessary to add passivation elements in CoCrFeNi HEA to improve its stability and protection. In the current studies, it is confirmed that the addition of Ti element promotes the formation of a stable passive film containing TiO2 and Ti2O3 on the surface of HEA coatings (Ref 20). Similarly, the addition of spontaneous passivation Al element in AlxCoCrFeNi alloy makes the passive film thicker and more dispersed (Ref 21). However, the addition of elements can also produce complex precipitates and destroy the uniformity of microstructure. For example, the addition of Ti element into the FeCoCrAlNi HEA will generate the Ti2Ni phase, resulting in a decrease in cavitation resistance. (Ref 22). Therefore, structure-control is also an important part of the preparation of corrosion-resistant HEA coatings. For example, the high-density grain boundaries of ultrafine-grained Cantor alloy tend to be the weak point against corrosion, while improved corrosion resistance could be achieved by coarsening the grains (Ref 23). In previous research, the effective structure-control methods for improving corrosion resistance were aging, annealing and remelting (Ref 24,25,26).
In this study, a small amount of passivation elements of Ti and Al were incorporated into CoCrFeNi to form (CoCrFeNi)94Ti1.5Al4.5 feedstock. (CoCrFeNi)94Ti1.5Al4.5 HEA coatings were then prepared by plasma cladding and plasma spray methods. The (CoCrFeNi)94Ti1.5Al4.5 HEA coating was annealed after cladding process. The effects of composition, processing, and annealing on the corrosion resistance of HEA coatings were investigated. Finally, the evolution process of corrosion was discussed.
Experimental Procedures
Preparation of HEA Coatings
CoCrFeNi and (CoCrFeNi)94Ti1.5Al4.5 feedstock powders were synthesized by the atomization method. The morphology and chemical composition are shown in Fig. 1. The CoCrFeNi and (CoCrFeNi)94Ti1.5Al4.5 HEA coatings were prepared on a Q235 steel substrate (100 mm×100 mm×10 mm) by plasma cladding. To study the influence of heat treatment, as-cladded (CoCrFeNi)94Ti1.5Al4.5 HEA coating was annealed at 800 °C for 18 h. And for comparison, (CoCrFeNi)94Ti1.5Al4.5 HEA coating was fabricated on the same size Q235 steel substrate by atmospheric plasma spraying (APS). The parameters of the two processes are listed in Table 1. The four samples and their numbers are listed in Table 2.
Analysis of Microstructure
The phases in the HEA coatings were determined by x-ray diffraction (XRD, Shimadzu 7000, Kyoto, Japan) using Cu Kα radiation over a scanning angle in the range of 20-100° at a scanning rate of 4 deg/min. The morphology of the HEA coating cross section and corrosion surface was observed by field-emission scanning electron microscopy (FESEM, GeminiSEM 300, Germany) in secondary electron (SE) and backscattered electron (BSE) mode, which was equipped with energy dispersive spectrometry (EDS, Oxford INCA x-act, Britain) for elemental analysis on the specimen surface.
Electrochemical Measurements
Corrosion behavior was investigated by electrochemical analysis. The corrosion samples were electrically connected by a copper wire and mounted by epoxy resin in cylindrical silicone molds. The exposed area of sample to the solution was 1 cm2. Three electrode cells were used for the corrosion investigation. The working, counter and reference electrodes were the prepared specimen, platinum electrode, and saturated calomel electrode (SCE), respectively. The electrolyte used in this study was a 3.5 wt.% NaCl solution at room temperature, in which each type of alloy was tested at least three times to ensure the accuracy of data.
The open circuit potential (OCP) was recorded for 3000 s to yield the steady-state potential. Potentiodynamic polarization tests were performed at a scan rate of 1 mV/s for potential ranging from − 0.25 VOCP to the final potential corresponding to the current density of 1 mA/cm2. Electrochemical impedance spectroscopy (EIS) was recorded in a range of frequencies from 100 KHz to 10 mHz. The amplitude of sinusoidal signals was 10 mV around the OCP.
Results and Discussion
Phase Constitutions
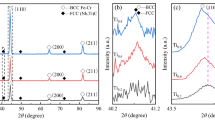
X-ray diffraction patterns of the non-TA, TA1, TA2, and TA3 HEA coatings are shown in Fig. 2(a). The phase of the non-TA coating presents a typical simple FCC crystal structure, and diffraction peaks appear at 43.84°, 50.94°, 75.10°, 91.16°, and 96.55°, corresponding to the lattice planes of (111), (200), (220), (311), and (222), respectively. With the addition of Al and Ti elements, no new peaks appear, indicating that minor Al and Ti atoms formed a solid solution with the CoCrFeNi matrix, and there was no new phase formed. A magnified view of the diffraction pattern over an angular range from 40° to 55° is shown in Fig. 2(b). The diffraction angle, full width at half maxima (FWHM), and peak height are listed in Table 3.
With the addition of Ti and Al elements, the diffraction angles of both TA1 and TA2 shift toward small angle as compared with non-TA. According to Bragg's law:
where d is the interplanar distance, θ is the diffraction angle, n is the diffraction level, and λ is the wavelength of the x-ray. The smaller diffraction angle corresponds to the larger interplanar distance, which is attributed to Al atoms with larger radii that result in lattice distortion. Since the Al atoms were oxidized during spraying and were not alloyed, the diffraction angle of TA3 did not shift. The grain size of the HEA solid solution can be calculated by Scherrer's formula:
where D is the microcrystal size, k is the Scherrer constant, γ is the wavelength of the x-ray, B is the FWHM, and θ is the diffraction angle. The FWHM of TA1 and TA2 is larger than that of non-TA, indicating that the average grain size of TA1 and TA2 is smaller due to the grain refinement caused by the addition of Ti element (Ref 27). The volume fraction of the FCC phase in non-TA is the largest, corresponding to the highest peak height, which is reduced in sprayed TA3 due to the oxidation of elements. The lattice distortion caused by Al element also reduces the diffraction intensity of the FCC phase, so the peak heights of TA1 and TA2 are lower than that of non-TA. The effect of the latter on peak height is significantly greater than that of the former. The effect of crystal structure on corrosion resistance is mainly reflected by the increase in grain boundary density due to grain refinement, which will be discussed in the following text.
Microstructural and Chemical Composition
Figure 3(a) shows the cross-section morphology of the TA1 coating, which presents the expected uniform structure without element segregation and precipitation. As shown in Fig. 3(b) and (c), the network dendrite structure which consists of the white dendrite (DR) region and the black interdendrite (ID) region appeared after annealing. Interestingly, the orientation of dendrite growth is different, which is due to the difference in heating direction during cladding. The inset of Fig. 3(c) shows the magnified morphology at the grain boundaries, which shows that nano-scale spherical precipitates appear in the dendrite region, and the particle size in the grain boundaries is larger than that in the dendrite region. These precipitates may affect the corrosion behavior of the HEA coating, which will be discussed in detail in the following text. In the cladded TA1 and TA2 coatings, as shown in Fig. 4, the segregation of Ti and Al elements in the dendrite region and white particle region is opposite to that of Co, Cr, and Fe elements, while it is more obvious after heat treatment. The growth of dendrites, and the formation of precipitation, and the aggravation of segregation together indicate that heat treatment promotes the diffusion of elements.
As shown in Fig. 3(d), the sprayed TA3 coating presents a flat layered microstructure that consists of four typical structures: thick lamellar alloy splats, thin lamellar alloy-oxide splats, alloy unmelts, and discontinuous oxide splats (marked with A, B, C, and D in the figure, respectively). Due to in-flight oxidation of feedstock powder in spraying, the oxygen content in the sprayed coating is approximately 20.32 at.%, which is much higher than that in the cladded coatings (less than 2 at.%). Figure 4 shows that the main elements of oxidation are Ti, Al, and Cr elements. The accurate quantitative analysis results are shown in Table 4. This complex microstructure affects the stability of the passive film structure, and makes pitting and galvanic corrosion easier (Ref 28). All these facts show that as compared to plasma spraying technology, the plasma cladding method is more suitable for the preparation of HEA coatings with uniform microstructure which are expected to have high corrosion resistance.
Corrosion Analysis
Potentiodynamic Polarization Measurements
Figure 5 shows the potentiodynamic polarization curves of the non-TA, TA1, TA2, and TA3 HEA coatings detected in the 3.5 wt.% NaCl solution at room temperature, and the parameters of corrosion behavior such as corrosion potential (Ecorr), corrosion current density (icorr), passivation potential (Ep), current density in the passive region (ipass), breakdown potential (Eb), and length of the passive zone (ΔE) were extracted, as shown in Table 5. Each of the four curves shows a similar spontaneous passive behavior, indicating that the oxide film is formed in the electrochemical corrosion process and hinders electron exchange. The passivation region of TA1 is wider than that of non-TA, and secondary passivation occurs, which can be attributed to the contribution of passivation elements. However, icorr and ipass also increased due to the porosity and weak protection of passive films containing Al element (Ref 29), which indicates that the protection of the passive film decreased. The addition of elements broadens the passivation region, increases the corrosion surface activity and makes the passivation film loose, thereby reducing the corrosion resistance of the coating. Therefore, it is necessary to post-treat the coating after adding passivation elements. After annealing at 800 °C for 18 h, the TA2 coating has the smallest icorr and ipass, indicating that the annealed microstructure has better corrosion resistance. However, the passivation region of TA3 is narrow, which is caused by the precipitation phase observed in section 3.2. The precipitation phase can form a galvanic cell with dendrites, pitting corrosion is more likely to occur, and the passive film is more unstable (Ref 30). The TA3 coating has the narrowest passivation region and the largest icorr and ipass, which corresponds to the worst corrosion resistance. This shows that compared with APS, the plasma cladding method is more suitable for the preparation of high performance corrosion-resistant HEA coatings. However, the values of icorr and ipass are calculated in the Tafel region, and the effect of passive film is not reflected. The corrosion resistance of the coating under a certain applied potential will be discussed in the later corrosion morphology characterization. The current fluctuation in polarization curve indicates metastable pitting corrosion (Ref 31), which is attributed to dissolution and re-passivation. During the growth of pitting, anodic dissolution causes the current to rise slowly, while re-passivation causes the current to decay suddenly (Ref 32). The fluctuation in TA1 is significantly smaller than that in TA0, because the HEA containing passivation elements re-passivates faster.
EIS Test
Figure 6 shows the Nyquist and Bode plots of the non-TA, TA1, and TA2 HEA costings. In Fig. 6(a) and (b), all three curves deviate from the y-axis, indicating that the corrosion surface is inhomogeneous (Ref 33). This reflects the property that the electrode double-layer deviates from the ideal capacitance. An inductive reactance arc appears in the low frequency part of the TA2 impedance diagram, as shown in Fig. 6(b), indicating that there are intermediate products in the electrode reaction, which are adsorbed on the surface of the working electrode to produce surface adsorption complexes. The inductive reactance arc appears because the relaxation time constant of the adsorption process is much larger than that of the charge discharge process composed of an electric double-layer capacitor (Ref 34), which is confirmed in Fig. 6(d).
An equivalent electrical circuit is shown in Fig. 7, which is composed of electrolyte, corrosion product film, double electrode, and HEAs coating. To simulate the inhomogeneous surface, constant phase element (CPE) is used to instead of capacitance. The circuit elements in the figure are solution resistance (Rs), charge transfer resistance (Rct) and corresponding double layer CPEdl, oxide layer resistance (Rf) and corresponding CPEf, and inductance (L). The fitting results of the equivalent circuit are shown in Fig. 6. The results indicate that the equivalent electrical circuit matches the actual situation. The parameters of the impedance spectra are listed in Table 6. The resistance value of TA2 is much smaller, indicating that the formation of surface adsorption complexes affects the corrosion resistance of the alloy. The TA1 and TA2 HEA coatings have a higher oxide layer resistance than non-TA, indicating that the protection ability of passive film is stronger with the addition of Ti and Al elements. These viewpoints are consistent with the analysis results of polarization curves.
Figure 8 shows the corrosion surface morphologies of the non-TA, TA1, TA2, and TA3 coatings which were linear scanned to 0.4 VSCE. As shown in Fig. 8(a-c), the corrosion surfaces of the non-TA, TA1, and TA2 coatings are smooth surfaces with a small number of pits. The extent of corrosion is evaluated by the number of pitting. TA1 is the most serious and TA2 is the lightest, which is related to their breakdown potential and microstructure. With the addition of passivation elements, the breakdown potential of TA1 is higher than that of non-TA. The passive film of non-TA was damaged and failed under a specific applied potential, while the passive film of TA1 was still protective. Therefore, TA1 coating is less corrosive. After annealing treatment, both dendritic segregation and large-scale precipitates at grain boundaries can induce pitting corrosion. The obvious dendrite structure can be observed in Fig. 8(c), and pitting occurs mostly at grain boundaries. Therefore, the corrosion of the TA2 coating is the most serious. These phenomena indicate that the protection of passive film is an important factor determining the corrosion resistance of HEA coatings. Therefore, it is important to improve the protection and stability of the passivation film during the preparation of corrosion resistance HEA coatings. As compared to the cladded coating, the corrosion of the sprayed coating is more serious. As shown in Fig. 8(d), dense and deep corrosion pits often occur at the junction of alloy and oxide. The mechanical bonding between the oxide and alloy is weak, which is easily attacked by Cl ions, and then local corrosion occurs, resulting in oxide shedding and eventually forming corrosion pits. A schematic diagram is shown in Fig. 9.
Evolution of Corrosion
As mentioned above, the cladded HEA coating has better protection than sprayed HEA coatings, so in this section, TA1 will be taken as an example to study its corrosion behavior. According to the morphologies of different corrosion extent shown in Fig. 10, a series of mechanisms of cladded (CoCrFeNi)94Ti1.5Al4.5 HEA coating for corrosion behavior in chloride solution can be proposed. There are three stages of corrosion evolution: first, the pitting (Fig. 10a) becomes larger, and filled with smaller holes at its outer edge (Fig. 10c). Figure 10(a) shows the selective leaching corrosion around the pitting, which is illustrated in Fig. 10(b). The chemical compositions of regions A, B, and C are shown in Table 7. The atomic percentages of Fe, Co, and Ni elements in the post-corrosion region (region B) decreased obviously, indicating that these elements dissolved out of the alloy first. The propagation of cracks around the white titanium oxide particles (region C) causes the surface film structure to fall off and the pitting hole become larger. Second, the pitting holes are interconnected (Fig. 10d) to form strip corrosion pits (Fig. 10e). At this stage, the applied potential (approximately 1.5 VSCE) exceeds the breakdown potential, indicating that the protection of the coating has basically disappeared. Finally, the surface film is completely shedding, exposing the porous HEA coating surface, as shown in Fig. 10(f). The corrosion evolution process of cladded HEA coatings is shown in Fig. 11.
Conclusions
-
(1)
In (CoCrFeNi)94Ti1.5Al4.5 HEA coatings, minor Ti and Al elements formed solid solution with the CoCrFeNi matrix, and maintained the FCC structure. Both the lattice distortion caused by Al atoms and in-flight oxidation during spraying lead to a decrease in the volume fraction of the FCC phase.
-
(2)
The cladded HEA coatings presented a network dendrite microstructure. After annealing, nano-scaled precipitates appeared in dendrite regions. The sprayed HEA coating showed a different flat layered microstructure.
-
(3)
Corrosion resistance varied with composition and structure. With minor addition of Ti and Al elements, the passivation region became wider, but corrosion resistance decreased. The corrosion resistance of the annealed HEA coating was the best, but due to the existence of precipitates, it was prone to pitting corrosion and the passivation zone was narrow. The corrosion resistance of the sprayed coating was worse than that of the cladded coating.
-
(4)
During the corrosion evolution, the pitting corrosion expanded due to selective dissolution, and then interconnected and formed strip corrosion pits, finally causing the surface film to fall off completely, exposing the porous HEA coating surface.
References
D.B. Miracle and O.N. Senkov, A Critical Review of High Entropy Alloys and Related Concepts, Acta Mater., 2017, 122, p 448-511.
J.W. Yeh, S.K. Chen, S.J. Lin, J.Y. Gan, T.S. Chin, T.T. Shun, C.H. Tsau and S.Y. Chang, Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes, Adv. Eng. Mater., 2004, 6(5), p 299-303.
Z. Lyu, C. Lee, S.Y. Wang, X.S. Fan, J.W. Yeh and P.K. Liaw, Effects of Constituent Elements and Fabrication Methods on Mechanical Behavior of High-Entropy Alloys: A Review, Metall. Mater. Trans. A, 2019, 50(1), p 1-28.
J.W. Yeh, S.J. Lin, T.S. Chin, J.Y. Gan, S.K. Chen, T.T. Shun, C.H. Tsau and S.Y. Chou, Formation of Simple Crystal Structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V Slloys with Multiprincipal Metallic Elements, Metall. Mater. Trans. A, 2004, 35(8), p 2533-2536.
S.Y. Jiang, Z.F. Lin, H.M. Xu and Y.X. Sun, Studies on the Microstructure and Properties of AlxCoCrFeNiTi1-x High Entropy Alloys, J. Alloys Compd., 2018, 741(1), p 826-833.
Y. Fu, J. Li, H. Luo, C.W. Du and X.G. Li, Recent Advances on Environmental Corrosion Behavior and Mechanism of High-Entropy Alloys, J. Mater. Sci. Technol., 2021, 80, p 217-233.
B.Q. Jin, N.N. Zhang, H.S. Yu, D.X. Hao and Y.L. Ma, AlxCoCrFeNiSi High Entropy Alloy Coatings with High Microhardness and Improved Wear Resistance, Surf. Coat. Technol., 2020, 402(4), p 126328.
D.Y. Lin, N.N. Zhang, B. He, X. Gong, Y. Zhang, D.Y. Li and F.Y. Dong, Structural Evolution and Performance Changes in FeCoCrNiAlNbx High-Entropy Alloy Coatings Cladded by Laser, Spray. Tech., 2017, 26, p 2005-2012.
Y.F. Liu, T. Chang, X.B. Liu, Y. Zhu, G. Wang, Y. Meng, J. Liang and J.C. Xie, Research Progress on Tribological Properties of High-Entropy Alloy Coatings, Surf. Technol., 2021, 50(8), p 156169.
J. Sienkiewicz, S. Kuroda, H. Murakami, H. Araki, M. Gizynski and K.J. Kurzydlowski, Fabrication and Oxidation Resistance of TiAl Matrix Coatings Reinforced with Silicide Precipitates Produced by Heat Treatment of Warm Sprayed Coatings, J. Therm. Spray. Tech., 2018, 27, p 1165-1176.
D.X. Hao, N.N. Zhang, Y. Zhang and D.Y. Li, Effect of Vanadium Addition on Microstructure and Properties of Al0.5Cr0.9FeNi2.5 Multi-Principal Alloys, J. Iron Steel Res. Int., 2021, 28, p 586-596.
W.J. Hao, R.L. Sun, W. Niu, X.L. Li, M. Gu and R.Y. Zuo, Study on Microstructure and Corrosion Resistance of CoCrFeNiSix High-Entropy Alloy Coating by Laser Cladding, Surf. Technol., 2021, 50(8), p 343-348.
B. Ren, Z.X. Liu, D.M. Li, L. Shi, B. Cai and M.X. Wang, Corrosion Behavior of CuCrFeNiMn High Entropy Alloy System in 1 M Sulfuric Acid Solution, Mater. Corros., 2015, 63(9), p 828-834.
A. Meghwal, A. Anupam, B.S. Murty, C.C. Berndt, R.S. Kottada and A.S.M. Ang, Thermal Spray High-Entropy Alloy Coatings: A Review, J. Therm. Spray. Tech., 2020, 29(11), p 857-893.
J. Wang, B. Zhang, Y. Yu, Z. Zhang and Z. Wang, Study of High Temperature Friction and Wear Performance of (CoCrFeMnNi)85Ti15 High-Entropy Alloy Coating Prepared by Plasma Cladding, Surf. Coat. Technol., 2020, 384, p 125337.
Y.A. Alshataif, S. Sivasankaran, F.A. Al-Mufadi, A.S. Alaboodi and H.R. Ammar, Manufacturing Methods, Microstructural and Mechanical Properties Evolutions of High-Entropy Alloys: A Review, Met. Mater. Int., 2020, 26(8), p 1099-1133.
Y. Shi, B. Yang, X. Xie, J. Brechtl, A.K. Dahmen and P.K. Liaw, Corrosion of AlxCoCrFeNi High-Entropy Alloys: Al-content and Potential Scan-rate Dependent Pitting Behavior, Corros. Sci., 2017, 119(5), p 33-45.
A. Meghwal, A. Anupam, B.S. Murty, C.C. Berndt and A.S.M. Ang, Thermal Spray High-Entropy Alloy Coatings: A Review, J. Therm. Spray. Tech., 2020, 29(11), p 857-893.
P. Muangtong, A. Rodchanarowan, D. Chaysuwan, N. Chanlek and R. Goodall, The Corrosion Behaviour of CoCrFeNi-x (x = Cu, Al, Sn) High Entropy Alloy Systems in Chloride Solution, Corros. Sci., 2020, 172, p 108740.
J. Liu, H. Liu, P.J. Chen and J.B. Hao, Microstructural Characterization and Corrosion Behaviour of AlCoCrFeNiTix High-Entropy Alloy Coatings Fabricated by Laser Cladding, Surf. Coat. Technol., 2019, 361, p 63-74.
Y.F. Kao, T.D. Lee, S.K. Chen and Y.S. Chang, Electrochemical Passive Properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) Alloys in Sulfuric Acids, Corros. Sci., 2010, 52(3), p 1026-1034.
C.L. Wu, S. Zhang, C.H. Zhang, H. Zhang and S.Y. Dong, Phase Evolution and Cavitation Erosion-corrosion Behavior of FeCoCrAlNiTix High Entropy Alloy Coatings on 304 Stainless Steel by Laser Surface Alloying, J. Alloys Compd., 2017, 698, p 761-770.
Z.H. Han, W.N. Ren, J. Yang, A. Tian, Y.Z. Du, G. Liu, R. Wei, G.J. Zhang and Y.Q. Chen, The Corrosion Behavior of Ultra-fine Grained CoNiFeCrMn High-Entropy Alloys, J. Alloys Compd., 2020, 816, p 152583.
W. Wang, J. Wang, Z. Sun, J. Li and X. Yang, Effect of Mo and Aging Temperature on Corrosion Behavior of (CoCrFeNi)100-xMox High-Entropy Alloys, J. Alloys Compd., 2020, 812, p 152139.
X. Zhang, J. Guo, X. Zhang, Y. Song, Z. Li, X. Xing and D. Kong, Influence of Remelting and Annealing Treatment on Corrosion Resistance of AlFeNiCoCuCr High Entropy Alloy in 3.5% NaCl Solution, J. Alloys Compd., 2019, 775, p 565-570.
Y. Qiu, M.A. Gibson, H.L. Fraser and N. Birbilis, Corrosion Characteristics of High Entropy Alloys, Mater. Sci. Technol., 2015, 31(10), p 1235-1243.
J. Pan, T. Dai, T. Lu, X. Ni, J. Dai and M. Li, Microstructure and Mechanical Properties of Nb25Mo25Ta25W25 and Ti8Nb23Mo23Ta23W23 High Entropy Alloys Prepared by Mechanical Alloying and Spark Plasma Sintering, Mater. Sci. Eng., 2018, 738(19), p 362-366.
W.R. Zhang, P.K. Liaw and Y. Zhang, Science and Technology in High-Entropy Alloys, Sci. China Mater., 2018, 61(1), p 2-22.
C.P. Lee, C.C. Chang, Y.Y. Chen, J.W. Yeh and H.C. Shi, Effect of the Aluminium Content of AlxCrFe1.5MnNi0.5 High-Entropy Alloys on the Corrosion Behaviour in Aqueous Environments, Corros. Sci., 2008, 50(7), p 2053-2060.
J.Y. Pang, T. Xiong, X.X. Wei, Z.W. Zhua, B. Zhang, Y.T. Zhou, X.H. Shao, Q.Q. Jin, S.J. Zheng and X.L. Ma, Oxide MnCr2O4 Induced Pitting Corrosion in High Entropy AlloyCrMnFeCoNi, Materialia, 2019, 6, p 100275.
C.W. Lu, Y.S. Lu, Z.H. Lai, H.W. Yen and Y.L. Lee, Comparative Corrosion Behavior of Fe50Mn30Co10Cr10 Dual-phase High-Entropy Alloy and CoCrFeMnNi High-Entropy Alloy in 3.5 wt.% NaCl Solution, J. Alloys Compd., 2020, 842, p 155824.
Y.Z. Shi, J.K. Mo, F.Y. Zhang, B. Yang, P.K. Liaw and Y. Zhao, In-situ Visualization of Corrosion Behavior of AlxCoCrFeNi High-Entropy Alloys During Electrochemical Polarization, J. Alloys Compd., 2020, 844, p 156014.
P. Bommersbach, C. Alemany-Dumont, J.P. Millet and B. Normand, Formation and Behaviour Study of an Environment-friendly Corrosion Inhibitor by Electrochemical Methods, Electrochim. Acta, 2005, 51(6), p 1076-1084.
Z.H. Jin, H.H. Ge, W.W. Lin, Y.W. Zong, S.J. Liu and J.M. Shi, Corrosion Behaviour of 316L Stainless Steel and Anti-corrosion Materials in a High Acidified Chloride Solution, Appl. Surf. Sci., 2014, 322(15), p 47-56.
Acknowledgments
This work was financially supported by Natural Science Foundation of Liaoning Province (No. 2019-MS-247), Liao Ning Revitalization Talents Program (XLYC1807178) and Chinese National Natural Science Foundation (52001216).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a special topical focus in the Journal of Thermal Spray Technology on High Entropy Alloy and Bulk Metallic Glass Coatings. The issue was organized by Dr. Andrew S.M. Ang, Swinburne University of Technology; Prof. B.S. Murty, Indian Institute of Technology Hyderabad; Distinguished Prof. Jien-Wei Yeh, National Tsing Hua University; Prof. Paul Munroe, University of New South Wales; Distinguished Prof. Christopher C. Berndt, Swinburne University of Technology. The issue organizers were mentored by Emeritus Prof. S. Ranganathan, Indian Institute of Sciences.
Rights and permissions
About this article
Cite this article
Xing, B., Zuo, X., Li, Q. et al. Influence of Microstructure Evolution on the Electrochemical Corrosion Behavior of (CoCrFeNi)94Ti1.5Al4.5 High Entropy Alloy Coatings. J Therm Spray Tech 31, 1375–1385 (2022). https://doi.org/10.1007/s11666-022-01364-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-022-01364-6